- Academic Editor
†These authors contributed equally.
Cardiovascular modulation in response to movement and gravitational forces can be influenced by vestibular input or peripheral baroreflex mechanisms. Galvanic vestibular stimulation (GVS) is a widely used, noninvasive method for activating neural pathways within the vestibular system, as well as associated pathways such as vestibulo-spinal, oculomotor, and vestibulo-autonomic circuits. Research on vestibulo-autonomic function via GVS has primarily focused on its effects on cardiovascular modulation and sympathetic muscle and nerve activity. However, inconsistencies in GVS application protocols across studies have made it challenging to reach a consensus regarding its effectiveness in modulating the vestibulo-autonomic pathway. Evidence suggests that GVS induces transient autonomic changes by stimulating a neural pathway sensitive to otolith input. This review collates the parameters used in GVS application and examines their effects on autonomic neural pathways by analyzing variations in amplitude, frequency, and electrode montage to understand their impact on autonomic responses, including changes in heart rate (HR), blood pressure (BP), and sympathetic muscle or nerve activity (MSNA). By analyzing stimulation parameters and experimental protocols, we aim to determine their impact on autonomic activation and evaluate their potential for precise autonomic modulation. Finally, based on the evidence generated in populations with neurological disorders and motion sickness, we discuss the potential of GVS as a complementary neuromodulation strategy to treat autonomic dysregulation.
Over the past two decades, galvanic vestibular stimulation (GVS) has been extensively employed in both basic and clinical research to investigate the vestibular system and its associated neural pathways [1]. While most studies have primarily focused on posture and ocular movements, GVS has also been used to explore other vestibular-related systems in both healthy and diseased populations [2, 3]. The vestibulo-sympathetic system is no exception, as it plays a critical role in regulating blood flow following head movement to ensure adequate circulation to vital and peripheral organs. Early studies demonstrated c-Fos activation of the lateral tegmental field, nucleus of the solitary tract (NTS), ventrolateral medulla, and the vestibular nucleus of the rostral medulla, during the elicitation of excitatory cardiac reflexes in bradykinin-treated cats [4]. In humans, numerous studies have used GVS as an analog to mechanical stimuli, measuring heart rate (HR), blood pressure (BP), muscle sympathetic nerve activity (MSNA), and skin sympathetic nerve activity (SSNA) for evaluation parameters. However, the complex interplay between movement, acceleration, and cardiovascular response regulated the vestibular apparatus, vestibular nuclei in the brainstem, chemoreceptive centers in the medulla oblongata, the reticular formation, and the hypothalamus, remains insufficiently understood, as do the neural mechanisms underlying disorders that involve vestibular activity such as motion sickness [5, 6], orthostatic hypotension [7], and vasovagal syncope [8].
Given its ability to stimulate the vestibular system without inducing fluid shifts that could interfere with baroreceptor function, GVS has emerged as a valuable tool for investigating autonomic responses under controlled experimental conditions. In this sense, this review provides an overview of cardiovascular and MSNA responses, associated to stimulation parameters, such as current intensity and frequency, exploring the possibility that its configuration may activate vestibular pathways associated to the autonomic activity under study.
GVS has been used to study cardiovascular sympathetic modulation in both animal models and human subjects. Research has primarily focused on understanding the effects of gravitational force or vestibular input, using electrical stimulation, on HR and BP. While findings have been varied, the approach and specific stimulation settings may influence the observed outcome in each case. A considerable number of studies have utilized the anesthetized rat models to determine the effects of GVS on cardiovascular responses [9, 10]. A key distinction between animal models and human studies is the electrode type. In animal models, subdermal electrodes are positioned either over the mastoid process in a bilateral bipolar configuration or implanted in the tympanic bulla. This alters the distance between the source of electrical current and the activated neural tissue, affected by the heterogeneity of the various interfaces’ impedance [11].
Regarding stimulation frequency, animal model protocols typically use lower
values than those applied in human studies. For instance, one study found that,
in rats, sinusoidal Galvanic Vestibular Stimulation (sGVS) at 0.008–0.4 Hz and
1–4 mA led to a decrease in HR and BP, with gradual recovery within
approximately six minutes [12]. In human subjects, a 1 Hz and
Another variable of relevance in the reviewed studies is electrode montage. The most commonly used configurations are unilateral, with both electrodes placed in the periauricular region, and bilateral, with the cathode and anode placed on opposite mastoids [17]. Again, studies in both animal models and human experiments have revealed that vestibular stimulation using monolateral/bilateral GVS induces changes in cardiac modulation. A study comparing monolateral and bilateral stimulation in anesthetized rats using 3 mA sGVS at frequencies of 0.025–0.5 Hz demonstrated that both electrode configurations induced HR and BP oscillations at twice the stimulation frequency, being the most effective range of 0.025–0.05 Hz [18].
Gravitational force has emerged as an interesting input variable in experimental
protocols that evaluate cardiovascular regulation using GVS, both in animal
models and in human subjects. In rodents, it was demonstrated that bilateral
biphasic square-wave GVS at 1 Hz and
To explore the potential interconnections between the vestibular system and
cardiovascular modulation, some studies using GVS should be mentioned. For
instance, Holstein et al. (2012) [20] examined the distribution of
c-Fos-positive neurons in rats following 30 minutes of sGVS at 2 mA and 0.025 Hz.
This paradigm resulted in significant c-Fos expression within the spinal, medial,
and superior vestibular nuclei, as well as the parasolitary nucleus, all of which
are known to receive substantial otolithic input. Notably, the highest density of
c-Fos-labeled neurons was observed in the parvocellular region of the medial
vestibular nucleus, a key area that projects efferent signals to the rostral
ventrolateral medulla (RVLM), which in turn modulates preganglionic sympathetic
neurons. Given that GVS activates both the otolith organs and the semicircular
canals, it is likely that cardiovascular modulation is provoked by fore and
lateral movement [21]. However, otolith input alone may not be the only factor
influencing changes in HR and BP. As seen with sGVS, pulsed infrared light beam
stimulation at 0.05 Hz targeting specifically the anterior and posterior
semicircular canals also resulted in a decrease in cardiovascular variables in
rats [22]. A complementary study using FluoroGold c-Fos protein and triple-label
immunofluorescence after five cycles of 2 mA sGVS at 0.025 Hz identified
glutamatergic and GABAergic populations in both the RVLM and caudal ventrolateral
medulla (CVLM). Vestibulo-sympathetic glutamate-immunofluorescent neurons were
predominantly found in the spinal and medial vestibular nuclei, projecting to the
RVLM and CVLM, whereas vestibulo-sympathetic
Other studies have focused on the influence of the medial vestibular nucleus (MVN) on cardiac activity through epinephrine release via the vestibulo-sympathetic reflex. While microinjection of a glutamate receptor agonist into the MVL increased BP, the administration of a glutamate receptor antagonist reduced epinephrine release in response to hypotension induced by sodium nitroprusside. These findings highlight the role of a vestibulo-spinal-adrenal axis modulating a sympathetic response, particularly in epinephrine-mediated cardiovascular modulation aimed at preventing syncope during hypotensive episodes. Consistently, an increased number of c-Fos-positive neurons were observed in the intermediolateral cell column of the spinal cord, at T4–T7 levels, following administration of glutamate receptor agonists, whereas this increment was not present when glutamate receptor antagonists were administered in combination with the hypotension-inducing agent [24]. Another study demonstrated that direct application of GABA (61 ng or 100 µg) or glutamate (2 µg) into the rat posterodorsal amygdala elicited opposing effects on heart rate variability parameters. While GABA enhanced cardiac parasympathetic activity, glutamate provoked a sympathetic behavior [25]. Still, the neural pathways connecting the amygdala with vestibular inputs remain under investigation, together with the possibility of eliciting sympathetic or parasympathetic outputs by modifying GVS parameters (Fig. 1, Ref. [26, 27, 28]).
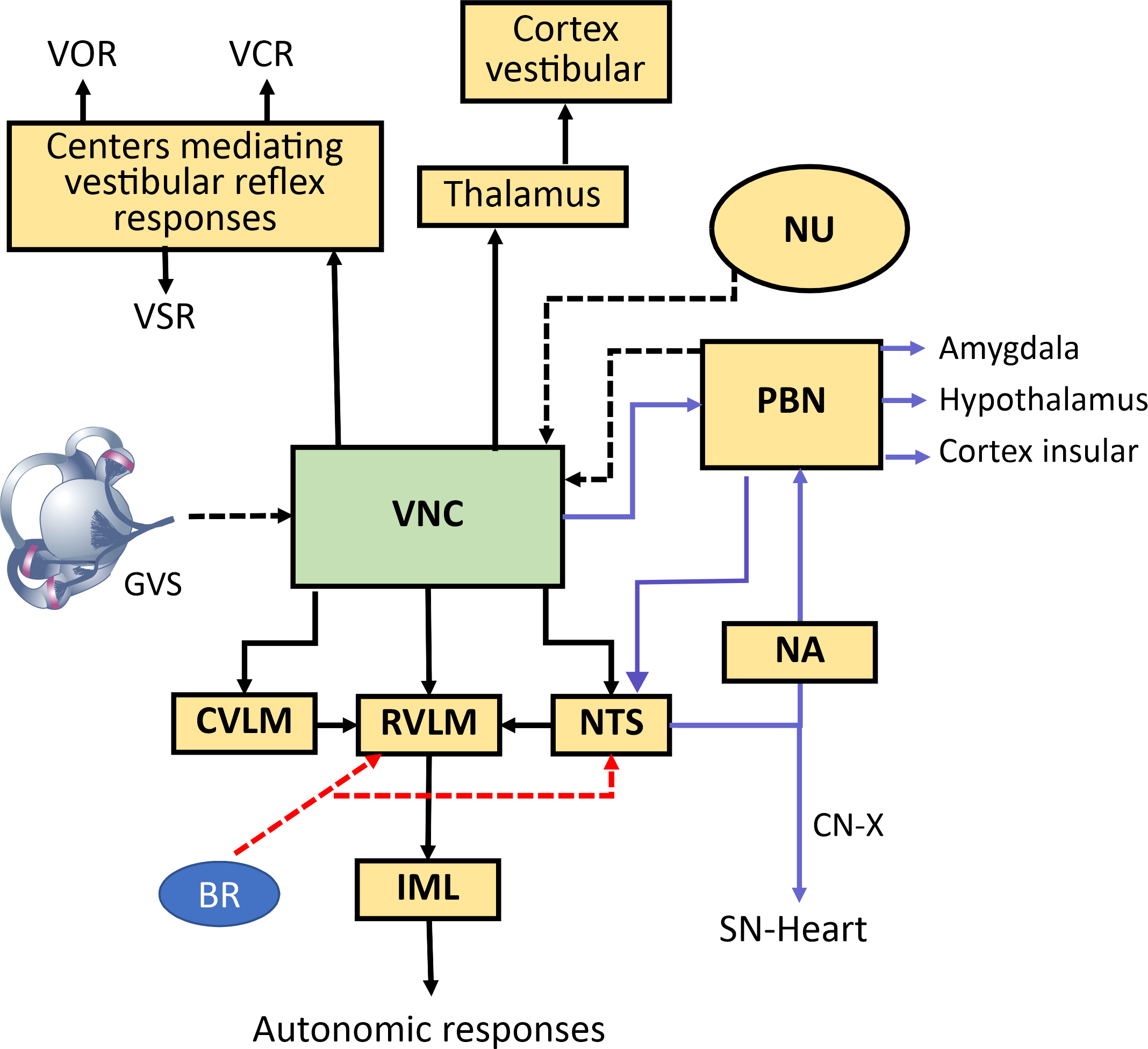 Fig. 1.
Fig. 1.
Neural pathways mediating vestibulo-autonomic responses. Schematic diagram illustrating the central pathways connecting vestibular inputs to autonomic nervous system outputs. Vestibular information from the vestibular nuclei complex (VNC) influences classic vestibulo-ocular (VOR), vestibulocollic reflexes (VCR), and vestibulospinal reflexes (VSR). Simultaneously, the VNC projects to autonomic control centers, primarily via two streams (dashed lines input to vestibular nuclei (VN), solid lines efferent paths from VNC) : (1) a primary reflex pathway to the nucleus tractus solitarius (NTS), rostral ventrolateral medulla (RVLM), and caudal ventrolateral medulla (CVLM) to integrate with baroreceptor (BR) signals and modulate blood pressure and heart rate via the intermediolateral nucleus (IML), influencing sympathetic nerve activity (skin sympathetic nerve activity (SSNA), muscle sympathetic nerve activity(MSNA)) [26, 27, 28]; and (2) a parallel pathway (purple lines) via the parabrachial nucleus (PBN) and NTS to the nucleus ambiguous (NA) for parasympathetic control of the sinus node, and to limbic (Amygdala) and homeostatic (Hypothalamus, Insular Cortex) regions. The red arrows show the input from the BRs to the RVLM and NTS. Vestibular signals also reach cortical vestibular areas via the thalamus, contributing to spatial awareness. The solid lines show the main output from vestibular nucleus and other interconnections related to vestibular autonomic responses. VNC, vestibular nuclei complex; VOR, vestibulo-ocular reflex; VCR, vestibulocolic reflex; VSR, vestibulospinal; VN, vestibular nuclei; NTS, nucleus tractus solitarius; RVLM, rostral ventrolateral medulla; CVLM, caudal ventrolateral medulla; BR, baroreceptor; IML, intermediolateral nucleus; SSNA, skin sympathetic nerve activity; MSNA, muscle sympathetic nerve activity; PBN, parabrachial nucleus; NA, nucleus ambiguous; NU, nodulus-uvula; CN-X Cranial nerve X; GVS, galvanic vestibular stim-ulation.
Vaso-vagal syncope is typically triggered by factors such as prolonged upright
posture, emotional stress, or impaired thermoregulation. These triggers can
induce a sudden drop in HR and BP, leading to nausea, dizziness, pallor, and
palpitations, and may ultimately result in transient loss of consciousness [29].
Considering that 49.3% of students susceptible to motion sickness were also
prone to vaso-vagal syncope [30], the prevalence of this condition appears to be
considerably high within certain populations. As previously mentioned,
low-frequency sGVS has been employed in animal models to induce HR and BP
reductions. Some research groups have proposed that these signs may resemble
those observed during vaso-vagal syncope. If the use of sGVS as a method to
induce syncope-like symptoms were validated, it could enable the exploration for
both therapeutic and pharmacological strategies to counteract such responses.
Several strategies have been employed to induce pathological vaso-vagal responses
using sGVS. For instance,
The cardiovascular response to sGVS is so consistent that it has enabled the development of a computational model, based on experimental data from rats exposed to sGVS (1–4 mA, 0.008–0.5 Hz) [35]. The model replicates the role of BP drops in triggering vasovagal responses. It incorporates physiological parameters such as low-frequency vasovagal oscillations, baroreflex sensitivity, response thresholds, system saturation, and target BP levels. The model concludes that a reduction in the desired BP is the primary driver of vasovagal syncope [36]. Moreover, the model can be employed to manipulate these variables in order to explore potential strategies for mitigating the symptoms that culminate in a vasovagal syncope.
To investigate the vestibular projections that influence sympathetic reflexes,
sGVS in combination with MSNA and SSNA recordings in humans has been used to
study the presence of neural pathways that link the vestibular system to
autonomic function. Similar to the modulation of BP and HR, research has focused
on the sGVS stimulation parameters that elicit the strongest entrainment of MSNA
and SSNA. Using this approach, it was found that the most effective
modulation-to-stimulus ratio occurred at low frequencies, specifically between
0.05 and 0.5 Hz, from a range of sGVS frequencies 0.05–5 Hz. This effectiveness
was quantified through cross-correlation histograms comparing SSNA with the GVS
waveform which revealed two distinct peaks. Some have proposed that the two
modulation peaks observed in response to sGVS correspond to the positive and
negative phases of the sinusoidal stimulus [37]. It has also been suggested that
these two peaks in MSNA modulation are independently associated with cardiac and
vestibular activity, respectively, and can be distinguished based on the phase
relationship between heart beat and sGVS at
Given that GVS is considered analogous to mechanical stimulation of the
vestibular system, it has served as an input that does not induce fluid shifts
and, consequently, does not interfere with the baroreceptor activity involved in
the orthostatic reflex [43]. This similarity has been corroborated by the MSNA
response to sGVS, which was found to be comparable to that elicited by sinusoidal
linear acceleration of
The differential responses of MSNA and SSNA to sGVS suggest that it is possible to selectively activate distinct sympathetic pathways by adjusting stimulation parameters. It has been proposed that MSNA is primarily associated with peripheral vasoconstriction mediated by otolith input, whereas SSNA is more closely linked to thermoregulatory processes and blood pressure modulation [47]. Although the origins of SSNA remains unclear, evidence indicates that its occurrence is not sensible to body temperature [48]. Both sympathetic outflows are sensitive to vestibular input, presumably via projections from the otolith organs to the RVLM for MSNA and to the medullary raphe for SSNA, respectively [40]. Representative studies that alter autonomic function, provoking a sympathetic or parasympathetic response by activating neural pathways using GVS, are shown in Table 1 (Ref. [10, 12, 14, 16, 18, 31, 32, 34, 37, 39, 42, 49, 50, 51, 52, 53]).
| Authors | Subject type | Stimulation parameters | Electrode configuration | Autonomic effect |
| Cohen et al. (2011) [12] | Animal model (Long-Evans rat) | 1–4 mA, 0.008–0.5 Hz, 15–30 min | Binaural | Instant decrease in HR and BP followed by increment |
| Cohen et al. (2013) [31] | Animal model (Long-Evans rat) | 3 mA, 0.025–1 Hz, 15 min | Binaural | Induced vasovagal response |
| Yakushin et al. (2014) [18] | Animal model (Long-Evans rat) | 3 mA, 0.025–0.5 Hz, 1–5 min | Binaural and monaural | Monaural and binaural stimulation induced vasovagal oscillations |
| Abe et al. (2009) [10] | Animal model (S-D rat) | Binaural | GVS inhibited BP and HR hypergravity response | |
| Tanaka et al. (2014) [14] | Human (healthy) | 2 mA, 0.2–10 Hz, 250 s | Binaural | R-R electrocardiographic interval increased |
| Matsugi et al. (2021) [16] | Human (healthy elder) | Binaural | Noisy GVS has no effect on BP and HR variability | |
| McBride et al. (2016) [32] | Animal model (Long-Evans rat) | 2–4 mA, 0.025–0.5 Hz, 3 min | Binaural | Hypotension, bradycardia, decreased cerebral blood flow |
| Quinn et al. (2015) [34] | Human (healthy young) | < |
Binaural | GVS provokes placebo-induced nausea |
| Singh et al. (2019) [37] | Human (healthy young) | 0.05–5.0 Hz, |
Binaural | Modulation is of MSNA is highest at 0.05 Hz and lowest at 5 Hz |
| James & Macefield (2010) [39] | Human (healthy) | Binaural | MSNA was weakest at sGVS cardiac frequency | |
| Klingberg et al. (2015) [42] | Human (healthy) | Binaural | SSNA was significantly larger in subjects experiencing nausea. MSNA did not increase | |
| Javaid et al. (2019) [49] | Human (healthy) | Binaural | SSNA, BP, HR, and respiratory rate changed distinctively in subjects experiencing nausea due to sGVS | |
| Allred et al. (2025) [50] | Human (healthy elder) | 0.01–4.0 mA, 0.275–0.325 Hz, 40 min | Binaural | Motion sickness was increased by Detrimental GVS and reduced by Beneficial GVS |
| Yamamoto et al. (2005) [51] | Human (PD) | 0.33 |
Binaural | Noisy GVS increased short time scales HR variability |
| Lotfi et al. (2021) [52] | Human (DM2) | Binaural | GVS reduced BP and Body Mass Index | |
| Viirre et al. (2025) [53] | Human (overweight and obesity) | 1 mA, 0.5 Hz, 60 min | Binaural | Visceral adipose tissue loss increased in the active group |
DM2, Diabetes mellitus type 2; PD, Parkinson’s disease; R-R, R wave–R wave interval; S-D, Sprague-Dawley; sGVS, sinusoidal Galvanic Vestibular Stimulation.
In this regard, the accumulated body of evidence on the sympathetic activation, for instance, linking abdominal obesity, elevated BP, and increased MSNA [54], must be reconsidered in light of incorporating GVS. The combined experimental approach of GVS and MSNA in obese populations, may uncover relevant strategies to enhance body fat loss, profiting from the potential of MSNA to identify individuals who may derive the greatest benefit from weight loss interventions under hypocaloric dietary conditions [55].
A wide range of neurological pathologies, primarily motor and cardiovascular,
have been evaluated during and after GVS, including vestibular and auditory
disorders, Parkinson’s disease, ischemic lesions, myelopathies, and mental
impairments [56]. GVS has also proven valuable in the study of symptoms related
to motion sickness [57]. For instance, sGVS at
Another example of a potential application focuses on the modern variant of
motion sickness induced by prolonged exposure to video games and virtual reality
(VR), cybersickness, GVS has gained attention as a method for both quantifying
and attempting to mitigate the symptoms of this technology-induced condition.
Typical symptoms of cybersickness such as nausea, headache, dizziness, or
salivation, were assessed with and without GVS (sinusoidal, 10 Hz,
Although GVS and the modulation of autonomic variables remains limited, with most studies focusing on HR, BP, MSNA and SSNA outcomes, its effect on autonomic variables is not negligible. A wide range of stimulation parameters vary considerably among studies: lower frequencies are typically employed to influence cardiovascular variables, while higher frequencies are more effective in modulating muscular and cutaneous sympathetic activity. However, most studies have analyzed either cardiovascular variables or those related to sympathetic and cutaneous muscular activity, but not both simultaneously, making it difficult to determine whether sinusoidal sGVS predominantly enhances sympathetic or parasympathetic activity. Nonetheless, continued exploration of GVS across a broader range of autonomic functions could lead to the development of targeted therapeutic protocols. There is growing evidence that GVS can modulate various autonomic functions and, in certain pathological conditions, may contribute to restoring physiological parameters toward values considered within a healthy range. Among the potential applications, the use of GVS has gained relevance, preventing nausea and motion sickness in sensory-conflict scenarios such as VR video games, normalizing blood glucose levels in individuals with type 2 diabetes mellitus, and promoting abdominal fat loss in individuals with obesity.
It is important to note that GVS is unlikely to produce responses analogous to natural vestibular stimulation. The low specificity of the electrical field may result in heterogeneous activation of neural pathways associated with the otolith organs and semicircular canals, in combination to other neural structures such as the auricular branch of the vagus nerve and cutaneous receptors. This could lead to autonomic responses with no natural equivalent. Moreover, GVS allows for the use of frequency oscillations that would be unachievable with mechanical stimuli, enabling selective activation of structures near the periauricular region without engaging other sensory systems, such as baroreceptors, typically stimulated by physical movement.
In summary, the stimulation parameters of GVS, such as frequency, current intensity, and signal waveform, may play a critical role in determining sympathetic or parasympathetic activation, producing varied effects across autonomic end-organs. Additionally, the potential for long-term therapeutic use of GVS to induce neuroplastic changes in specific autonomic functions remains a promising area for future research.
ANS, autonomic nervous system; BP, blood pressure; BR, Baroreceptor; CVLM, caudal ventrolateral medulla; DM2, type 2 diabetes mellitus; GABA,
AP and ES conceived and wrote the manuscript. Both authors contributed to editorial changes in the manuscript. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
During the preparation of this work the authors used Microsoft Copilot in order to check spelling and grammar. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
