- Academic Editor
Chronic fatigue syndrome is primarily caused by myalgic encephalomyelitis (ME)-associated dysfunction of the central nervous system. Postural instability or disequilibrium is a typical neural sign and is classified as static or kinetic.
A total of 160 ME patients (53 males and 107 females) with a mean age of 37 ± 12 years were enrolled in this study. They underwent both the Romberg test for static disequilibrium and the tandem gait test with turn and return for kinetic disequilibrium.
Static disequilibrium was found in 40 (25%) patients who showed instability when standing with both feet together and eyes either open (n = 7, 4%) or closed (n = 33, 21%). Kinetic disequilibrium was found in 71 (44%) patients, with 57 (36%) being positive for the straight tandem gait test. Fourteen (9%) patients were negative for the straight tandem gait test, but showed a positive result after turning and returning. Almost all patients with static disequilibrium also had kinetic disequilibrium (39/40, 98%). Patients with static and/or kinetic disequilibrium had a significantly higher prevalence of orthostatic intolerance, diagnosed as failure to complete the 10-min standing test, compared with patients without disequilibrium. They also had a significantly higher median performance status score (0–9) for restricted activities of daily living. Both types of disequilibria were recovered in 11 (85%) of 13 patients treated with repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral prefrontal cortex and primary motor area in the brain, suggesting a central vestibular origin.
Static disequilibrium related to orthostatic intolerance, and kinetic disequilibrium related to gait disturbance are both prevalent in patients with ME and are important central neural signs that restrict activities of daily living. rTMS treatment effectively alleviated these disequilibria.
The study has been registered on https://jrct.mhlw.go.jp/ (registration number: jRCT1042240065; registration date: July 30, 2024).
Chronic fatigue syndrome (CFS) is characterized by severe disabling fatigue not resolved by rest, prolonged post-exertional malaise, and unrefreshing sleep. It markedly reduces the ability to perform activities of daily living and impairs quality of life [1]. CFS is an important health problem that affects many young people, mainly females, and markedly reduces working activity [1]. Despite the public health burden of this disease, effective treatment and prevention strategies are still not available. Recent research and clinical experience strongly point to widespread inflammation and multisystemic neuropathology in CFS [2]. In 2011 it was therefore accepted as more appropriate and correct to use the term myalgic encephalomyelitis (ME) for this condition [2].
Postural stability is essential for performing several daily activities, including orthostasis and gait. Our recent studies found that many ME patients present with postural instability or disequilibrium [3, 4, 5]. This neurogenic disorder has long been overlooked, despite several studies reporting disequilibrium in some patients [6, 7]. Orthostatic intolerance is known to be associated with daily functional capacity in patients with ME/CFS [3, 4, 5, 8]. Most orthostatic intolerance symptoms are related to cardiovascular and cerebral blood flow reduction, and to exaggerated sympathetic nervous system activation, which are frequently associated with postural orthostatic tachycardia [2, 9]. Central vestibular dysfunction-related disequilibrium was recently implicated in the pathogenesis of orthostatic intolerance [3, 4, 5]. Orthostatic intolerance is characterized by the inability to remain upright without severe signs and symptoms such as hypotension, palpitations, faintness, light-headedness, pallor, fatigue, weakness, dizziness, diminished concentration, tremulousness, and nausea [9, 10]. Patients may become intolerant to sitting and become bedridden, with further progression of orthostatic intolerance [2, 4].
Disequilibrium is classified as static (unstable upright posture) or kinetic (unstable gait). Our recent studies suggest that static disequilibrium causes postural instability, thereby resulting in orthostatic intolerance [3, 4, 5]. On the other hand, kinetic disequilibrium often results in gait disturbance [11, 12, 13, 14]. The present study evaluated static disequilibrium in ME patients using the Romberg test, whereby patients were asked to stand with their feet together and eyes closed. In addition, kinetic disequilibrium was diagnosed using the tandem gait test, thus allowing assessment of the prevalence and clinical significance of each type of disequilibrium in ME.
Recently, we reported that repetitive transcranial magnetic stimulation (rTMS) in patients with ME ameliorated their symptoms, especially static disequilibrium and orthostatic intolerance [15]. Only static equilibrium was examined in this earlier study. In the present study, we examined the possible therapeutic effects of rTMS on both static and kinetic disequilibria.
Consecutive patients with ME who visited the Miwa Naika Clinic from September 2018 to May 2022 and were able to stand and walk were included in the study. ME was diagnosed according to the International Consensus Criteria [2].
Patients with significant complications unrelated to ME were excluded from the
study, as were pregnant or lactating females. Of the 160 patients, 53 were
male and 107 were females, with a mean age of 37
All study participants provided written informed consent. The study was approved by the Toyama Prefectural Medical Association Ethics Committee (approval#: 2016-010) and the Ethics Committee of the Toyama Prefectural Rehabilitation Hospital & Support Center for Children (approval# 47), and was conducted in accordance with the Declaration of Helsinki. Additionally, each participant provided written informed consent for the possible publication of identifying information/images in an online open-access publication.
All study patients underwent neurologic testing, including the Romberg test and tandem gait test to evaluate possible static and kinetic disequilibrium, performance status (PS) scoring, and a conventional active 10-min standing test. Patients with disequilibrium underwent rTMS and were evaluated after treatment.
Participants were evaluated to determine the presence of widespread pain and tenderness, which are characteristic of fibromyalgia [16].
The severity of patient fatigue was scored using the Chalder fatigue questionnaire (CFQ) [17].
Information concerning activities of daily living was obtained from each patient and verified by a physician. PS was graded on a 10-point scale according to symptom severity, as previously reported [4, 5] (Table 1).
| PS 0: | The patient can perform usual daily living and social activities without malaise. |
| PS 1: | The patient often feels fatigue. |
| PS 2: | The patient often needs to rest because of general malaise or fatigue. |
| PS 3: | The patient cannot work or perform usual activities for a few days in a month. |
| PS 4: | The patient cannot work or perform usual activities for a few days in a week. |
| PS 5: | The patient cannot work or perform usual activities but can perform light work. |
| PS 6: | The patient needs daily rest but can perform light work on a good day. |
| PS 7: | The patient can take care of himself/herself but cannot perform usual duties. |
| PS 8: | The patient needs help to take care of himself/herself. |
| PS 9: | The patient needs to rest the entire day and cannot take care of himself/herself without help. |
To diagnose static disequilibrium, participants were asked to stand with their feet together and eyes closed for 10 s [3, 4, 5]. Unstable standing with wide body sway or oscillations and possibly a fall with feet together and eyes open and/or closed was diagnosed as positive for static disequilibrium [3, 4, 5]. Kinetic disequilibrium was diagnosed using the 2-m tandem gait test. The patient carefully walked 2 m on a straight, flat floor, completing toe-to-heel touch with each step. The patient then turned around and repeated the tandem gait along the same line. Step-out, misstep, and/or body sway with the whole head displaced from the vertical line of gravity at the midpoint between both feet were deemed positive for kinetic disequilibrium and static disequilibrium. None of the 30 healthy control subjects were diagnosed with disequilibrium by the diagnostic criteria used in this study.
The conventional active 10-min standing test was performed as previously reported [5]. Participants were asked to remain standing still with their feet approximately shoulder width apart. Patients were scored positive for orthostatic intolerance when they experienced difficulty during the test due to symptoms such as palpitations, light-headedness, pallor, fatigue, dizziness, and nausea. Because of these symptoms, they were unable to maintain the standing posture and gave up standing.
Patients with both static and kinetic disequilibria and who provided written informed consent were assigned to the rTMS study group. rTMS was performed as described in a previous report [15].
High-frequency rTMS was delivered using a MagstimRapid 2 instrument (Miyuki Giken, Tokyo, Japan) equipped with a figure-of-8 stimulating coil. All rTMS patterns consisted of bursts containing three pulses at 50 Hz and an intensity of 80% of the active motor threshold, repeated at 200-ms intervals (5 Hz). A 2-s train of theta burst stimulation (TBS) pattern was repeated every 10 s for a total of 190 s (600 pulses) in the intermittent TBS (iTBS) pattern [18]. Burst Stimulator (Medical Try System, Tokyo, Japan) is a magnetic stimulation control software employed for the delivery of TBS. The dorsolateral prefrontal cortex (DLPFC) and primary motor area (M1) of the left hemisphere were selected as target areas for iTBS, irrespective of the patients’ right- or left-handedness. DLPFC was identified using magnetic resonance imaging. M1 was determined by measuring the motor threshold. The stimulating coil was first placed on the left DLPFC and then on M1. The stimulation intensity was initially set to 80% of the resting motor threshold, and subsequently reduced according to the patient’s tolerance. The patients underwent rTMS treatment, including 10 sessions each for the DLPFC and M1, over 2 weeks of hospitalization.
Continuous variables are presented as the mean
Tables 2,3,4,5 and Figs. 1,2,3,4,5 show the prevalence of static (Table 2 and Fig. 1) and kinetic disequilibrium (Table 3 and Figs. 2,3) in participants with ME, comparative data on participants with and without static or kinetic disequilibrium (Table 4), the distribution of PS scores with and without disequilibrium (Fig. 4), and treatment effects with rTMS (Table 5 and Fig. 5). The details of these results are described below. Proprioceptive sensation was not impaired in any of the participants, suggesting no cases of sensory ataxia that may have caused a positive Romberg test.
| With static disequilibrium | Without static disequilibrium | p | |||
| Number of patients | 40 (25%) | 120 (75%) | |||
| Female | 32 (80%) | 75 (63%) | 0.05 | ||
| Age (years) | 38 |
37 |
0.85 | ||
| Body mass index (kg/m2) | 21 |
22 |
0.52 | ||
| ME disease duration (years) | 6.5 |
4.2 |
|||
| Fibromyalgia | 23 (58%) | 19 (16%) | |||
| Fatigue score (CFQ) | 27 |
19 |
|||
| Performance status score | 4–9 | 3–8 | |||
| Median score | 7 | 5 | |||
| 24 (60%) | 34 (28%) | ||||
| 10 (25%) | 57 (48%) | 0.02 | |||
| Orthostatic intolerance | 22 (55%) | 5 (4%) | |||
| Kinetic disequilibrium | 39 (98%) | 32 (27%) | |||
ME, myalgic encephalomyelitis; Static disequilibrium, instability upon standing
with feet together and eyes closed; Orthostatic intolerance, failure to complete
the 10-min standing test; CFQ, Chalder fatigue questionnaire. Values are
presented as the mean
| With kinetic disequilibrium | Without kinetic disequilibrium | p | |||
| Number of patients | 71 (44%) | 89 (56%) | |||
| Female | 59 (83%) | 48 (54%) | |||
| Age (years) | 38 |
37 |
0.30 | ||
| Body mass index (kg/m2) | 22 |
22 |
0.92 | ||
| ME disease duration (years) | 5.5 |
4.2 |
0.18 | ||
| Fibromyalgia | 32 (45%) | 10 (11%) | |||
| Fatigue score (CFQ) | 24 |
18 |
|||
| Performance status score | 3–9 | 3–8 | |||
| Median score | 7 | 5 | |||
| 38 (54%) | 21 (24%) | ||||
| 19 (27%) | 48 (54%) | ||||
| Orthostatic intolerance | 27 (38%) | 0 (0%) | |||
| Static disequilibrium | 39 (55%) | 1 (1%) | |||
Kinetic disequilibrium, unstable tandem gait with
turn and return. Values are presented as the mean
| With static or kinetic disequilibrium | Without static or kinetic disequilibrium | p | |||
| Number of patients | 72 (45%) | 88 (55%) | |||
| Female | 60 (83%) | 47 (53%) | |||
| Age (years) | 39 |
36 |
0.26 | ||
| Body mass index (kg/m2) | 22 |
22 |
0.91 | ||
| ME disease duration (years) | 5.5 |
4.2 |
0.22 | ||
| Fibromyalgia | 32 (44%) | 10 (11%) | |||
| Fatigue score (CFQ) | 24 |
18 |
|||
| Performance status score | 3–9 | 3–8 | |||
| Median score | 7 | 5 | |||
| 38 (53%) | 21 (24%) | ||||
| 20 (28%) | 47 (53%) | ||||
| Orthostatic intolerance | 27 (38%) | 0 (0%) | |||
| Static disequilibrium | 40 (56%) | 0 (0%) | |||
| Kinetic disequilibrium | 71 (99%) | 0 (0%) | |||
Values are presented as the
mean
| Patient# | Age/Sex | History (years) | Handedness | Intensity (%) | CFQ score | Static disequilibrium | Kinetic disequilibrium | Standing test | PS score | ||||||||||
| rTMS | rTMS | rTMS | rTMS | rTMS | |||||||||||||||
| before | after | before | after | before | after | before | after | before | after | ||||||||||
| 1 | 53/F | 1 | Right | 60 | 31 | 22 | + | – | + | – | 8′30′′ | C | 8 | 4 | |||||
| 2 | 24/F | 3.5 | Left | 70 | 26 | 18 | + | – | + | – | 5′ | C | 6 | 2 | |||||
| 3 | 34/F | 3 | Right | 80 | 27 | 16 | + | – | + | – | 8′40′′ | C, POT | 6 | 3 | |||||
| 4 | 30/M | 0.7 | Right | 72 | 24 | 15 | + | – | + | – | 8′40′′ | C, POT | 6 | 3 | |||||
| 5 | 46/F | 12 | Right | 60 | 26 | 15 | + | – | + | – | C | C | 4 | 1 | |||||
| 6 | 44/F | 1.7 | Left | 65 | 30 | 24 | + | – | + | – | C, OH | C, OH | 8 | 6 | |||||
| 7 | 61/F | 12 | Right | 70 | 31 | 25 | + | – | + | – | 9′ | C | 7 | 5 | |||||
| 8 | 51/F | 6 | Right | 65 | 29 | 24 | + | – | + | – | C | C | 7 | 5 | |||||
| 9 | 30/F | 15 | Right | 60 | 28 | 22 | + | – | + | – | 8′ | C | 7 | 5 | |||||
| 10 | 25/F | 2.5 | Right | 60 | 29 | 21 | + | – | + | – | 8′ | C, POT | 6 | 4 | |||||
| 11 | 32/F | 7 | Right | 50 | 26 | 21 | + | – | + | – | 5′ | C′ | 6 | 4 | |||||
| 12 | 46/F | 2 | Right | 70 | 30 | 29 | + | + | + | + | C | C | 8 | 8 | |||||
| 13 | 19/F | 1.4 | Right | 55 | 32 | 30 | + | + | + | + | 15′′ | 20′′ | 8 | 8 | |||||
| Total | 38 1M | 5.2 | 11Right | 64 | 28 | 22* | median | ||||||||||||
| 2Left | 13+ | 2+* | 13+ | 2+* | 4C | 12C* | 7 | 4* | |||||||||||
Mean
+, present; –, absent;
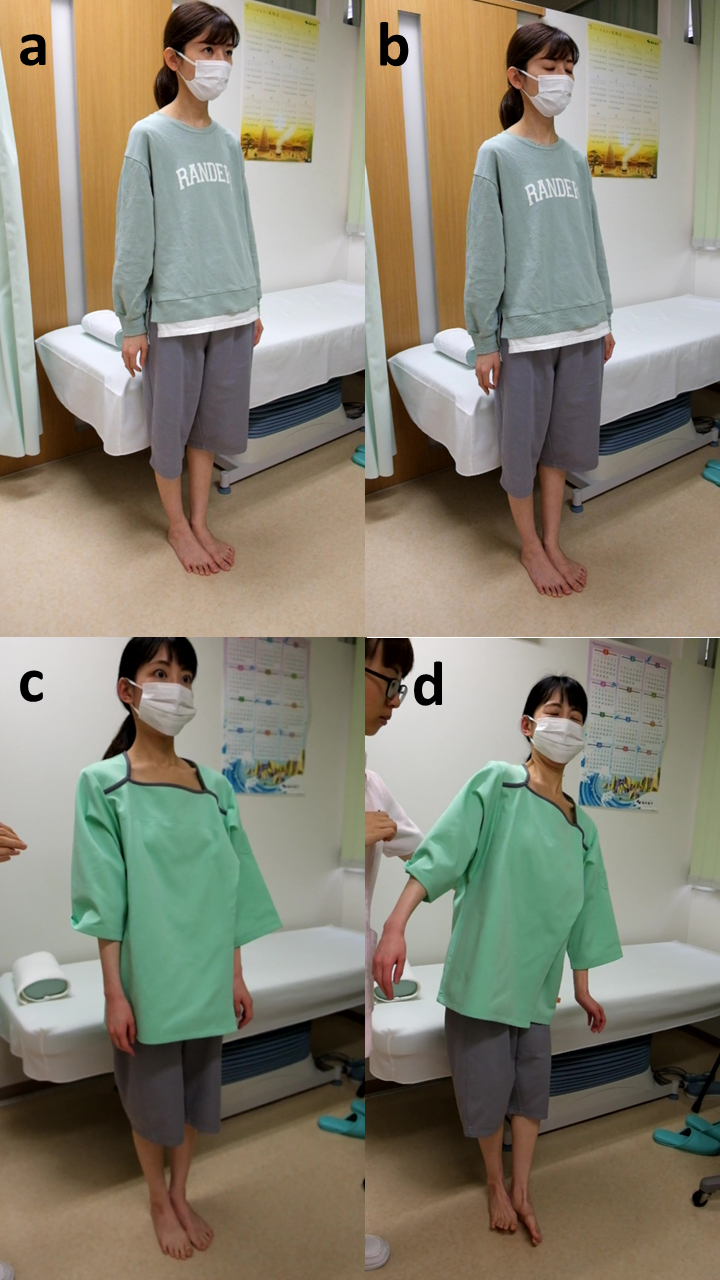 Fig. 1.
Fig. 1.
Comparative Romberg test in ME patients with and without static disequilibrium. Upper (a,b): a 30–year–old female patient without static disequilibrium remained stable with both feet together and with eyes open (a) and closed (b). Lower (c,d): in contrast, a 32-year-old female patient with static disequilibrium remained standing with both feet together and eyes open (c), but had wide body sway and finally fell down with eyes closed (d).
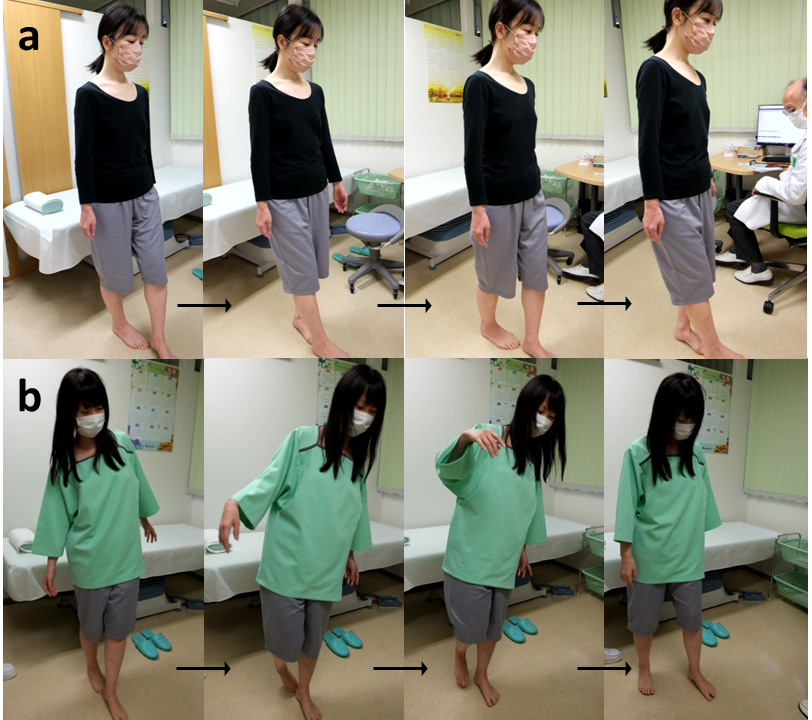 Fig. 2.
Fig. 2.
Comparative tandem gait test in ME patients with and without kinetic disequilibrium. (a) A 31-year-old female patient without kinetic disequilibrium showed stable performance in the tandem gait test. (b) In contrast, a 36-year-old female patient with kinetic disequilibrium exhibited wide body sway and missteps during the tandem gait test.
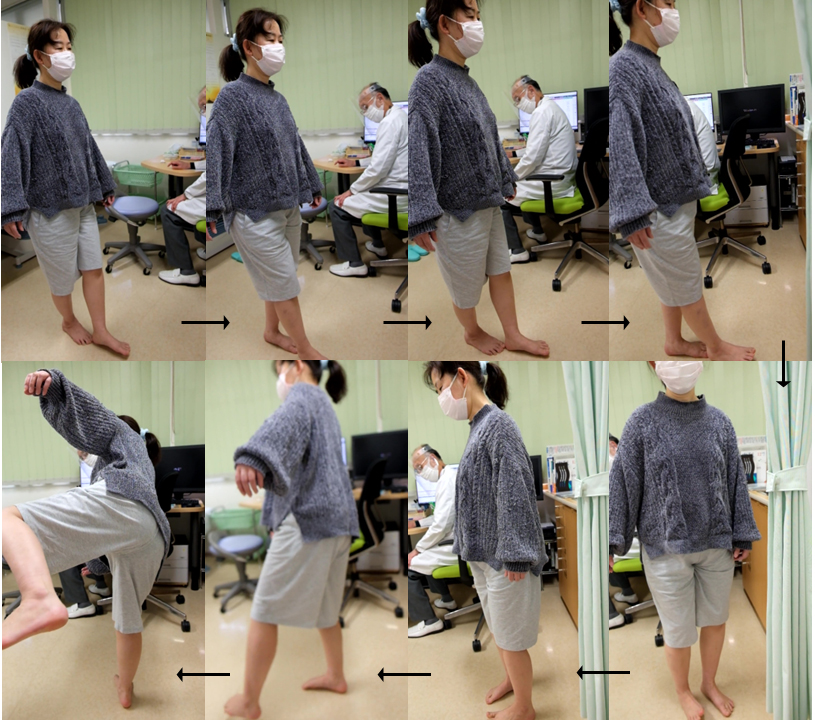 Fig. 3.
Fig. 3.
Tandem gait test with turn and return in a 60-year-old female ME patient with kinetic disequilibrium. The patient performed a stable tandem gait in the first straight line, but lost balance and almost fell during the return after turning.
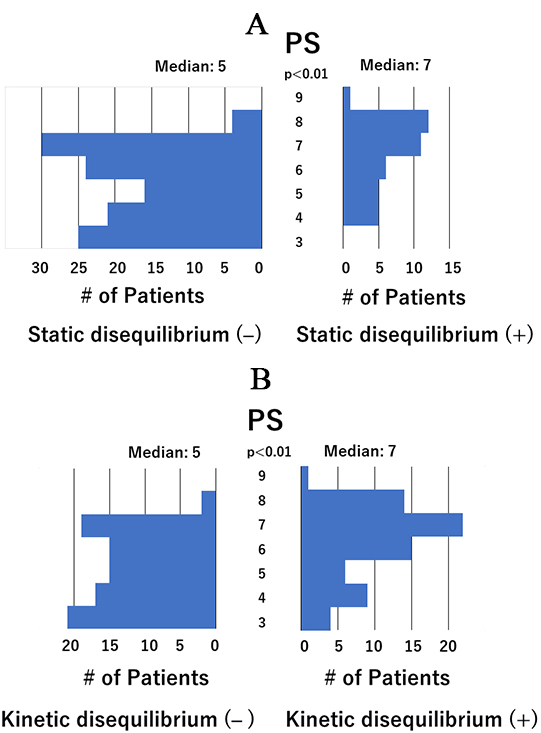 Fig. 4.
Fig. 4.
Distribution of performance status scores. (A) Distribution of performance status (PS) scores in patients with and without static disequilibrium. The median PS score was significantly higher in patients with static disequilibrium than in those without (7 vs. 5). (B) Distribution of PS scores in patients with and without kinetic disequilibrium. The median PS score was significantly higher in patients with kinetic disequilibrium than in those without (7 vs. 5).
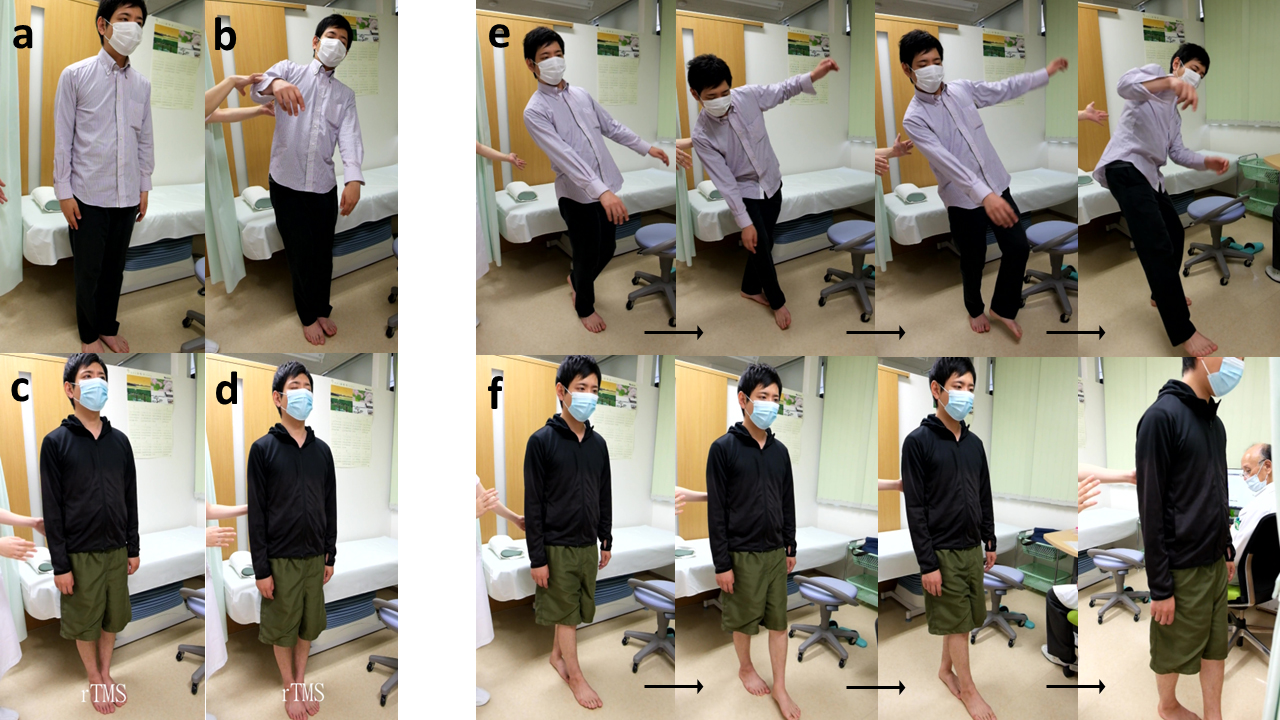 Fig. 5.
Fig. 5.
Comparison of the Romberg test (left) and tandem gait test (right) by a 30-year-old male ME patient with both static and kinetic disequilibrium before and after treatment with rTMS. Left: Romberg test for static disequilibrium. Before rTMS treatment, the patient was able to stand with both feet together and eyes open (a), but had wide body sway with eyes closed (b). In contrast, after treatment he could stand stably with both feet together and eyes open (c), as well as with eyes closed (d). Right: Tandem gait test for kinetic disequilibrium. Before rTMS treatment, the patient exhibited significant body sway and missteps, demonstrating kinetic disequilibrium (e). In contrast, after treatment he had a stable tandem gait, demonstrating the absence of kinetic disequilibrium (f).
Among the 160 participants, unstable standing with feet together and eyes open was noted in 7 (4%) cases, and with eyes closed in an additional 33 (21%) cases. Thus, static disequilibrium as demonstrated by unstable standing during the Romberg test was positive in 40 (25%) patients. The duration of ME disease was significantly longer in patients with static disequilibrium than in those without (Table 2). These patients also showed a significantly higher mean CFQ score. Typical Romberg test results for patients with and without static disequilibrium are shown in Fig. 1a–d.
Truncal instability and/or missteps were observed in 57 (36%) of the 160 participants during the first straight tandem gait for 2 m. Typical results for the first straight tandem gait in patients with and without kinetic disequilibrium are shown in Fig. 2a,b. Additionally, instability or missteps in tandem gait after turning 180° was detected in 14 (9%) patients who had normal tandem gait before turning. Fig. 3 shows a typical unstable tandem gait after turning 180° in a patient with kinetic disequilibrium. A total of 71 (44%) patients were diagnosed as positive for kinetic disequilibrium, with a significantly higher prevalence amongst female patients (Table 3). The mean CFQ score was significantly higher in patients with kinetic disequilibrium than in those without.
Medications before the study are shown in Supplementary Table 1. Those medications appeared to be not different between patients with and without static or disequilibrium.
Of the 160 participants with ME, 42 (26%) were diagnosed with fibromyalgia (Table 2). This condition was significantly more prevalent in patients with static disequilibrium (Table 2) and in patients with kinetic disequilibrium (Table 2) than in those without.
The distribution of PS scores in patients with and without static disequilibrium
is shown in Fig. 4A, and with and without kinetic disequilibrium in Fig. 4B. The
median PS score was significantly higher in patients with static or kinetic
disequilibrium than in those without. Moreover, the median PS score was
significantly higher in the 39 patients with both static and kinetic
disequilibrium than in those without (score of 7 vs. 5, p
Orthostatic intolerance was found in 27 (17%) of the 160 participants, as demonstrated by failure to complete the 10-min standing test. Furthermore, orthostatic intolerance was significantly more prevalent in patients with static disequilibrium (55%) than in those without (4%) (Table 2), and in patients with kinetic disequilibrium (38%) compared to those without (0%) (Table 3).
Both static and kinetic disequilibrium were observed in almost all patients with static disequilibrium (39/40, 98%), and in about half of patients with kinetic disequilibrium (39/71, 55%). Only one patient with static disequilibrium exhibited no kinetic disequilibrium. Female prevalence, prevalence of combination with fibromyalgia, prevalence of orthostatic intolerance, mean CFQ score, and median PS score were all significantly higher in the 72 patients with any type of disequilibrium than in the 88 patients showing no disequilibrium (Table 4).
For most patients, the disbalancing motion was not sudden but instead rather slow, as if pulling out, and in one direction only.
The rTMS study was carried out on 13 patients with both static and kinetic
disequilibrium. The clinical features of these patients and the therapeutic
effects are summarized in Table 4. In most patients, the intensity of stimulation
was lowered due to intolerance to discomfort, headache, and dizziness. The CFQ
score decreased significantly following treatment (28
The present study demonstrated that both static and kinetic disequilibrium are prevalent in patients with ME and impair their activities of daily living. The mean CFQ score was significantly higher in patients with disequilibrium than in those without, suggesting that disequilibrium causes worse fatigue. Both static and kinetic disequilibrium were alleviated in most patients with ME after treatment with rTMS, suggesting a central vestibular origin.
Disequilibrium of central origin is a typical neural sign and includes both static and kinetic disequilibrium. Its prevalence in ME patients is suggestive of a neural disease.
Static and kinetic balance for postural stability is essential for performing daily activities [11, 19, 20]. Contradictory results have been reported with regard to disequilibrium in patients with CFS. Komaroff [21] estimated that 30–50% of patients with CFS experience some degree of disequilibrium, whereas Merry [22] suggested the incidence could be as high as 70% in all patients. Our recent studies suggested that disequilibrium may be involved in the development of orthostatic intolerance [3, 4, 5]. In contrast, Paul et al. [23] found no significant difference in postural stability between patients with CFS and control subjects, as measured by postural sway under eyes-open and eyes-closed conditions. In the present study, standard neurological examinations revealed abnormal results, such as instability when standing with both feet together and eyes open and/or closed, and unstable tandem gait. This suggests disequilibrium or truncal ataxia in a considerable number of patients with ME. A positive Romberg test was previously reported in 10–20% of CFS patients, and an abnormal tandem gait test in 15–25% [6, 7]. The present study found a higher incidence of positive Romberg test (21%) in patients with ME, as well as an abnormal tandem gait test even before turning around (36%). The International Consensus Criteria allows the identification of ME patients who are more physically debilitated and have greater physical and cognitive impairments, thus differentiating patients with ME from those who are depressed [2]. The higher incidence of both a positive Romberg test and an abnormal tandem gait test observed in ME patients may be due to the greater severity of the disease compared to the CFS cases evaluated in previous studies. In the present study, patients with any disequilibrium had higher CFQ and PS scores, suggesting more severe fatigue and restricted activities of daily living. Disequilibrium, or postural instability, is a useful indicator of advanced disease. Recently, Hayes et al. [24] evaluated postural sway during a 30-s static standing test and suggested that people with long coronavirus disease (Long COVID) and those with ME/CFS have similarly impaired balance and physical capacity.
Static balance and postural stability are regulated by a postural adjustment system [19, 20]. Multiple types of sensory information, such as somatosensory, visual, and vestibular sensations, are processed by various brain areas in the multisensory cortical area and then in the corticocortical vestibular network to achieve adaptable postural control. The automatic process of postural control is termed postural reflex and includes head-eye coordination accompanied by appropriate body segment alignment [19, 20]. This control is mediated by descending pathways from the brainstem, with cooperation between the vestibulospinal, reticulospinal, and tectospinal tracts contributing to the process. The visual sensory-mediated system can compensate for central vestibular function to maintain postural control when the above-mentioned system is impaired in the brain cortex. Visual information from the eyes is projected directly to the vestibular and oculomotor nuclei in the midbrain to stimulate the head and external oculomotor muscles and maintain postural stability, thereby helping vestibular function in cooperation with the cerebellum. Visual compensation is therefore likely to be essential for maintaining postural stability in the presence of vestibular deficits [19, 20], explaining why many ME patients lost balance during the Romberg test when their eyes were closed. Regulation of kinetic stability is usually more difficult under locomotive conditions than stability under static conditions, especially with tandem gait and turning 180°, even with eyes open.
The exact cause of the disequilibrium observed in patients with ME remains unknown. However, it appears to have a central vestibular origin, consistent with previous results of vestibular function tests in patients with CFS [25, 26]. The pathogenesis for the observed neurologic defects in disequilibrium may be due to neural brain inflammation [27].
Previous studies have demonstrated significantly impaired functional connectivity or default mode network functional hypo-connectivity in the brain of ME/CFS patients [28, 29, 30]. Furthermore, the symptom severity of such patients was associated with functional connectivity in diverse brain regions. It was recently reported that COVID-19 infection may have a widespread effect on the functional connectome. This is characterized by lower functional connectivity and altered patterns of information processing efficiency and effective information flow, similar to ME/CFS [31].
The long-lasting neuromodulatory effects of rTMS are of great interest for therapeutic applications in various neurological and psychiatric disorders where the functional connectivity among brain regions is profoundly disturbed [32, 33, 34, 35]. rTMS and TBS show significant promise in improving the outcomes for adults with complex neurological and neurodegenerative conditions, such as Alzheimer’s disease, stroke, and Parkinson’s disease [35]. rTMS and TBS may impact neuroplasticity and functional connectivity, in particular through brain derived neurotrophic factor and tropomyosin-related kinase receptor type B. The plastic after-effects of rTMS result in large-scale changes at the network level that affect both local and remote activity within the stimulated network, as well as interactions between the stimulated and distinct functional networks.
The cortical organization of human vestibular information processing is poorly understood. rTMS of the DLPFC and M1 may facilitate the recovery of central vestibular function by enhancing neural network activity within the known vestibular function center sites located in the brainstem and in the cortex. The vestibular system provides information on head translation, rotation, and orientation in a gravitational environment [36]. The corticovestibular network is distributed throughout the brain and has a high degree of functional connectivity that can quickly buffer the network and reestablish network integrity in case of injury [36, 37, 38]. Central or cortical vestibular region disorders or lesions, such as cerebrovascular events and encephalitis, rarely cause vestibular deficits in the acute phase. Moreover, the symptoms do not persist, probably because of a highly robust and redundant vestibular cortical network. Neural inflammation in ME encompasses almost the entire central nervous system [37]. This is probably why disequilibrium associated with central vestibular dysfunction develops and persists in many patients with ME. The DLPFC and/or M1 appear to be extremely important modulators of this neural network. rTMS treatment probably corrects or enhances neural network function through the vestibular center, resulting in the amelioration of disequilibrium.
Kakuda et al. [39] conducted a pilot study in several patients with CFS in which they applied high-frequency rTMS over the DLPFC. They observed favorable therapeutic effects and reduced fatigue symptoms in most cases, and speculated the beneficial effect of rTMS was mediated through DLPFC neural activation. Hence, recovery or enhancement of the impaired corticocortical network may play a crucial role in the therapeutic effects of rTMS.
The present study found a close relationship between disequilibrium and orthostatic intolerance, as suggested by other recent reports. However, cerebral hypoperfusion and exaggerated sympathetic activation are thought to be the primary pathogenesis of orthostatic intolerance. A paradigm shift is therefore required [5].
Kinetic disequilibrium is also associated with gait disturbances. Patients with kinetic disequilibrium may experience difficulty walking in crowded streets, and need to make small, frequent and rapid changes in walking direction, as well as frequent and rapid changes in walking speed. Such patients are unable to walk for extended periods of time because of the effort required, and hence they soon become fatigued.
Fibromyalgia is significantly more prevalent in patients with static or kinetic disequilibrium than in those without. A recent study in patients with CFS suggested that poor balance was associated with poor self-reported physical health [40]. Although none of the patients in the present study reported that their disequilibrium was due to musculoskeletal pain, I cannot exclude that pain may have influenced the neurological tests results. Differences between the pathophysiology of ME in combination with fibromyalgia, and ME alone, remain to be elucidated and require further investigation.
This study had several limitations. First, there were no control patients with sham treatment for comparison with rTMS treatment. Second, rTMS treatment was applied to both the DLPFC and M1 in all participants. Consequently, the effect of this treatment could not be evaluated individually for the DLPFC and M1. Third, the long-term effects of rTMS treatment were not determined.
Disequilibrium is prevalent in ME patients and should be recognized as an important neural deficit. Static disequilibrium is related to orthostatic intolerance, while kinetic disequilibrium is related to gait. Both are important symptoms that restrict activities of daily living. rTMS treatment of both the DLPFC and M1 effectively alleviated disequilibrium and resolved orthostatic intolerance.
The paper is listed as, “Static and Kinetic Disequilibrium as Central Neural Signs in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome” as a preprint on (SSRN) at: https://ssrn.com/abstract=4232084 or http://dx.doi.org/10.2139/ssrn.4232084.
Data are available upon request, although availability may be limited due to patient confidentiality. The data associated with this study have not been deposited in any publicly available repository.
All work was conceived and completed by KM. KM contributed to editorial changes in the manuscript. KM read and approved the final manuscript. KM has participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All study participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Toyama Medical Association Ethics Committee (approval#: 2016-010) and the Ethics Committee of the Toyama Prefectural Rehabilitation Hospital & Support Center for Children (approval# 47).
I appreciate the contribution of Dr. Yukichi Inoue at the Toyama Prefectural Rehabilitation Hospital & Support Center for Children, who conducted the rTMS procedures as my co–researcher. Unfortunately, he died during the present study on April 16, 2021. I thank Ms. Takako Miwa for her technical help.
This research received no external funding.
The author declares no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/JIN25488.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
