- Academic Editor
Obstructive sleep apnea (OSA) is associated with widespread higher-order cognitive consequences, including deficits in memory and executive function. However, the specific cognitive architecture and underlying mechanisms that link the disease’s pathophysiology to these broad cognitive changes remain poorly understood. This study tested the hypothesis that a selective vulnerability of the working memory (WM) executive control system serves as a central hub, mechanistically mediating the relationship between OSA disease burden and memory retention.
Thirty male patients with OSA underwent comprehensive polysomnography and neurocognitive assessment. A data-driven Global Severity Index (GSI) was derived from principal component analysis of the most cognitively-relevant physiological metrics. A multi-task paradigm was used to dissociate performance on tasks of WM maintenance capacity from those requiring executive control. Hierarchical linear regression and mediation analyses were performed, controlling for relevant covariates.
A higher GSI was consistently associated with poorer performance across multiple tasks requiring executive control, but not with measures of WM maintenance capacity or attentional control. Critically, the a priori defined mediation model was supported: the relationship between the GSI and memory retention performance was fully mediated by a latent Executive Control Factor (ECF) derived from the executive tasks.
Our findings delineate a specific mechanistic pathway for the cognitive consequences of OSA. The disease’s pathophysiological burden is selectively associated with executive control performance, and this vulnerability appears to serve as a core mechanism that accounts for the disorder’s downstream impact on memory function. This work identifies executive control as a critical target for mitigating the broader cognitive impact of OSA.
Obstructive sleep apnea (OSA) is a prevalent sleep disorder characterized by recurrent episodes of upper airway collapse during sleep, affecting a substantial portion of the adult population [1, 2, 3]. These episodes lead to intermittent hypoxia and sleep fragmentation, which contribute to significant health consequences [1, 4, 5]. While excessive daytime sleepiness is the most recognized symptom, a growing body of evidence indicates that the impact of OSA extends far beyond sleepiness, inflicting considerable damage on cognitive functions [6, 7, 8, 9]. Numerous studies have consistently documented that patients with OSA exhibit deficits across a wide range of cognitive domains, with the most pronounced impairments often observed in higher-order functions such as executive functions, attention, and episodic memory [6, 7, 8, 9]. Crucially, mounting evidence from longitudinal studies suggests that these cognitive deficits are not merely transient functional impairments but may represent an elevated risk for long-term neurological decline, including the development of dementia and Alzheimer’s disease [10, 11, 12, 13, 14]. This positions OSA as not just a sleep disorder, but a significant and potentially modifiable risk factor for neurodegenerative disease that profoundly affects patients’ quality of life [15, 16, 17].
Given these widespread cognitive impairments, a critical question arises regarding the common neural substrate underlying these deficits. A substantial body of neuroimaging research indicates that the pathophysiological processes in OSA exert lasting, detrimental effects on the brain [1, 4, 5]. Task-based studies have consistently shown that OSA is associated with reduced activation of the prefrontal cortex—particularly the dorsolateral prefrontal cortex (DLPFC)—during cognitively demanding tasks [18, 19, 20]. Furthermore, resting-state studies have documented decreased functional connectivity and reduced hemodynamic responsiveness in the prefrontal cortex [21, 22]. These alterations specifically compromise the integrative functions of critical brain regions involved in higher-order cognitive control, which are foundational for both working memory (WM) and executive function.
The WM model provides a powerful theoretical framework for unifying these disparate cognitive findings. According to the influential multicomponent model, WM is not a unitary construct but a system composed of functionally distinct components [23, 24, 25]. Two core components are typically identified: a maintenance function for the short-term storage of information, associated with the parietal cortex, and an executive control function that supports the active manipulation and updating of information, which is predominantly mediated by the dorsolateral prefrontal cortex (DLPFC) [26, 27, 28]. Given the particular vulnerability of prefrontal networks to OSA-related pathophysiology, the executive component of WM may be disproportionately affected. Indeed, a growing body of evidence indicates that the impact of OSA on WM is not uniform but component-specific. Studies have revealed that patients with OSA exhibit significantly poorer performance on tasks involving manipulation and updating, while performance on simple maintenance tasks can remain relatively intact [29, 30]. This suggests that the widespread cognitive consequences of OSA may be driven by a selective disruption of the executive control hub within WM, rather than a global, undifferentiated cognitive decline.
The present study therefore aimed to systematically examine the differential effects of OSA on the core components of WM. To achieve this, we first sought to identify a multidimensional profile of OSA pathophysiology most relevant to cognitive impairment, departing from a reliance on single physiological markers. We then tested whether this cognitively-relevant disease burden was selectively associated with the executive control component of WM, while sparing maintenance capacity. Our primary hypothesis was that poorer performance in executive control would mediate the relationship between OSA disease burden and poorer memory retention, thereby providing a specific mechanistic account for the broader cognitive consequences of the disorder.
A total of 30 male patients diagnosed with OSA were recruited from the Department of Otolaryngology-Head and Neck Surgery at The First Affiliated Hospital of University of Science and Technology of China. The diagnosis of OSA was made according to the criteria of the International Classification of Sleep Disorders, 3rd Edition (ICSD-3).
Inclusion criteria required patients to have an Apnea-Hypopnea Index (AHI) of
The study protocol was approved by the Institutional Review Board of The First Affiliated Hospital of University of Science and Technology of China, and all participants provided written informed consent prior to their involvement in the study.
All participants underwent one night of in-hospital, attended PSG for diagnostic purposes. Recordings were performed using a standard clinical setup, capturing signals that included a six-channel electroencephalogram (F3, F4, C3, C4, O1, O2), electrooculogram, chin and bilateral leg electromyogram, nasal/oral airflow, thoracic and abdominal respiratory effort, body position, and peripheral oxygen saturation (SpO2), alongside synchronized video monitoring. All sleep stages and respiratory events were scored by certified technicians in accordance with the American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events (Version 3.0) [31].
An obstructive apnea was defined as a cessation of airflow for
• AHI: The total number of apneas and hypopneas per hour of sleep. • Obstructive Apnea Index (OAI) and Hypopnea Index (HI): The number of obstructive
apneas and hypopneas per hour of sleep, respectively. • Event Durations: The average and longest duration for all respiratory events
combined, as well as separately for obstructive apneas and hypopneas. • Hypoxemia Metrics: The minimum oxygen saturation reached during the night (SpO2
nadir) and the percentage of total sleep time spent with oxygen saturation below
90% (T90).
All participants underwent a battery of demographic, clinical and neurocognitive assessments including: the Epworth Sleepiness Scale (ESS) [32], the Montreal Cognitive Assessment (MoCA) [33] for general cognitive screening, the Generalized Anxiety Disorder 7-item scale (GAD-7) [34], and the 9-item Patient Health Questionnaire (PHQ-9) [35] for depression screening.
2.2.2.1 Digit Span Task
The digit span subtest from the Wechsler Adult Intelligence Scale (WAIS) was administered to assess two distinct components of WM: maintenance capacity (specifically of the phonological loop, via the digit span forward condition) and executive control involving information manipulation (via the digit span backward condition). In both conditions, an experimenter verbally presented sequences of digits at a rate of approximately one per second. In the digit span forward condition, the participant was required to recall the digits in the exact order they were presented. In the digit span backward condition, the participant was required to recall the digits in the reverse order. The sequence length progressively increased, and testing was discontinued when the participant failed both trials at a given length. The primary dependent variable for each condition was the span, defined as the number of digits in the longest sequence correctly recalled.
2.2.2.2 N-back Task
The n-back task was employed to assess the executive control component of WM, specifically the process of continuous information updating. The task comprised two conditions: a 2-back condition serving as the primary executive task, and a 0-back condition as an active baseline measuring sustained attention and processing speed (see Fig. 1).
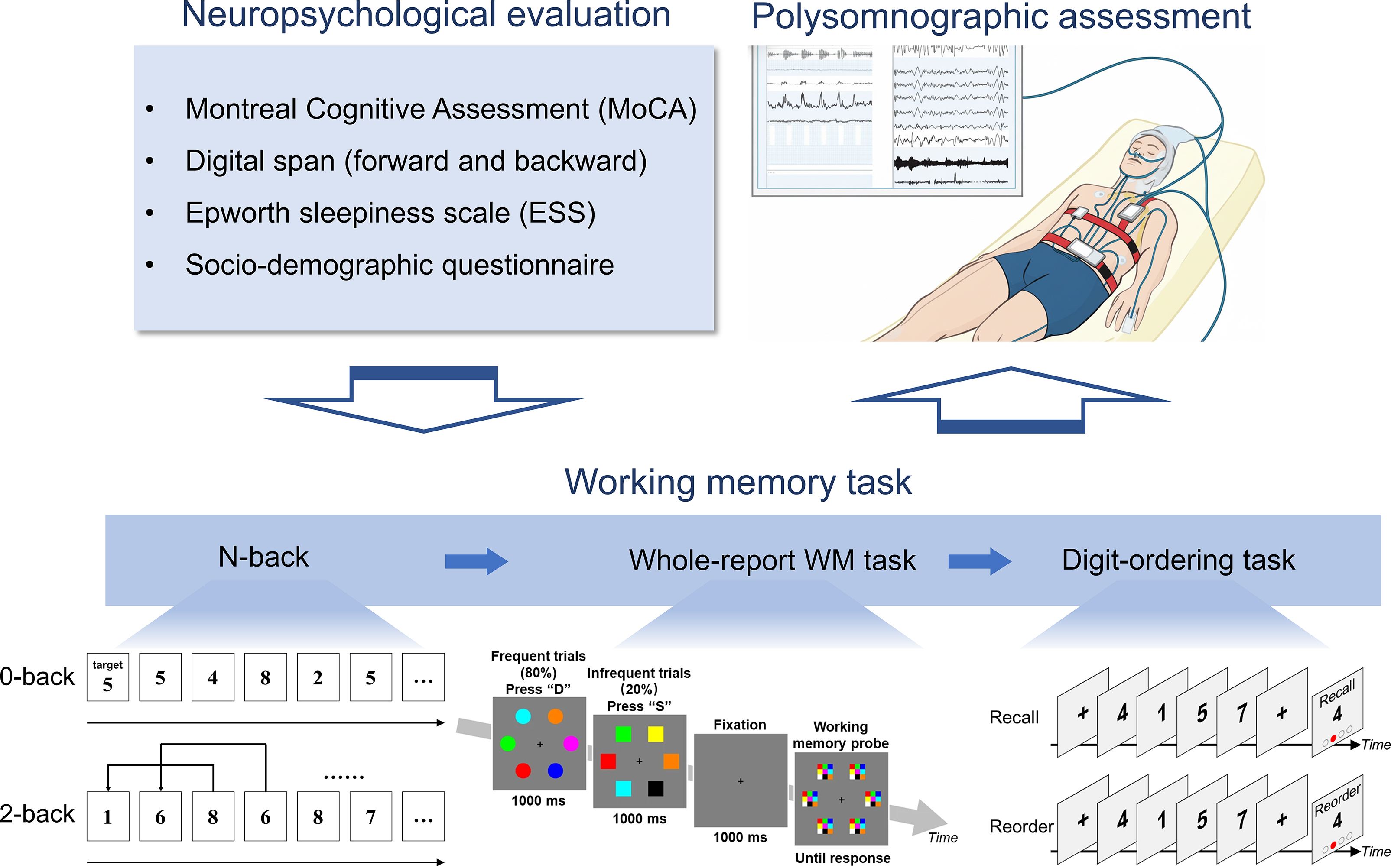 Fig. 1.
Fig. 1.
Study procedure. Participants underwent the entire experimental procedure in a single evening session at the sleep laboratory. After providing informed consent, they completed a battery of baseline assessments, including a socio-demographic questionnaire, the Epworth Sleepiness Scale (ESS), and the Montreal Cognitive Assessment (MoCA). Subsequently, they performed a series of computerized cognitive tasks designed to probe distinct components of working memory (WM) and attention. These included the Digit Span task (assessing maintenance capacity and executive control), the n-back task (assessing information updating), the Whole-report task (assessing attentional control and visuospatial WM capacity), and the Digit-ordering task (assessing information reorganization). Upon completion of all assessments, participants were fitted with the polysomnography (PSG) equipment for their overnight diagnostic sleep study. Figure created with Google Gemini (https://gemini.google.com/).
Stimuli were single digits (0–9) presented sequentially for 500 ms, followed by a 500 ms fixation cross, resulting in a 1-second stimulus-onset asynchrony. In the 0-back condition, participants responded to a pre-specified target digit. In the 2-back condition, they responded via key press to any digit that matched the one presented two positions earlier. The task consisted of five alternating blocks for each condition, separated by 20-second rest intervals. Each block contained 18 (2-back) or 20 (0-back) trials, with targets appearing on approximately 33% of trials. The total task duration was approximately 10 minutes. The primary dependent variable for each condition was mean accuracy (ACC).
2.2.2.3 Whole-report WM Task
This paradigm was designed to concurrently assess two distinct cognitive processes: baseline attentional control (including sustained attention and vigilance) and visuospatial WM capacity (see Fig. 1).
The task presented a continuous stream of shape-discrimination trials. In each trial, an array of six geometric shapes—either all circles (80% of trials; frequent condition) or all squares (20% of trials; infrequent condition)—was presented for 800 ms. Participants were instructed to identify the shape via a key press. On 50% of the infrequent (square) trials, this judgment was followed by a 1000 ms fixation and then a surprise memory test. In this test, participants were required to recall the colors of the six previously presented squares by clicking on a color grid. The task consisted of four blocks, each containing 200 shape-judgment trials and 20 surprise color-recall trials, for a total duration of approximately 34 minutes. Several dependent variables were derived from this task:
• Attentional Control: To quantify processing efficiency, we calculated the Linear
Integrated Speed-Accuracy Score (LISAS) [36]. This score combines both reaction
time and accuracy into a single metric, with higher scores indicating poorer
performance. LISAS was calculated separately for frequent trials (as a measure of
sustained attention) and infrequent trials (as a measure of vigilance). The
detailed calculation of LISAS is provided in the Statistical Analysis section. • WM Capacity: The primary dependent variable for the memory component was the
mean number of correctly recalled colors on the surprise test trials, serving as
a measure of visuospatial WM capacity.
2.2.2.4 Digit-ordering Task
The Digit-ordering task was employed to quantify a specific facet of executive control: the mental manipulation of information held in WM (see Fig. 1).
Each trial commenced with an encoding phase, during which a sequence of five randomly ordered digits was presented visually, each for a duration of 1 second. Following encoding, a retrieval cue designated one of two recall conditions for that trial:
• The Order condition served as a baseline, requiring participants to mentally
rehearse the sequence in its original presentation order. • The Reorder condition engaged the targeted executive process, requiring
participants to mentally reorganize the sequence into ascending numerical order.
Retrieval was assessed using a probed-recognition method. A single digit was presented on-screen, and participants had to verify, within a 5-second response window, if it matched the digit at a specified serial position within the just-rehearsed or reordered mental sequence. The task consisted of two blocks, each containing 18 trials (counterbalanced across conditions in a pseudorandom order), with a 20-second rest interval between them. The total task duration was approximately 8 minutes.
The primary dependent variable was the LISAS, computed separately for the Order and Reorder conditions. Contrasting the LISAS between these two conditions allowed for the quantification of the cognitive cost specifically attributable to the executive demand of information resequencing.
All statistical analyses were performed using SPSS (Version 26.0, IBM Corp., Armonk, NY, USA) and the PROCESS macro (Version 5.0, https://www.processmacro.org/) for SPSS [37]. The alpha level for statistical significance was set at 0.05. The overall analytical strategy was designed to first identify the multidimensional profile of OSA pathophysiology most relevant to general cognitive impairment, and subsequently to test our core hypothesis regarding the specific role of executive control in mediating poorer cognitive performance.
To characterize the study sample, participants were first categorized into
Severe OSA (AHI
LISAS [36]: For tasks involving a speed-accuracy trade-off, performance was quantified using LISAS, an integrated measure of cognitive efficiency. It is calculated for each subject j in each condition i as follows:
where RTij is the mean correct reaction time, PEij is the proportion of errors, and the ratio of the subject’s standard deviation of RT (SRTj) to the standard deviation of PE (SPEj) serves as a subject-specific weighting factor. Higher LISAS values indicate poorer performance.
Global Severity Index (GSI): As preliminary analyses of the multidimensional OSA profile revealed a high degree of multicollinearity among the cognitively-relevant metrics, Principal Component Analysis (PCA) was employed as a data-driven approach to distill these intercorrelated variables into a single, robust index. This analysis was conducted on the z-standardized scores of the clinical OSA metrics that showed a significant preliminary association with the MoCA total score. The first unrotated principal component was extracted to serve as the GSI.
Executive Control Factor (ECF): To derive a single latent variable representing executive control capacity, a second PCA was performed on the z-standardized scores of the three executive task metrics (i.e., 2-back accuracy, digit span backward score, and the inverted reorder LISAS from the digit-ordering task). The first unrotated component was extracted to serve as the ECF.
To address the potential concern that data-driven variables derived from a limited sample may lack reproducibility, we conducted a cross-validation analysis to test the stability of both the GSI and the ECF. We implemented a split-half cross-validation procedure repeated over 1000 iterations. In each iteration, the full sample (n = 30) was randomly partitioned into a training set (n = 15) and a test set (n = 15). The PCA was performed on the standardized data of the training set to derive the component loadings for the GSI and ECF. These loadings were then applied to the standardized data of the test set to compute their scores. This iterative process yielded a full set of cross-validated GSI and ECF scores for all 30 participants, with each score calculated without influence from the subject’s own data on model construction.
Hierarchical Linear Regression: A series of hierarchical linear regression
models were employed for two primary purposes: first, to identify which clinical
OSA metrics were most strongly associated with general cognitive performance
(MoCA total score), and second, to test the specific associations between the
resulting disease burden profile and the various cognitive outcomes. Step 1 of
each model included a standard set of covariates: age, education, Body Mass Index
(BMI), and mood scores (GAD-7, PHQ-9). For tasks with an internal baseline, the
baseline performance was also entered in Step 1. The GSI was entered in Step 2.
The unique contribution of the GSI was evaluated by the change in R-squared
(
Mediation Analysis: To test the hypothesis that executive control mediates the relationship between disease burden and memory performance (as measured by the MoCA delayed recall score), a mediation analysis (PROCESS macro, Model 4 [37]) was performed. GSI was entered as the independent variable (X), ECF as the mediator (M), and the MoCA delayed recall score as the dependent variable (Y). All covariates were included in the model. The significance of the indirect effect was determined using 95% confidence intervals derived from 50,000 bootstrap resamples.
To ensure the statistical robustness of our findings, a series of post-hoc power
analyses were conducted using G*Power (v3.1.9.6, G*Power:
https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower).
For the primary regression models linking the GSI to executive control outcomes,
the achieved power was consistently high (all
For illustrative purposes and to facilitate comparison with clinical standards,
participants were subsequently categorized into two subgroups based on AHI: a
Severe OSA group (AHI
| Characteristic | Severe OSA (n = 23) | Mild-to-Moderate OSA (n = 7) | t-statistic | p-value |
| Age (years) | 34.52 |
31.29 |
1.77 | 0.096 |
| Education (years) | 14.48 |
14.71 |
–0.28 | 0.788 |
| BMI (kg/m2) | 26.67 |
26.61 |
0.04 | 0.968 |
| GAD-7 Score | 2.13 |
4.43 |
–1.66 | 0.136 |
| PHQ-9 Score | 3.00 |
4.00 |
–0.60 | 0.570 |
| Epworth Sleepiness Scale (ESS) | 9.83 |
3.86 |
5.93 | |
| AHI (events/hr) | 56.27 |
20.87 |
7.56 | |
| SpO2 Nadir (%) | 71.43 |
84.14 |
–4.45 | |
| Time |
24.70 |
1.86 |
4.74 | |
| Average Event Duration (s) | 28.01 |
20.47 |
3.40 | 0.004 |
| Longest Event Duration (s) | 66.21 |
37.50 |
4.59 | |
| OAI (events/hr) | 27.44 |
3.59 |
6.38 | |
| OA Average Duration (s) | 29.28 |
19.96 |
2.05 | 0.077 |
| OA Longest Duration (s) | 63.88 |
24.87 |
5.63 | |
| HI (events/hr) | 22.08 |
17.03 |
1.45 | 0.160 |
| Hypopnea Average Duration (s) | 25.34 |
20.43 |
2.31 | 0.036 |
| Hypopnea Longest Duration (s) | 45.94 |
36.36 |
2.09 | 0.057 |
OSA, obstructive sleep apnea; BMI, body mass index; GAD-7, generalized anxiety disorder 7-item scale; PHQ-9, the 9-item patient health questionnaire; AHI, apnea-hypopnea index; SpO2 nadir, the minimum oxygen saturation; ESS, Epworth sleepiness scale; OAI, obstructive apnea index; OA, obstructive apnea; HI, hypopnea index.
Participants’ performance was evaluated on several primary neurocognitive measures: the Montreal Cognitive Assessment (MoCA) with its subscales, the Digit Span tasks, the n-back task (accuracy), and the performance metrics (LISAS) for the whole-report and digit-ordering tasks. The descriptive statistics for these measures are presented in Table 2.
| Category | Measure | Mean | SD | Min | Max | Range |
| Montreal Cognitive Assessment (MoCA) | Total Score | 26.000 | 2.070 | 22.000 | 29.000 | 7.000 |
| MoCA Subscales | Visuospatial/Executive | 4.670 | 0.480 | 4.000 | 5.000 | 1.000 |
| Naming | 2.930 | 0.250 | 2.000 | 3.000 | 1.000 | |
| Language | 2.670 | 0.610 | 1.000 | 3.000 | 2.000 | |
| Attention | 2.000 | 0.000 | 2.000 | 2.000 | 0.000 | |
| Abstraction | 2.000 | 0.000 | 2.000 | 2.000 | 0.000 | |
| Delayed Recall | 1.730 | 1.510 | 0.000 | 4.000 | 4.000 | |
| Orientation | 6.000 | 0.00 | 6.000 | 6.000 | 0.000 | |
| Digit Span | Forward Span | 8.700 | 1.560 | 6.000 | 12.000 | 6.000 |
| Backward Span | 5.730 | 1.860 | 3.000 | 10.000 | 7.000 | |
| N-Back Task | 0-Back Accuracy | 0.969 | 0.051 | 0.720 | 1.000 | 0.280 |
| 2-Back Accuracy | 0.932 | 0.059 | 0.789 | 0.989 | 0.200 | |
| Whole Report | Attended Memory Span | 2.540 | 0.707 | 0.870 | 4.318 | 3.449 |
| Sustained Attention (Accuracy) | 0.987 | 0.011 | 0.950 | 1.000 | 0.050 | |
| Sustained Attention (RT) | 0.452 | 0.071 | 0.321 | 0.592 | 0.271 | |
| Sustained Attention (LISAS) | –1.269 | 0.817 | –2.592 | 0.395 | 2.987 | |
| Vigilance (Accuracy) | 0.886 | 0.072 | 0.684 | 0.987 | 0.303 | |
| Vigilance (RT) | 0.550 | 0.072 | 0.422 | 0.688 | 0.265 | |
| Vigilance (LISAS) | 1.269 | 0.982 | –0.704 | 3.45 | 4.154 | |
| Digital Ordering | Ordered Responses (Accuracy) | 0.937 | 0.073 | 0.667 | 1.000 | 0.333 |
| Ordered Responses (RT) | 2.596 | 0.350 | 1.927 | 3.502 | 1.575 | |
| Ordered Responses (LISAS) | –0.971 | 0.832 | –2.632 | 0.695 | 3.326 | |
| Reordered Responses (Accuracy) | 0.867 | 0.156 | 0.222 | 1.000 | 0.778 | |
| Reordered Responses (RT) | 3.394 | 0.477 | 2.379 | 4.376 | 1.998 | |
| Reordered Responses (LISAS) | 0.971 | 1.658 | –1.849 | 6.668 | 8.518 |
SD, Standard deviation.
In line with our primary aim to identify a cognitively-relevant profile of OSA
pathophysiology, we first examined the individual associations between key
clinical metrics and the MoCA total score. To do so, we first built a series of
hierarchical linear regression models. Age, education, BMI, and mood scores were
entered as confounding factors in the first step, and each OSA severity
measurement was then added individually in the second step. After applying a
Bonferroni correction for multiple comparisons, we found that only the frequency
of OSA events (AHI:
While these significant factors represented different aspects of OSA severity,
preliminary analysis revealed a high degree of collinearity among them: the AHI,
SpO2 nadir, T90, and ESS score were strongly intercorrelated (
As expected, a hierarchical linear regression, controlling for age, education,
BMI, and mood scores, revealed that the GSI was a significant negative predictor
of the MoCA total score (
To confirm that the GSI primarily captured the variance in disease burden most
relevant to cognitive performance, we conducted a follow-up analysis. We
regressed the shared variance of the GSI from each of its constituent clinical
metrics (AHI, SpO2 nadir, T90, and ESS). The remaining residuals, representing
the unique variance of each metric, were then used to predict the MoCA total
score. None of the unique variance components significantly predicted general
cognitive performance: AHI (
To pinpoint the specific cognitive domain driving this association, we conducted
a follow-up analysis on the MoCA subscores. However, an examination of their
distributional properties revealed that six of the seven subscores (attention,
abstraction, orientation, language, naming, and visuospatial/executive functions)
were unsuitable for regression analysis due to pronounced ceiling effects, as
detailed in Table 2. This analytical process isolated the delayed recall subscore
as the sole component with sufficient variance for robust inferential analysis. A
final hierarchical regression confirmed that after controlling for all
covariates, the GSI was significantly associated with poorer delayed recall
performance (
Taken together, these analyses systematically demonstrate that OSA severity is associated with poorer general cognitive function. More specifically, this general association appears to be largely attributable to the relationship between disease burden and performance in short-term memory retention, as measured by the delayed recall task.
Having established the GSI as a robust index of cognitively-relevant disease burden, we next sought to test our primary hypothesis that its detrimental effects are selective to the executive control component of WM. To systematically investigate the relationship between the GSI and the distinct components of WM, we conducted a series of hierarchical linear regression models for each cognitive outcome measure. In each model, a set of common covariates—including age, education, BMI, and mood scores (GAD-7 and PHQ-9)—was entered in the first step. For tasks possessing an internal baseline (n-back, digit span, digit-ordering), the corresponding baseline measure was also included as a covariate. The GSI was then entered in the final step to assess its unique predictive power. A summary of all models is presented in Table 3.
| Cognitive domain | Outcome variable | Step 1: R2 | Step 1: F | Step 2: R2 | Step 2: F | ΔR2 | Fchange | pBonf | p | |
| Attention | Sustained Attention | 0.075 | 0.391 | 0.112 | 0.482 | 0.036 | 0.944 | 0.682 | 0.220 | 0.341 |
| Vigilance | 0.229 | 1.423 | 0.308 | 1.704 | 0.079 | 2.625 | 0.238 | 0.323 | 0.119 | |
| Maintenance Capacity | Digit Span Forward | 0.215 | 1.318 | 0.236 | 1.183 | 0.020 | 0.613 | 0.884 | –0.164 | 0.442 |
| Whole-Report Span | 0.218 | 1.336 | 0.308 | 1.709 | 0.091 | 3.014 | 0.192 | –0.346 | 0.096 | |
| Executive Control | 2-Back Accuracy | 0.254 | 1.308 | 0.582 | 4.380** | 0.328 | 17.264 | –0.663 | ||
| Digit Span Backward | 0.613 | 6.078** | 0.727 | 8.358*** | 0.113 | 9.136 | 0.018 | –0.393 | 0.006 | |
| Digit-Ordering | 0.325 | 1.849 | 0.535 | 3.610* | 0.209 | 9.888 | 0.015 | 0.529 | 0.005 |
GSL, global severity index; *** p
We first examined the association between disease burden and baseline attention,
but found no significant association between the GSI and either attention measure
derived from the whole-report task. Specifically, for sustained attention
(monitoring frequent targets), the GSI did not account for significant additional
variance (
We then investigated the maintenance capacity of WM, focusing on measures of
short-term memory span. When predicting forward digit span, the GSI did not
significantly predict performance (
In contrast, a consistent pattern of associations emerged across multiple tasks requiring executive control over information held in WM.
First, consistent with literature assessing n-back performance in patients with
OSA [38], we found that after controlling for covariates, including 0-back
accuracy, the GSI remained a significant negative predictor of 2-back accuracy, a
task that heavily taxes information updating and monitoring (
Second, converging evidence was found in the digit span backward task. This task
requires not just maintenance but also active manipulation of a sequence in
memory. After controlling for forward digit span (i.e., maintenance capacity),
the GSI was also a significant negative predictor of backward span performance
(
Finally, to assess the core executive process of active information
reorganization, we analyzed performance on the digit-ordering task. After
controlling for performance on the non-reordering condition, the GSI
significantly predicted lower cognitive efficiency (i.e., a higher LISAS score)
in the reordering condition (
Taken together, these findings provide converging evidence from multiple, distinct tasks for a clear dissociation between the core components of WM. While the GSI showed no significant linear relationship with measures of maintenance capacity, it selectively and consistently predicted poorer performance across a range of tasks requiring executive control, including continuous updating (n-back), sequence manipulation (digit span backward), and active information reorganization (digit-ordering task).
The previous findings demonstrated a clear dissociation, isolating executive control as the primary cognitive component associated with the GSI. To formally test our overarching hypothesis that performance in executive control mediates the relationship between disease burden and memory retention, we conducted the planned mediation analysis. To start, we performed a PCA to distill a single, robust latent variable representing a general executive control capacity based on the convergent evidence from the previous section. The PCA was conducted on the standardized scores of the three significant executive metrics (2-back accuracy, digit span backward score, and the inverted digit-ordering reorder LISAS). This yielded a dominant first principal component that explained 69.8% of the total variance, with strong and balanced loadings from all three indicators (loadings: 2-back, 0.59; backward span, 0.55; reorder, 0.59). This component was subsequently defined as the ECF, with a higher value representing a superior executive control capacity.
We then tested the hypothesis that this ECF mediates the association between disease burden (GSI) and memory retention (MoCA delayed recall subscore). A mediation analysis was conducted using the PROCESS macro (Version 5.0, Model 4) for SPSS [37], controlling for age, education, BMI, and mood scores. Significance was assessed via bootstrapping with 50,000 resamples.
The analysis first confirmed a significant total effect of GSI on Delayed Recall
(path c:
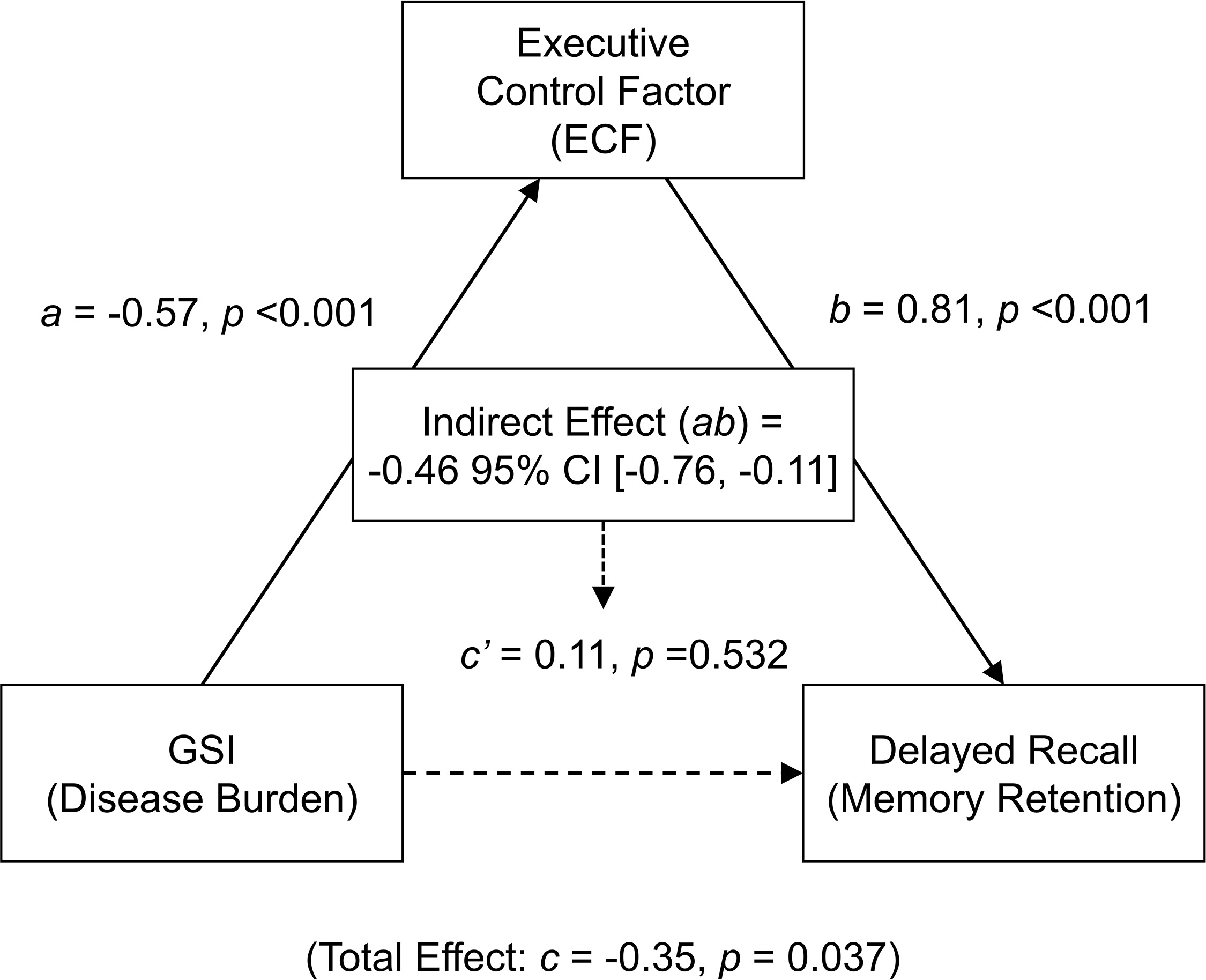 Fig. 2.
Fig. 2.
Path diagram of the mediation model. The model
illustrates that the Executive Control Factor (ECF) fully mediates the
relationship between the Global Severity Index (GSI) and MoCA delayed recall
performance. Path coefficients are standardized betas. Solid lines indicate
significant paths (p
These results provide strong evidence that the relationship between OSA disease burden and memory retention is fully mediated by performance in executive control. Notably, this full mediation remained significant even when the sustained attention and vigilance measures were added as covariates to the model (indirect effect ab = –0.45, p = 0.022, 95% Bootstrap CI [–0.80, –0.01], proportion mediated = 150.5%), further underscoring the specific role of executive control, independent of these attentional measures.
To ensure our primary finding was not an artifact of the specific sample used to derive the data-driven variables, we conducted a split-half cross-validation procedure, repeated over 1000 iterations, to test the stability of the mediation model. This process yielded 1000 sets of cross-validated GSI and ECF scores for all 30 participants.
We found that the cross-validated scores were highly consistent with the
original scores derived from the full sample, demonstrating strong correlations
for both the GSI (all 1000 iterations yielded r
Taken together, these results demonstrate that the full mediation of the relationship between OSA disease burden and memory retention by executive control is fairly robust, and likely not dependent on the specific sample composition used to derive the GSI and ECF.
Finally, to identify the core pathological events contributing to the GSI, we examined the relationship between the GSI and the specific characteristics of obstructive apnea versus hypopnea events. We conducted a series of hierarchical linear regression models, entering age, education, BMI, and mood scores as covariates in the first step. In the second step, we individually added each of the three key characteristics (frequency, average duration, and longest duration) for both event types to assess their unique association with the GSI.
After Bonferroni correction for multiple comparisons, a clear pattern emerged.
Significant associations with the GSI were found for all three metrics of
obstructive apnea events: frequency (
These convergent findings provide strong evidence that obstructive apnea events, rather than hypopneas, are the primary physiological correlate of the global disease burden captured by the GSI. This suggests that the severity and frequency of complete airway obstruction, in particular, may be the key pathological process underlying the variance in our index of cognitively-relevant disease burden.
The present study systematically investigated the relationship between a multidimensional profile of OSA disease burden and the specific components of WM. Our findings revealed a specific pattern of associations rather than a uniform cognitive decline. A cognitively-relevant index of disease severity (GSI) was significantly associated with performance on tasks requiring executive control, but not with measures of attentional control or maintenance capacity. Furthermore, a mediation analysis demonstrated that the association between this disease burden and memory retention performance was fully statistically mediated by performance on these executive control tasks. At a physiological level, this disease burden was primarily associated with the characteristics of obstructive apneas rather than hypopneas. Taken together, these findings suggest that the widespread higher-order cognitive consequences of OSA may be fundamentally underpinned by a selective vulnerability of the WM executive control system to the disease’s underlying pathophysiology.
Our central finding—that OSA’s cognitive impact is not uniform but disproportionately greater on the executive component of WM—offers a more refined, mechanistic perspective than previously available. This clear dissociation between executive control and maintenance capacity aligns with longstanding theoretical models positing that WM is not a unitary construct but a system of distinct components subserved by different neural networks [23, 24, 25, 39, 40]. Specifically, the executive component is highly dependent on the integrity of prefrontal cortical networks, particularly the dorsolateral prefrontal cortex (DLPFC), whereas maintenance functions are more associated with the parietal cortex [26, 27, 28, 39, 40]. This component-specific pattern strongly suggests that the prefrontal cortex is a site of particular vulnerability to OSA-related pathophysiology, which can be best understood as the confluence of the OSA’s two primary pathophysiological insults—intermittent hypoxia and sleep fragmentation—acting upon a region with unique biological fragilities. These insults contribute to neural injury through distinct yet complementary pathways. On one hand, intermittent hypoxia functions as a chronic ischemia-reperfusion injury, initiating a cascade of cellular harm via oxidative stress, neuroinflammation, and glutamate-mediated excitotoxicity [41, 42, 43, 44, 45], ultimately leading to measurable neuronal injury indicated by markers like reduced N-acetylaspartate (NAA) [46]. Concurrently, sleep fragmentation from recurrent arousals severely disrupts essential sleep-related restorative processes, such as synaptic repair, while also promoting chronic sympathetic overactivity and systemic inflammation [45, 47]. This combination of direct cellular insults and disrupted neural restoration is believed to drive the structural brain changes, such as gray matter loss [48], that form the neuroanatomical basis for the resulting neurocognitive dysfunction [45, 49].
The prefrontal cortex, and particularly the DLPFC, emerges as a preferential target for these combined insults for several key reasons. Its status as one of the brain’s most metabolically active regions renders it exceptionally susceptible to the neuroinflammatory cascade initiated by intermittent hypoxia [46]. Furthermore, its profound reliance on the very sleep-related restorative processes that are systematically dismantled by recurrent arousals makes it highly vulnerable to the effects of sleep fragmentation [45]. This convergence of high metabolic demand and critical dependence on sleep homeostasis provides a clear pathophysiological basis for why executive functions subserved by the DLPFC are so consistently impaired in OSA. This interpretation is substantiated by a wealth of neuroimaging evidence demonstrating that OSA is associated with reduced activation, decreased functional connectivity, and attenuated hemodynamic responsiveness specifically within the prefrontal cortex [18, 19, 20, 21, 22]. In line with our results, Naëgelé and colleagues [30] employed paradigms to separate storage from executive operations, revealing that patients with OSA exhibited significantly poorer performance on tasks involving manipulation and updating, while performance on simple maintenance tasks remained relatively intact. Similarly, Grenèche et al. [29] reported more pronounced impairments in the central executive domain compared to maintenance subsystems. By delineating a selective behavioral deficit that maps onto a known pattern of neural vulnerability, our study provides a critical bridge between the cognitive and neurophysiological consequences of OSA, suggesting that prefrontal dysfunction represents a core pathway through which the disorder impairs higher-order cognition.
While our finding of a selective executive control deficit is consistent with contemporary models of prefrontal vulnerability, it is important to situate it within the diverse landscape of the literature. It is well-established that OSA is associated with a wide range of cognitive impairments, including deficits in attention, memory, and executive functions [50]. However, some earlier studies did not find deficits in what was termed “general executive control” [51]. This apparent divergence likely reflects differences in the specific cognitive constructs being assessed. Whereas earlier studies often utilized tasks measuring broad cognitive flexibility and strategic search, such as the Wisconsin Card Sorting Test, our study was specifically designed to tax the executive components within the working memory system—namely, the active updating and manipulation of information. Thus, our findings do not necessarily contradict prior work but rather refine it, suggesting a specific vulnerability in the online management of information held in working memory. This heterogeneity in the literature is likely also compounded by differences in analytical approaches (e.g., the use of multi-component severity indices versus traditional group comparisons) and patient cohort characteristics across studies.
Our mediation analysis was grounded in the theoretical framework that views memory retention not as a passive storage process but as a dynamic one, critically dependent on prefrontal-dependent executive control [52, 53]. Effective encoding, organization, and retrieval of memories rely on the central executive’s ability to actively manipulate information and suppress interference—all core functions subserved by the prefrontal cortex [54, 55, 56, 57]. Our results provide direct support for this framework, demonstrating that the statistical relationship between the GSI and memory retention performance was fully accounted for by performance in executive control. This finding suggests that the well-documented memory difficulties in OSA may not be a primary deficit in storage, but rather a downstream consequence of a compromised executive control system [58, 59]. Notably, this conclusion is strengthened by our analysis showing the full mediation remained robust even after statistically controlling for measures of baseline attention and vigilance, underscoring the specific role of higher-order executive processes.
The specific pattern of associations delineated in this study carries significant clinical implications for the assessment and management of OSA-related cognitive changes. Firstly, for assessment and monitoring, our findings suggest that tasks specifically probing executive control may be more sensitive indicators of the cognitive consequences of OSA than global screeners like the MoCA. The observation that executive performance tracks closely with a multidimensional index of disease severity provides a critical insight that helps to unify the varied results seen across the literature. Specifically, from our findings that the shared variances embedded in GSI accounted for the behavioral differences while the unique variances of individual metrics did not, we may infer that the well-documented links between OSA and cognitive deficits likely stem from a ‘general disease burden’. Different studies have previously reported associations using various single metrics (e.g., AHI, SpO2 nadir, T90, ESS), and our results suggest these metrics were likely significant because they all serve as proxies for this underlying shared pathological core. Secondly, these results identify executive control as a promising therapeutic target. Interventions, whether behavioral (e.g., cognitive training) or physical (e.g., neuromodulation), could be tailored to enhance these specific prefrontal-dependent processes, potentially offering a more targeted and effective approach to cognitive rehabilitation in this population.
Our finding that obstructive apneas, rather than hypopneas, primarily drove the GSI further refines this concept and highlights that not all respiratory events are equal in their potential for neurological harm [60, 61, 62]. The greater detriment of complete obstructions likely stems from a more severe pathophysiological cascade. By definition, an apnea causes more profound and rapid oxygen desaturation—a key predictor of executive dysfunction [60, 63, 64] that triggers a hostile neurochemical environment [41, 47, 61]. This severe hypoxic insult, combined with the more acute hypercapnia resulting from the complete cessation of airflow [62], acts synergistically to provoke a stronger sympathetic activation and greater cerebrovascular stress [41, 47, 62, 65, 66]. Furthermore, terminating a complete obstruction requires a more intense cortical arousal, which leads to more severe sleep fragmentation—itself a strong predictor of cognitive impairment [49, 60]. Therefore, we propose that obstructive apneas constitute a more potent “triple-hit” of severe hypoxemia, acute hypercapnia, and greater arousal intensity, providing a potential mechanistic basis for why they were the stronger predictors of our cognitively-relevant disease burden index.
Finally, our mechanistic findings offer a crucial insight into the link between OSA and long-term neurological risk [67, 68]. By demonstrating that executive control performance statistically mediates the relationship between disease burden and memory retention, we provide a strong theoretical rationale for how treating OSA may preserve broader brain health. This positions OSA as a highly actionable, modifiable risk factor for cognitive decline. Early and effective intervention, such as continuous positive airway pressure (CPAP) therapy, which has been shown to improve executive control, may therefore not only alleviate immediate cognitive complaints but could also play a pivotal role in mitigating the risk of dementia by restoring the integrity of the executive control system.
Several limitations of the present study warrant consideration. The findings
should be interpreted with caution given the relatively modest sample size. In
addition, the imbalanced distribution of disease severity, with a majority of
patients in the severe category, also precluded meaningful subgroup comparisons
and necessitated our regression-based approach across the severity continuum.
Nonetheless, post-hoc power analyses confirmed that our key regression and
mediation models achieved adequate to high statistical power (all
In conclusion, our findings delineate a specific mechanistic pathway for the cognitive consequences of OSA. We propose that a selective vulnerability in the executive control component of WM serves as a central hub, linking the disease’s pathophysiological burden to its broader effects on higher-order cognition, such as performance in memory retention.
Due to their clinical nature, the data presented in this study will be available upon reasonable request from the corresponding authors.
YL, LH and XH designed the research study. YL, LH, YJ, XR and YB performed the research. YL and LH analyzed the data. YL and LH wrote the manuscript, XZ, XH and ZS acquired funding, supervised and reviewed the manuscript, providing critical interpretations of the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki. The research protocol was approved by the Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China (Ethic Approval Number: 2023KY472), and all of the participants provided signed informed consent.
The authors thank all the participants, kept anonymous, who provided data for this study.
This research was funded by the Research Start-up Fund of USTC.
The authors declare no conflict of interest.
During the preparation of this work the authors used Gemini 2.5pro in order to check spell and grammar. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
