- Academic Editor
Current intrathecal (IT) catheter techniques in rats are problematic due to complex surgical procedures and frequent blockages. This study developed a simpler, faster, and more reliable method for long-term IT catheter placement.
Fifty adult male Sprague-Dawley rats were randomly divided into three groups: IT group (n = 30), Sham group (n = 10), and Control group (n = 10). We inserted a polytetrafluoroethylene (PTFE) catheter (0.5-mm outer diameter, 0.3-mm inner diameter) into the cauda equina, reaching a depth of 0.5–1 cm via the L5–L6 intervertebral space. Then catheter was tunneled subcutaneously, exiting at the dorsal neck, and held in place with mechanical compression. We assessed safety and efficacy over 12 weeks through behavioral testing, functional evaluations, and immunofluorescence analysis.
Surgery took an average of 7.2 ± 1.8 min, with a 93.3% first-attempt success rate. Remarkably, all catheters remained patent throughout the 12-week study period (100% patency). Behavioral tests showed no changes in pain sensitivity, although rats did experience a temporary reduction in weight gain during the first postoperative week (p < 0.01). Lidocaine testing confirmed proper catheter function, with motor block occurring rapidly (onset: 30 ± 5 s), followed by complete recovery. Lipopolysaccharide doses of 3, 15, and 30 μg demonstrated clear dose-dependent inflammatory responses, confirming accurate drug delivery. Western blot analysis confirmed no chronic inflammation, with interleukin 1 beta (IL-1β), IL-6, and tumor necrosis factor alpha expression in the IT-Saline group comparable with controls (p > 0.05). Immunofluorescence analysis revealed no significant activation of microglia (ionized calcium-binding adaptor molecule 1) or astrocytes (glial fibrillary acidic protein) based on mean fluorescence intensity, with preserved neuronal density (NeuN-positive cells) comparable with controls.
Our L5–L6 approach effectively minimized the risk of spinal cord injury. The choice of PTFE material proved crucial, as it enabled 100% long-term patency, a result not achieved with other materials. Combined with the neck-mounted external design, this technique offers an improved approach for repeated IT drug delivery in rat models, but more studies are needed to confirm its effectiveness in a wider range of pharmacological applications.
Intrathecal (IT) drug delivery, which involves injecting drugs directly into the subarachnoid space, has become an indispensable technique in neuroscience research. By circumventing the blood–brain barrier, it enables targeted treatment of the central nervous system with remarkable efficiency. The compelling advantages of this approach including low drug dosages, rapid onset of action, and minimal systemic side effects, which make it particularly valuable for pain mechanism research, analgesic drug screening, and investigating neurodegenerative diseases [1, 2]. However, current IT catheter technology still faces limitations, such as complex surgical procedures and catheter blockage, which necessitate the development of improved methods for long-term and reliable application.
Since Yaksh and Rudy [3] pioneered the chronic IT catheter technique in rats in 1976, the field has experienced continuous technological advancements. The early atlanto-occipital approach required inserting the catheter through the foramen magnum and advancing it 8–9 cm into the lumbar region. While this method allowed for precise lumbar drug delivery, the long catheter in the narrow vertebral canal could lead to spinal cord compression, resulting in progressive neurological deficits [2]. Størkson et al. [4] reported that among 40 rats catheterized via the atlanto-occipital route, 2 died and 11 developed neurological dysfunction, whereas no such complications occurred in the lumbar group. Similar concerns about the safety of the atlanto-occipital approach were also shown by Dib [5] and Ineichen et al. [2], who emphasized the advantages of shorter lumbar catheterization routes to minimize neurological injury. By contrast, none of the 32 animals in the direct lumbar group experienced death or neurological dysfunction. Dib [5] also reported complications associated with the atlanto-occipital approach.
To address these serious complications, researchers have explored the direct lumbar puncture approach. Martin et al. [6] were the first to report catheter insertion through the intervertebral space, thereby shortening the IT catheter length, which helps reduce spinal cord compression. Størkson et al. [4] further optimized the puncture procedure by developing the “catheter-through-needle” technique, increasing the success rate to 85%. LoPachin et al. [7] and Milligan et al. [8] made improvements to catheter materials and fixation methods, respectively. In recent years, the 32 G polyurethane (PU) catheter (outer diameter [OD] 0.2–0.25 mm) has gradually become the “gold standard” due to its improved performance [9, 10].
However, existing technologies still face numerous unresolved issues. First, while ultrafine catheters reduce tissue damage, they are highly susceptible to blockage. Jasmin and Ohara [11] reported that, during a 9-month observation period, the functional catheter rate of 32 G PU catheters was 83% (10/12) at 3 months, 58% (7/12) at 6 months, and 50% (6/12) at 9 months. Second, the selection of catheter exit site remains a challenge for researchers. Exit sites on the back or lateral abdomen are easily damaged, requiring single-cage housing, which causes social isolation and adversely affects animal behavior and physiological responses [12, 13]. Hatch et al. [12] described the “isolation syndrome” in rats, and recent studies have confirmed that social isolation can alter pain thresholds and stress responses [13, 14]. Third, existing sealing methods, such as heat sealing and three-way stopcock valves, are complex and costly. Finally, most methods still require 20–30 min of surgical time, which limits their large-scale application [15].
Based on a systematic analysis of existing technologies and a deep understanding of rat spinal cord anatomy, this study proposes an innovative catheter placement strategy. We selected the L5–L6 intervertebral space as the puncture site, which lies below the level of the conus medullaris and contains only the cauda equina, providing a relatively spacious anatomical area [16]. The selection of appropriate sealing methods is critical for maintaining catheter patency while preventing contamination. Although PU catheters have become the mainstream choice [9], this study explored polytetrafluoroethylene (PTFE) catheters for their specific advantages including: (1) low surface energy (18–22 mN/m) minimizing protein adsorption; (2) chemical inertness ensuring drug stability; (3) mechanical properties preventing kinking; and (4) proven biocompatibility in clinical applications [17, 18]. The neck-external design cleverly avoids the problem of catheter damage [11], while the innovative dual-seal closure system ensures both ease of operation and flexibility in usage.
Fifty adult male Sprague-Dawley rats (specific-pathogen-free [SPF] grade),
weighing 180–220 g, were purchased from Lanzhou Veterinary Research Institute
(SCXK Gansu 2020-0002). The animals were housed in an SPF-grade laboratory at
Lanzhou University, under controlled conditions (22
The catheter was made from medical-grade PTFE (0.3 mm inner diameter [ID], 0.5 mm OD) and cut to a final length of 20 cm. The distal end of the catheter was slanted at a 45° angle using an 11-gauge surgical blade to create a sharp bevel for easier puncture, while the proximal end was kept flat for connection. The disinfection process involved soaking the catheter in 75% ethanol for 30 min, followed by three thorough rinses with sterile saline to remove any residual ethanol, and finally, autoclaving at 121 °C for 20 min. After sterilization, the catheter was designed in segments: the puncture segment (2 cm, measured from the level of the spinous process, with approximately 0.5–1 cm entering the IT space), subcutaneous tunneling segment (14 cm), and external fixation section (4 cm).
Rats were anesthetized with isoflurane (RWD, R510-22-16, Shenzhen, China) at a concentration of 3% and oxygen flow rate of 1 L/min, which was subsequently lowered to a maintenance dose of 1.5–2% after loss of the righting reflex. The rats were placed in the prone position and fixed to the operating table. The dorsal and neck areas were shaved, and the surgical site was disinfected sequentially with iodine tincture and 75% ethanol. The gap between the L5 and L6 spinous processes was identified by palpation, and a 1.5 cm longitudinal skin incision was made along the midline of the spine. The subcutaneous tissue was bluntly dissected to expose the spinous processes and interspinous ligaments. A 22 G puncture needle was inserted at a 70–80° angle toward the head, passing through the paraspinal muscles and interspinous ligaments, establishing a channel into the vertebral canal. Once the needle position was stable, a PTFE catheter, preloaded with a flexible steel core (0.15 mm diameter), was slowly advanced 1.5–2 cm through the needle sheath. Indicators of successful catheter insertion included cerebrospinal fluid (CSF) refluxing from the catheter and slight twitching of the rat’s tail or hind limbs. Once the catheter position was confirmed, the external catheter segment was stabilized and the steel core was slowly withdrawn, with clear CSF continuously flowing from the catheter. The puncture needle sheath was then carefully withdrawn, and the catheter was securely fixed to the paraspinal muscles using 5-0 sutures.
A 4 mm transverse incision was made in the region between the scapulae of the neck. Using micro-ophthalmic hemostatic forceps, the catheter end was clamped and bluntly dissected subcutaneously from the lumbar incision toward the neck, establishing a subcutaneous tunnel. After the catheter was advanced through the neck incision, its external length was set to 4 cm. The lumbar incision was sutured in layers: first with 4-0 absorbable sutures for the muscle layer and then with 5-0 silk sutures for the skin. The neck catheter exit site was sutured using a purse-string suture technique to secure the catheter and prevent infection. At the end of the procedure, antibiotic ointment (Erythromycin Ointment, 149059B, Guangdong Hengjian Pharmaceutical Co., Ltd., Jieyang, China) was applied locally.
(1) Mechanical Compression Sealing Method: The tip of the IT catheter was sealed by mechanical compression using a small surgical pressure clamp. The applied force, estimated at approximately 20–30 N, was sufficient to flatten the PTFE catheter and occlude its lumen, a task easily performed with one hand.
(2) Sterile Surgical Film Sealing Method: A 1
(1) Behavioral Testing: Baseline measurements were taken 3 days and 1 day before
surgery, and tests were performed once a week post-surgery for 12 weeks. Testing
was conducted in a temperature-controlled environment (22
Mechanical Paw Withdrawal Threshold: Von Frey filaments (Stoelting Co., Wood Dale, IL, USA) were used. After allowing the rats to acclimate on a metal grid for 15 min, a 50% withdrawal threshold was determined using the up-down method. Starting with a 2.0 g filament, vertical stimulation was applied to the plantar area of the hind paw for 6–8 s. Based on the presence or absence of a withdrawal response, the next filament was selected. The threshold was calculated by averaging the results of three tests performed on each paw.
Thermal Pain Threshold: Measured using a hot plate apparatus set at 48 °C. The latency until the rat licked its paw or jumped was recorded, with a cutoff time of 50 s. Each rat underwent three tests, with 10-min intervals between tests, and the average value was calculated.
Cold Pain Threshold: Acetone spray method. Using a micro-injector, 100 µL of acetone was sprayed on the hind paw, and the withdrawal response within 30 s was observed. Each side was tested three times, in 5-min intervals.
(2) Body Weight Monitoring: The body weight of all rats was recorded on the day of IT catheter placement and measured weekly thereafter. Body weight gain rate was calculated using the following formula:
Body weight gain rate = (final body weight – initial body weight) / initial
body weight
(3) Catheter Function Verification (Week 12): Catheter patency was assessed daily by visual observation, with more comprehensive functional checks including CSF reflux and injection resistance performed in week 6 (n = 10) and full functional verification in week 12.
Lidocaine Test: 10 µL of 2% lidocaine (H20133209, Hubei Tianyao Pharmaceutical Co., Ltd., Xiangyang, China) was injected followed by 15 µL of saline to flush the catheter. The onset and recovery times of motor block were recorded.
Dye Distribution: 20 µL of 0.5% (M9140-25G, Sigma-Aldrich, St. Louis, MO, USA) methylene blue and 15 µL of saline were injected. The dye spread was visible after 10 min.
Computed tomography (CT) Imaging: 30 µL of contrast agent (240705DJ, Ioversol Injection, Jiangsu Hengrui Pharmaceuticals Co., Ltd., Lianyungang, China) was injected, and small animal CT scanning was performed to determine the catheter location.
(4) Immunofluorescence: The L1-L6 spinal cord was collected, fixed in 4% paraformaldehyde for 24 h, cryoprotected in 30% sucrose, and sectioned at a thickness of 20 µm. Sections were incubated overnight at 4 °C with anti-glial fibrillary acidic protein (GFAP) (1:500; ab7260, Abcam, Cambridge, MA, USA), anti-ionized calcium-binding adapter molecule 1 (Iba-1) (1:1000; OB-PGP049-01, Oasis Biofarm, Huzhou, China), and anti-NeuN (1:500; 84401-4-RR, Proteintech, Wuhan, China) primary antibodies, followed by incubation with fluorescent secondary antibodies (1:300; BD9010, BioDragon, Suzhou, China) for 2 h. DAPI was used for nuclear staining. Images were captured using a fluorescence microscope with consistent parameters. Mean fluorescence intensity (MFI) was measured in three randomly selected regions per section using ImageJ software (v1.52a; National Institutes of Health, Bethesda, MD, USA). Five sections per animal were analyzed.
In week 12, 24 rats from the IT group (n = 30) were randomly selected for the lipopolysaccharide (LPS) dose-response gradient experiment, and 6 rats from the Control group were selected as uncatheterized controls. The experimental animals were randomly divided into five groups (n = 6): Control group (uncatheterized normal control), IT-Saline group (IT saline), IT-LPS 3 µg group, IT-LPS 15 µg group, and IT-LPS 30 µg group.
LPS from Escherichia coli O55:B5 (L2880, Sigma-Aldrich, St. Louis, MO, USA) was prepared in sterile saline at concentrations of 0.3, 1.5, and 3.0 µg/µL. The IT-Saline group and IT-LPS groups received 10 µL of the corresponding solution via catheter injection, followed by a 15 µL saline flush. The Control group received no treatment. After 24 h, the rats were euthanized, and the L3-L5 segment of the spinal cord was snap-frozen in liquid nitrogen and stored at –80 °C.
Western blot analysis was used to detect the expression of inflammatory cytokine
proteins. Spinal cord tissue was lysed using RIPA lysis buffer containing
protease inhibitors, and protein concentration was measured using the BCA method.
Protein (30 µg) was loaded onto each lane, separated by 12.5% sodium
dodecyl sulfate polyacrylamide gel electrophoresis, and electrotransferred to
PVDF membranes. The membranes were blocked in 5% skim milk for 1 h and incubated
overnight at 4 °C with the following primary antibodies: rabbit
anti-interleukin 1 beta (IL-1
All rats were euthanized using a two-step protocol: isoflurane anesthesia (3% concentration, 1 L/min oxygen flow) until loss of righting reflex, followed by intraperitoneal administration of pentobarbital sodium (200 mg/kg, C1887623, Nanjing Huangtai Pharmaceutical Co., Ltd., Nanjing, China). Death was confirmed by monitoring for absence of heartbeat, respiration, and corneal reflex for a minimum of 5 minutes.
Data were analyzed using GraphPad Prism 8.0.2 (GraphPad Software Inc., La Jolla,
CA, USA). All quantitative data are presented as mean
Of the 30 rats in the IT group, 28 had successful puncture on the first attempt
(93.3%), while 2 required a second puncture, ultimately resulting in successful
catheter placement for all rats. The average surgery time was 7.2
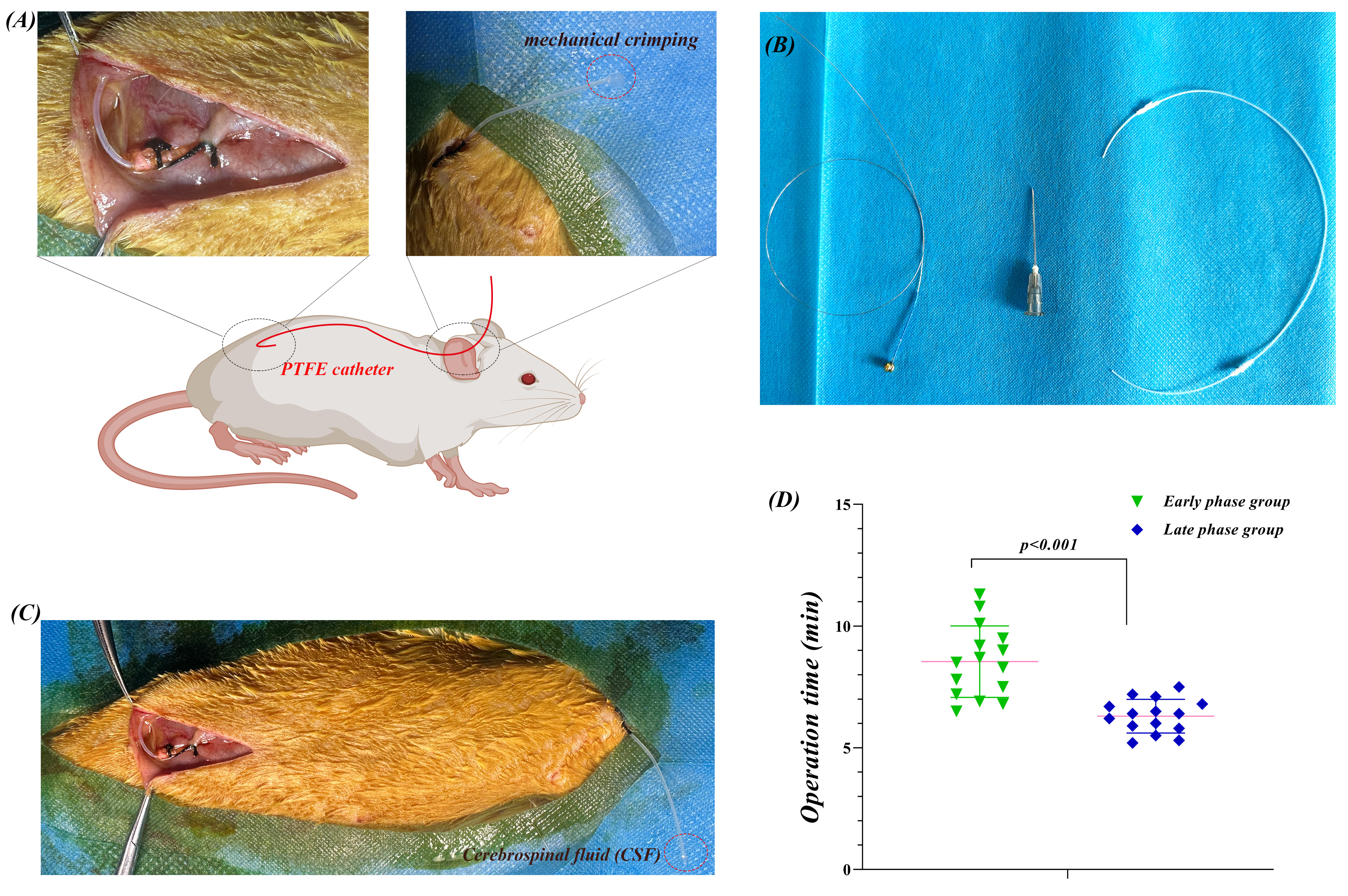 Fig. 1.
Fig. 1.
L5-L6 approach for chronic IT catheterization and surgical
learning curve in rats. (A) Schematic of the modified technique for IT
catheterization. Top left: L5-L6 intervertebral space puncture with a 22 G needle
inserted at a 70–80° angle, and a PTFE catheter (0.5 mm OD) inserted
0.5–1 cm into the cauda equina region. Top right: Neck-external catheter
placement with mechanical compression sealing. Center: Catheter path, from the
lumbar puncture site through a 14 cm subcutaneous tunnel to the neck. (B) PTFE
catheter (0.3 mm ID, 0.5 mm OD), with the distal end beveled at 45°, and
4 cm left externally. (C) CSF reflux confirmation. Clear CSF flow was observed
after the catheter was successfully placed into the subarachnoid space. (D)
Surgical learning curve for procedure time. Early group (first 15 rats): 8.1
All rats regained consciousness within 5 min after discontinuation of isoflurane inhalation and regained activity within 15 min. One rat (3.3%, 1/30) exhibited mild lameness 1–2 days post-surgery, which fully recovered within 1 week. No severe complications, such as infection, paralysis, death, or catheter dislodgement, occurred. Over the 12-week observation period, all catheters remained secure.
Long-term behavioral monitoring confirmed that the L5-L6 IT catheter did not have a significant effect on rat pain perception. Over the 12-week observation period, the IT, Sham, and Control groups exhibited highly consistent response patterns across multiple pain assessment modalities.
(1) Pain Sensitivity Assessment: No differences were observed between groups in any of the pain tests. The mechanical pain sensitivity (von Frey test) remained stable across all groups, with no signs of abnormal hyperalgesia or hypoalgesia (Fig. 2A). The cold pain response time remained within the normal range, with high consistency between groups (Fig. 2B). The thermal pain latency showed a normal physiological extension trend, which was observed synchronously across all three groups, reflecting a normal thermal adaptation process rather than pathological changes (Fig. 2C). Importantly, the stability of these pain parameters confirmed that catheter placement did not damage the pain transmission pathways in the dorsal horn of the spinal cord.
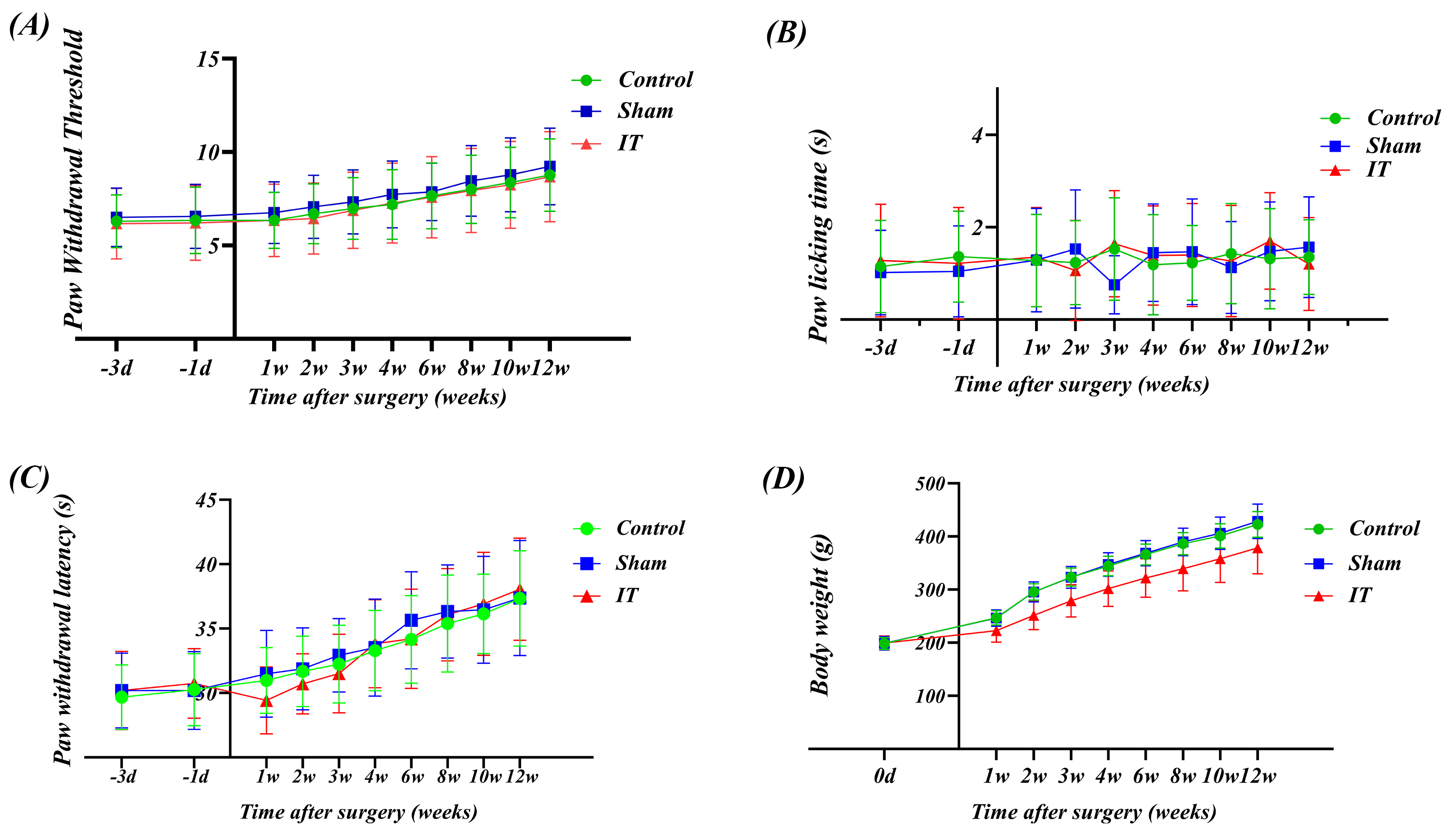 Fig. 2.
Fig. 2.
Long-term assessment of the modified L5–L6 intrathecal
catheterization technique’s effects on pain sensitivity and body weight in rats.
(A) Mechanical Pain Threshold: 50% withdrawal threshold measured using von Frey
filaments. Baseline measurements at three days (–3 d) and one day (–1 d) before
surgery, and post-surgery measurements were taken every 1–2 weeks for 12 weeks.
No significant differences were observed between the groups, indicating that
catheter placement had no effect on mechanical pain sensitivity. (B) Cold Pain
Response: Paw withdrawal duration measured using the acetone test for cold pain
response. The response time for each group remained stable within the
physiological range, with high consistency between groups, confirming that
catheter placement did not affect the cold pain transmission pathway. (C) Thermal
Pain Latency: Latency to paw withdrawal or jumping measured using the hot plate
test (48 °C). All three groups exhibited the same adaptive extension
trend, with the latency increasing from approximately 30 s at baseline to 38 s,
reflecting a normal thermal adaptation process. (D) Body Weight Growth Curve: The
IT group showed a slight delay in weight gain during the first week post-surgery,
but thereafter, the growth trajectory was parallel to that of the other two
groups. From the second week onward, the growth trajectory of all three groups
converged, indicating that the surgical stress effects were self-limiting. Data
are expressed as mean
(2) Body Weight Growth Dynamics: The IT group showed mild delayed weight gain only during the first week post-surgery. This transient phenomenon can be attributed to stress and temporary changes in feeding behavior due to surgery. Notably, from the second week onward, the weight gain trajectory of the IT group was parallel to that of the Control group, indicating that the surgical effects were self-limiting. By the end of the observation period, there were no significant differences in body weight among the three groups, suggesting that the catheter placement technique had no long-term negative impact on the rats’ nutritional status or metabolic function (Fig. 2D).
The consistency of the behavioral data not only validated the safety of the surgical technique but more importantly, ensured baseline consistency for subsequent pharmacological experiments. The complete preservation of pain perception function is particularly critical for pain mechanism studies, as any neural functional changes caused by the catheter itself would introduce confounding factors in the interpretation of experimental results.
The catheter patency was assessed using a stepwise evaluation strategy. Daily observations showed that all catheters remained transparent and stable throughout the 12 weeks. In week 6, 10 rats were randomly selected for inspection, and 2 rats showed CSF reflux with no resistance to the injection of 10 µL saline. In week 12, during the full evaluation, 28 rats (93.3%) maintained complete patency, while 2 rats (6.7%) required slight increases in injection pressure but were still able to deliver the drug successfully. Based on functional standards, a 100% patency rate was achieved at week 12, with no incidence of catheter-related infections.
Functional verification in week 12 confirmed the long-term efficacy of the catheters. The lidocaine test (n = 10) showed an onset time of 30 s, with all animals displaying typical paralysis of both hind limbs, which lasted approximately 20 min before full recovery (Fig. 3A). The dye distribution test (n = 3) showed that methylene blue was primarily distributed between L2-L5 (Fig. 3B). CT verification (n = 3) confirmed that the catheter tip was positioned at the L4 level (Fig. 3C). These validations, along with successful implementation of the LPS experiment, confirmed the long-term reliability of this method.
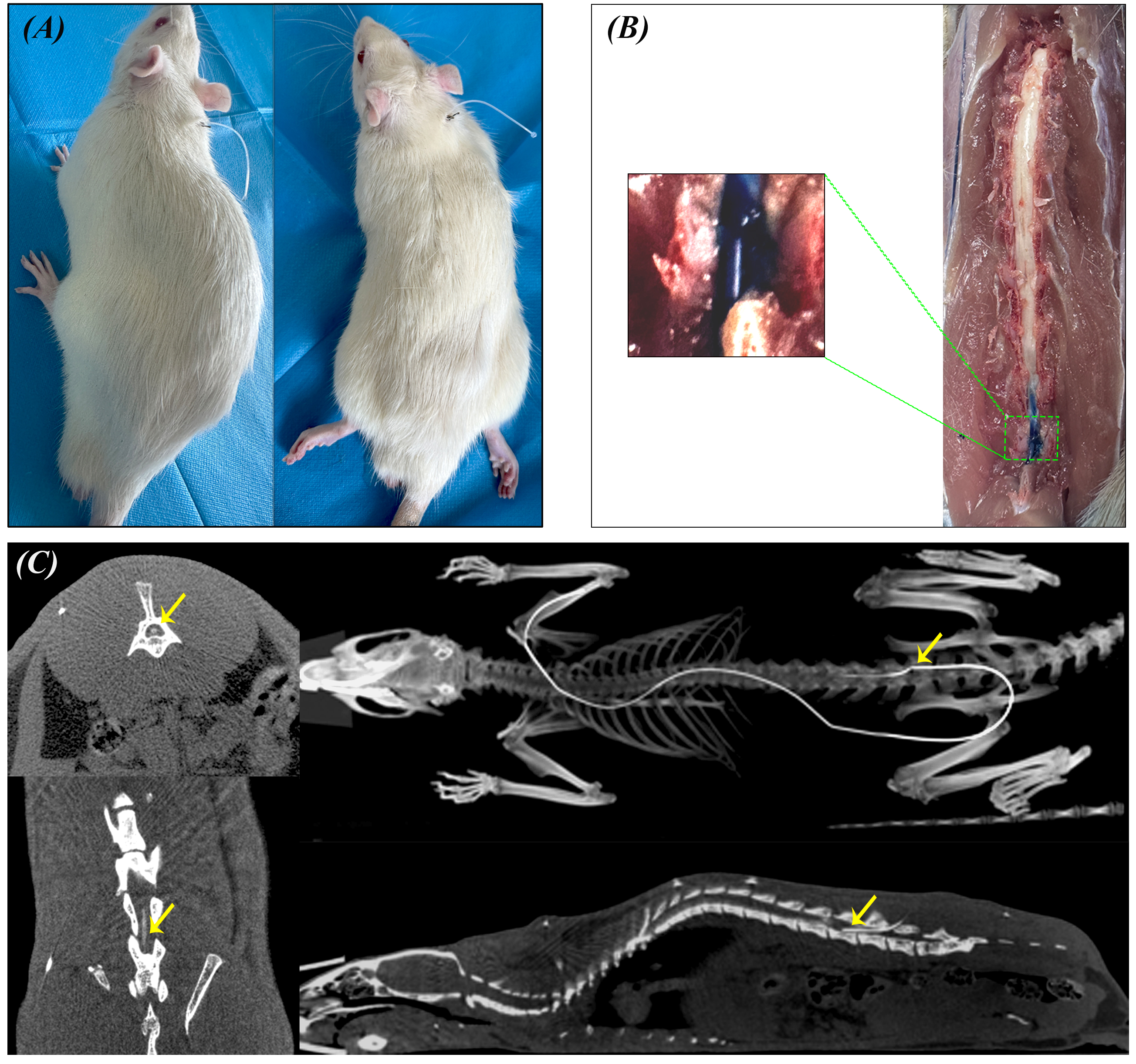 Fig. 3.
Fig. 3.
Functional verification of L5-L6 IT catheter at 12 weeks. (A) Lidocaine Motor Block Test. Left: Before injection; Right: After 10 µL of 2% lidocaine was injected intrathecally, resulting in bilateral hind limb paralysis. (B) Methylene Blue Dye Distribution. 20 µL of 0.5% methylene blue was injected intrathecally. After 10 min, sagittal spinal sectioning revealed dye distribution (blue-stained area). Inset: Magnified view of the dye distribution. (C) Micro-CT Imaging Verification of Intrathecal Catheter Placement. Left: Axial CT images at different spinal levels demonstrating catheter position within the spinal canal (yellow arrows indicate catheter cross-sections). Right top: Three-dimensional skeletal reconstruction showing the complete catheter trajectory (white line) with the catheter tip position marked (yellow arrow) at the L5-L6 level. Right bottom: Mid-sagittal CT reconstruction of the vertebral column displaying the IT catheter course and tip location (yellow arrow) within the lumbar subarachnoid space. CT, Computed tomography.
Immunofluorescence analysis at 12 weeks revealed minimal glial activation and
preserved neuronal integrity. Quantitative analysis showed no significant
difference between IT and Control groups in GFAP MFI (p
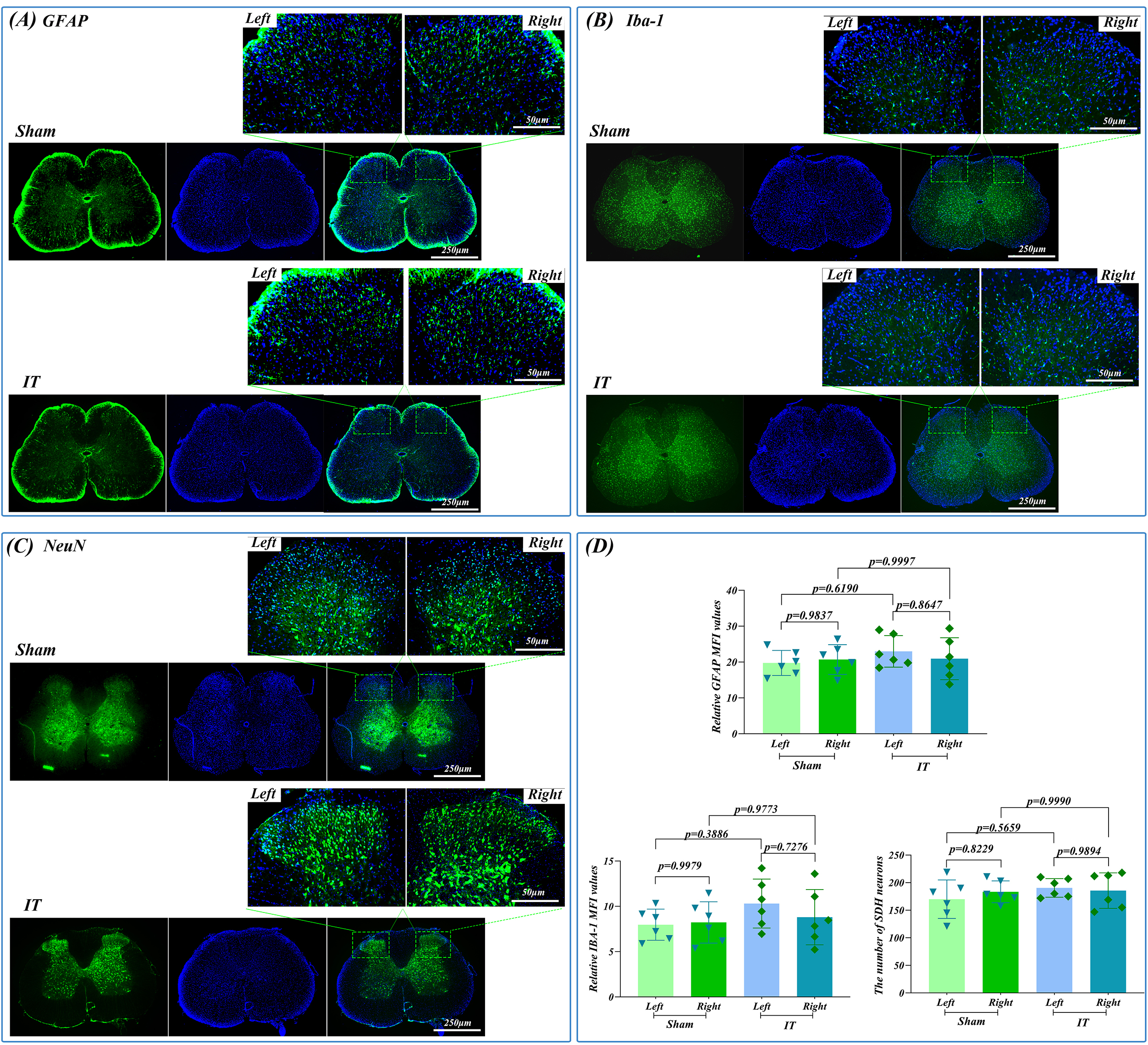 Fig. 4.
Fig. 4.
Immunofluorescence analysis of glial activation and neuronal
preservation 12 weeks after L5-L6 IT catheterization. (A) GFAP
immunofluorescence (green). (B) Iba-1 immunofluorescence (green). (C) NeuN
immunofluorescence (green). (D) Quantitative analysis of GFAP MFI, Iba-1 MFI, and
NeuN-positive neuronal counts in dorsal and ventral horn regions. Scale bars: 250
µm (overview), 50 µm (magnified). DAPI: blue (n = 6). Quantitative
data represent mean
To further validate the safety of long-term catheterization and confirm the
accuracy of drug delivery doses, we conducted an LPS dose-gradient experiment in
rats 12 weeks after catheter placement. Western blot analysis showed that
IL-1
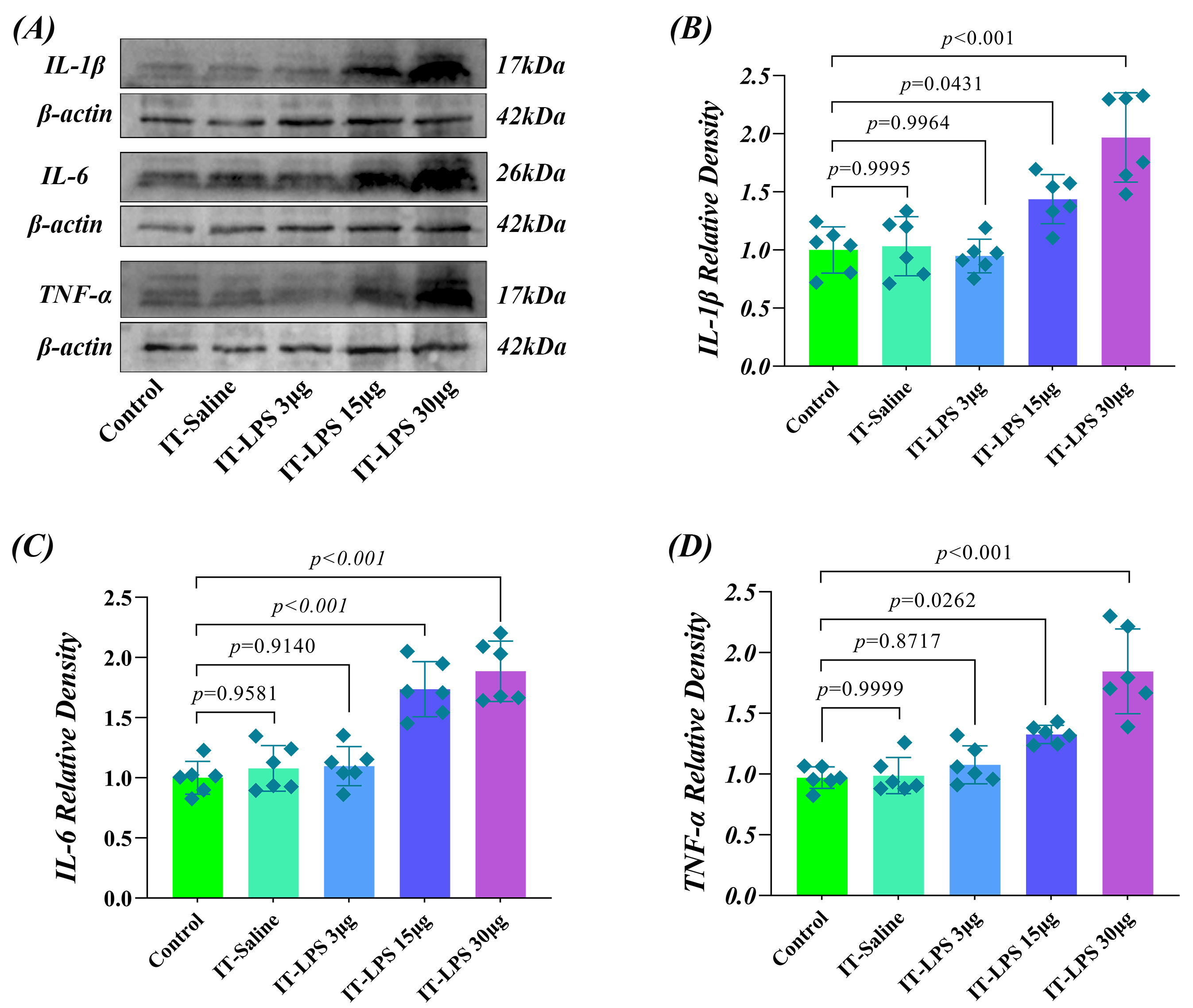 Fig. 5.
Fig. 5.
Dose-effect response of spinal cord inflammatory cytokines to LPS
in long-term IT catheterized rats. (A) Representative Western blot analysis of
IL-1
Twenty-four hours after IT injection of varying doses of LPS, inflammatory
cytokine expression showed a clear dose-dependent change. In the IT-LPS 3
µg group, the expression of all three inflammatory cytokines was not
significantly higher compared to baseline levels (p
These results show that inflammatory cytokine expression in the IT-Saline group did not differ significantly from the Control group, confirming that long-term catheterization itself does not induce chronic inflammatory responses. The LPS dosage groups showed a clear dose-response relationship: 3 µg was a subthreshold dose, 15 µg induced moderate inflammation, and 30 µg caused a strong inflammatory response. These findings confirm that the catheterization method retains good biocompatibility and drug delivery accuracy even after long-term use.
The modified IT catheterization technique established in this study successfully addressed key challenges of traditional methods through systematic innovations. The choice of the L5–L6 approach was based on a precise understanding of rat spinal cord anatomy, whereas the PTFE material provides a balance between ease of operation and long-term efficacy. The neck-external catheter design and dual sealing system demonstrate practical considerations.
The lumbar-sacral enlargement of the rat spinal cord is located at the L3–S1 level [19], and the L5–L6 approach is at the caudal end of the spinal cord, primarily containing the cauda equina. This anatomical feature is the basis for the success of this method. In comparison with the classic method of Yaksh and Rudy [3], we avoided the risk of compression associated with an 8–9 cm long catheter traversing the entire spinal cord. The traditional atlanto-occipital approach may result in neurological complications, including spinal cord compression, nerve root injury, and paralysis [4]. Only 3.3% (1/30) of our rats showed temporary lameness, which completely recovered. Compared to the L4–L5 approach by Martin et al. [6], the L5–L6 position is lower, providing a larger safety margin. Størkson et al. [4] developed the “catheter-through-needle” technique for direct lumbar puncture; however, using the PE-10 polyethylene catheter, we have demonstrated that a thicker catheter, when placed in the appropriate position, is also safe.
The physiological characteristics of the cauda equina enable the use of a 0.5 mm catheter. Histological analyses showed the absence of glial cell activation, with both astrocytes and microglia maintaining their resting morphology, and importantly, neuronal density remained intact with no evidence of neuronal loss in either dorsal or ventral horn regions, which is comparable to the safety profile demonstrated by Sakura et al. [9] using a 32 G catheter. This challenges the long-held belief that “thinner is always better” and suggests that, at specific anatomical sites, the relationship between catheter diameter and damage is not linear. The selection of PTFE was a carefully considered decision. While PU is currently the mainstream material [11], PTFE offers unique advantages. Its low surface energy (approximately 18–22 mN/m) [17, 18] minimizes protein adsorption, which may be key to achieving a 100% high patency rate. By contrast, Jasmin and Ohara [11] reported that, over a 9-month observation period, the functional patency rate of 32 G PU catheters was 83% (10/12) at 3 months, 58% (7/12) at 6 months, and only 50% (6/12) at 9 months. The chemical inertness of PTFE ensures the stability of drugs, which is particularly crucial for protein-based drugs.
The average surgery time of 7.2 min has important practical implications. Several new methods have reported similarly quick operation times, such as a 3–4 min drug delivery time [20], while surgery times for long-term catheter placement vary across different techniques, with lumbar puncture taking 20 min and the atlanto-occipital approach taking 10 min [2]. Our improvements have significantly increased efficiency, which not only reduces the risks associated with anesthesia but more importantly, lowers surgical stress. The body weight gain rate supports this view as rats were only affected in the first week, and quickly recovered thereafter. This means that formal experiments can begin 1 week after catheter placement, greatly reducing the recovery period compared to the 2–3 weeks traditionally recommended. The rapid surgery technique also aids in standardization. We observed a clear learning curve effect (significant time differences between the first and last 10 rats), but even beginners could complete the procedure within 10 min. This reproducibility is crucial for multicenter studies.
The stability of the three pain modalities (mechanical, thermal, and cold) supports the method’s safety. This is consistent with the results of a previous study using fine catheters [21], but our method is simpler to operate. Symmetrical responses from both sides eliminate the possibility of unilateral injury, a factor often overlooked in previous studies. Although this study still required single-cage housing to prevent rats from gnawing on each other’s catheters, the neck-external design has distinct advantages over traditional back or side abdominal exit designs: (1) limited neck mobility, making it difficult for rats to gnaw on the catheter; (2) ease for the researcher to observe the catheter’s condition and administer drugs; and (3) reduced catheter displacement caused by scratching or friction from the rats. Although social isolation has been shown to affect pain thresholds [14, 22], no significant changes in pain sensitivity were observed in this study, possibly due to the rats’ adaptation to single-cage housing conditions. This also suggests that housing conditions may serve as a confounding factor in interpreting pain research results.
The LPS dose-gradient experiment and lidocaine functional verification create a complementary validation framework, confirming the reliability of the catheterization method from different perspectives. The lidocaine experiment confirmed both the accuracy and patency of the catheter placement through its motor block effect, whereas the LPS experiment demonstrated the method’s precision in drug delivery across different doses. The lack of differences in inflammatory markers between the IT-Saline group and the Control group molecularly confirmed the biocompatibility of the catheter 12 weeks after placement, corroborating the histological findings. Furthermore, the distinct dose-dependent gradient effect observed in the three LPS groups strongly supports the dose control precision and repeatability of this catheter technique. This dual verification strategy using both small molecules (lidocaine) and large molecules (LPS) demonstrates the method’s ability to deliver different types of substances in rat models, providing a methodological option for IT drug delivery studies.
The dual closure system design represents a combination of practicality and forward-thinking design. The mechanical compression closure method used in this study performed very well during the 12-week observation period: it only required a hemostatic clamp to operate, with each use consuming 0.5–0.6 mm of the catheter, and the 4 cm reserve theoretically allowed for over 60 uses, fulfilling the requirements for long-term IT drug delivery. This “low-tech” solution is highly accessible in resource-limited settings. With 100% patency and no infections in 30 rats, this method proved highly reliable. Although the infection rate of human IT catheters is relatively high, infection complications are uncommon in rodents [23]. The physical seal formed by mechanical compression blocks contamination at its source—unlike traditional open-ended designs relying on caps or valves—likely being a key factor in the high success rate. For high-frequency drug delivery needs (such as pharmacokinetic studies), we propose the sterile surgical film method as an alternative: inspired by the principle of clinical infusion ports, it allows for long-term repeated drug delivery without shortening the catheter, providing flexible options for different experimental needs.
In recent years, several research groups have attempted to improve the IT catheterization technique. Wu et al. [24] described a simpler and less invasive method for chronic lumbar IT catheterization in mice, which avoids major surgery and causes no neurological deficits. Mazur et al. [15] emphasized rapid catheterization, achieving a success rate of 93%, but still used the traditional PE-10 catheter. Ineichen et al. [2] published detailed protocols in Nature Protocols. Our method combines several innovations: anatomical targeting (L5–L6), material selection (PTFE), external catheter design (neck), and closure system (dual). This systematic improvement has led to significant performance enhancement, especially the 100% long-term patency rate, which is clearly superior to what has been previously reported in the literature.
The catheterization technique established in this study has certain technical limitations. First, the 15 µL dead volume in the catheter affects drug delivery accuracy. With conventional drug doses (15–30 µL), the drug dilution rate is 33–50%. Although this can be compensated by adjusting the drug concentration, for microdosing experiments with less than 10 µL, this limitation will significantly impact experimental precision. This dead volume limitation has specific implications for pharmacological studies. (1) In pharmacokinetic experiments requiring precise drug concentrations, the mixing zone created by the dead volume could alter initial drug distribution patterns. (2) For dose-response studies, especially at the lower end of the curve, the effective delivered dose may deviate significantly from the intended dose. (3) In repeated dosing protocols, residual drug from previous injections could create cumulative effects. Researchers should account for these factors when designing experiments, particularly when comparing results across different catheterization techniques with varying dead volumes. To address this issue, potential technical improvements include: (1) using a catheter with a smaller inner diameter (0.2 mm) to reduce the dead volume to 10 µL; (2) developing a pre-filled catheter system; and (3) designing a dual-lumen catheter for independent control of drug delivery and flushing. Second, the 0.5 mm catheter OD limits its application in certain specialized settings, particularly when the catheter needs to be extended to the thoracic spinal cord. However, based on literature analysis, more than 90% of IT pharmacological studies focus on lumbar administration, so the practical impact of this limitation is relatively small.
To advance the standardized application of this technology, future efforts should primarily focus on: (1) material optimization: enhancing the hydrophilicity of PTFE using plasma treatment and developing PTFE-PU composite catheters that balance biocompatibility with mechanical properties; (2) function expansion: integrating MEMS pressure sensors for patency monitoring and combining with implanted pumps for programmable drug delivery; (3) standardized system: developing a standard operating procedure that includes surgical videos, fault diagnosis flowcharts, and quality control indicators and establishing a multi-center verification mechanism; and (4) cross-species applicability: the neck-external design of this method effectively prevents animals from damaging the catheter, making it suitable for use in other experimental animals such as rabbits and dogs.
The L5–L6 IT catheterization method developed in this study represents a significant technological advancement in the field. Through innovative anatomical localization, material selection, external catheter design, and dual closure system, we achieved rapid (7.2 min), safe (93.3% success rate), and reliable (100% patency rate) long-term IT drug delivery. The key strength of this method lies in its optimal use of rat spinal cord anatomy, where using a relatively thicker PTFE catheter in the cauda equina region is not only safe but may also enhance long-term success rates by minimizing blockages. The neck-external design cleverly addresses the catheter protection issue, while the dual-closure system accommodates various experimental needs. Although the 15 µL dead volume poses a limitation for some precision experiments, it remains an acceptable technical compromise for most IT drug delivery studies. More importantly, this method significantly lowers technical barriers, increases experimental efficiency, and provides a practical tool for neuroscience research, particularly pain and neuropharmacology studies. As this technique is further validated and optimized, it may contribute to more efficient preclinical studies in central nervous system drug development and pain mechanism research.
All data from Western blot (WB) and immunofluorescence assays are available from the corresponding author upon reasonable request. Commercially available experimental reagents are described in detail in the Materials and Methods section. Further inquiries can be directed to zhanghh22@foxmail.com.
The corresponding authors HHZ and WN, as well as the first author WMZ, organized, designed and wrote the article. KZ, YQS, YBD, RR, GHZ and YCM made substantial contributions by refining the study design, performing experiments and data acquisition, conducting data analysis, preparing figures/tables, and conducting literature searches. All authors contributed to editorial changes in the manuscript. All authors have read and approved the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The experimental protocol involving animals was approved by the Animal Ethics Committee at the Second Hospital of Lanzhou University (Approval No. D2025-459). All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996) and the ARRIVE 2.0 guidelines.
Not applicable.
This work was supported by the National Natural Science Foundation of China (No. 82360435); Lanzhou University Second Hospital Cuiying Youth Fund Project (No. CY2022-QN-A03); Cuiying Science and Technology Innovation Program Project (No. 2022-MS-A10); Lanzhou Youth Science and technology Talents Innovation Project (2023-2-39); Natural Science Foundation of Gansu Province (No. 24JRRA1101).
The authors declare no conflict of interest.
We declare that ChatGPT was used solely as a language editing tool, similar to grammar-checking software or professional editing services, and did not contribute to the intellectual content of this research. All authors have reviewed and approved this declaration.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
