- Academic Editors
†These authors contributed equally.
Depression frequently manifests as a secondary affective disorder in individuals who have experienced a stroke. In laboratory rats subjected to stroke, prolonged exposure to chronic stress effectively replicates the physiological impairment and adverse environmental challenges encountered by stroke patients. Nevertheless, the complex mechanisms underlying these phenomena remain unclear.
To elucidate the mechanisms underlying these impairments, we established a poststroke depression model by combining middle cerebral artery occlusion (MCAO) with 70 minutes of ischemia and chronic unpredictable mild stress (CUMS) exposure. Behavioral assessments, along with analyses of purinergic ligand-gated ion channel 7 receptor (P2X7R) and nucleotide-binding oligomerization domain, leucine-rich repeats, and pyrin domain-containing protein 3 (NLRP3)-associated inflammatory protein levels and peripheral blood inflammatory cytokine levels, were conducted at 1, 2 and 4 weeks post-MCAO, and the results were compared with those of rats subjected to stroke alone.
Depression-like behaviors were induced by CUMS exposure for three weeks. These changes were accompanied by significant increases in the protein levels of interleukin-1β (IL-1β), caspase-1, NLRP3 and Iba-1 in the hippocampus. Additionally, an increase in the fluorescence intensity of Iba-1, P2X7R, and NLRP3 in the Cornu Ammonis 1 (CA1) region was observed, along with dysregulation of plasma IL-6, IL-4, IL-10, and IL-1β levels. Importantly, the interaction of CUMS exposure and time affected behavioral scores and the levels of IL-1β. Notably, intraperitoneal administration of Brilliant blue G reversed depression-like behaviors and reduced the expression of NLRP3, caspase-1, IL-1β and IL-18 in the affected hippocampus.
These findings are consistent with the involvement of P2X7R/NLRP3 signaling in hippocampal impairment and inflammation/immune dysregulation in the context of depression-like behaviors induced by CUMS. In particular, behavioral scores may be affected by the interaction between CUMS exposure and time.
Poststroke depression (PSD) is an affective disorder that emerges in the aftermath of a stroke and is characterized primarily by diminished euphoria, reduced interest, low mood, feelings of worthlessness, and disrupted sleep patterns [1, 2]. The prevalence of PSD varies, ranging from 23.30% to 36.0% among individuals recently affected by a stroke [2, 3, 4]. In more than 80% of related study, the presence of affective disorders at the time of the stroke correlated with the worsening of symptoms during subsequent follow-up [5]. Furthermore, PSD not only adversely affects quality of life and the ability of affected individuals to engage in daily activities but also increases the likelihood of recurrent strokes, morbidity, and mortality [1, 4, 5].
To identify the optimal strategies for modeling chronic stress exposure following stroke, we previously conducted a systematic review of rodent depression models and related assessment methods [6] and reported pertinent experimental findings [7]. According to the literature [8, 9], 6–12 weeks of chronic unpredictable mild stress (CUMS) exposure is typically required for the induction of depression-like behavior in rats, whereas other reports have shown that only 4 weeks of CUMS exposure may be necessary [10, 11]; however, the outcomes are contingent upon the particular protocol utilized. Ischemic stroke can induce depression-like phenotypes in rats; however, these phenotypes are typically brief in duration and tend to resolve within one week [6, 12]. Therefore, a stroke-only model is not suitable for long-term studies of PSD-like phenotypes. Combining stroke induction with CUMS exposure is a frequently used method for generating PSD models and often reduces the duration of CUMS exposure required to induce PSD symptoms [6]. Researchers believe that this phenomenon may be related to the fact that short-term stress exposure can exacerbate damage after stroke [13]. However, whether the duration of chronic stress or CUMS exposure determines whether depression-like behavior develops after stroke remains unknown. In particular, to date, no studies have reported whether depressive-like behavior in rats subjected to stroke is affected by the interaction between stress and time.
Purinergic ligand-gated ion channel 7 receptor (P2X7R) is an adenosine
triphosphate (ATP)-dependent cationic channel protein expressed at varying levels
on the membranes of neurons, glia, and endothelial cells [14]. Pathological
stimuli such as hypoxia, inflammation, and hypoglycemia have been shown to
trigger ATP release into the intercellular space [14, 15]. The subsequent
excessive accumulation of ATP induces the opening of the membrane receptor P2X7,
leading to intracellular K+ efflux and the activation of the
nucleotide-binding oligomerization domain, leucine-rich repeats, and pyrin
domain-containing protein 3 (NLRP3) inflammasome during neurovascular injury [14, 16]. The NLRP3/caspase-1 pathway is a well-known molecular signaling cascade
associated with pyroptosis [17, 18]. In this pathway, interleukin-1
Previous studies have also highlighted the involvement of sensitized microglia in mediating central immune and inflammatory processes in response to both endogenous and exogenous stressors [28, 29]. Upon exposure to inflammatory factors, heat shock responses, or oxidative stress, resting microglia are activated, experience microenvironmental changes, and transform into phagocytic amoeboid cells [28, 29, 30]. Our previous work showed that CUMS promotes microglial sensitization in the ischemic corpus striatum and frontal lobe of rats subjected to stroke [7]. However, a comprehensive understanding of the dynamic changes in hippocampal microglia and the associated inflammatory responses is lacking. The aim of this study was to elucidate the potential mechanisms of PSD to address the limitations of prior studies.
Specific-pathogen-free male Sprague‒Dawley rats were obtained from Hunan Slake Jingda Co., Ltd. [Changsha, Hunan, China, certificate No. SYXK (Xiang) 2020-0017]. The body weights of the rats ranged from 250 to 280 g. Throughout the experimental period, all the animals were housed at our institution’s animal research center at a constant humidity and temperature with consistent lighting conditions, and they were acclimated to the environment for 1 week before the experiments. During this acclimatization period, the rats were provided unrestricted access to food and water.
A protein electrophoresis system (MINI-PROTEAN3; Bio-Rad, Hercules, CA, USA), Chemiluminescence detection system (1708370; Bio-Rad), Image Lab software (6.0.1; Bio-Rad), a high-speed refrigerated centrifuge (3.16R; Hengnuo, Changsha, Hunan, China), a fluorescence microscope (BA410T; Motic, Xiamen, Fujian, China), a filament (2636-A4; Xinon, Beijing, China), ImageJ software (V.2.0.0; NIH, Bethesda, MD, USA) and Microwave oven (M1-L202B; Midea, Foshan, Guangdong, China) were used in this study.
The main reagents used in this study were as follows. Radioimmunoprecipitation
assay (RIPA) buffer (KGB5203; KeyGen, Nanjing, Jiangsu, China), BBG (B0770; Sigma-Aldrich,
St. Louis, MO, USA) and 4% paraformaldehyde (G1101; Servicebio, Wuhan, Hubei, China)
was used. Primary antibodies against P2X7R (PA5-77665, rabbit anti-rat, Thermo
Fisher Scientific, Waltham, MA, USA), NLRP3 (ab263899, rabbit
anti-rat, Abcam, Cambridge, MA, USA), Iba-1 (MA5-27726, mouse anti-rat, Thermo Fisher
Scientific), caspase-1 (AF5418, rabbit anti-rat,
Affinity, Nanjing, Jiangsu, China), IL-18 (DF6252, rabbit anti-rat, Affinity), IL-1
In addition, there are some other auxiliary reagents and consumables, such as: 75% alcohol (67182260463; Annjet, Dezhou, Shandong, China), 0.9% physiological saline (XY4950; QiDuYaoYe, Zibo, Shangdong, China), sodium pentobarbital (BC1040; Protein Biotechnology, Beijing, China), EDTA Buffer (CM12684; Proteintech), nonfat dry milk (GC310001; Servicebio), phosphate buffered saline with tween (PBST, G2157-1L; Servicebio, Wuhan, China), goat serum (50012-8615; Everygreen, Hangzhou, Zhejiang, China), sucrose water (XY4797; JinJianYaoYe, Changsha, Hunan, China), Pure water (G4701-500ML; Servicebio), antifluorescence quencher (G1401-5ML; Servicebio), phosphate buffered saline (PBS, BL302A; Biosharp, Hefei, Anhui, China), Xylene (N1-5L; Taikangyiliao, Wuhan, Hubei, China), Paraffin (C0175A-500g; Beyotime, Shanghai, China), and eppendorf (EP) tube (EP-150-M; Servicebio).
Following a one-week adaptation period, the rats were subjected to right middle cerebral artery occlusion (MCAO). The model rats were subsequently divided into three groups, the stroke (n = 30), stroke + CUMS (n = 20) and stroke + CUMS + intervention (n = 10) groups, utilizing random numbers generated by SPSS 24.0 software (IBM Corp., Chicago, IL, USA). To determine the impacts of CUMS exposure for different durations, the rats in the stroke + CUMS group were further divided into two subgroups and examined at 2 weeks (n = 10) or 4 weeks (n = 10) after MCAO. The stroke-only group was divided into three subgroups and studied at 1 week (n = 10), 2 weeks (n = 10), or 4 weeks (n = 10) after MCAO. Notably, the data collected from rats that had not undergone CUMS at one week after MCAO were used as common baseline data for the stroke + CUMS and stroke-only groups. Notably, two subsets of rats in the stroke + CUMS + intervention group were administered either normal saline (NS; n = 5) or BBG (n = 5) at the beginning of the CUMS protocol (Fig. 1).
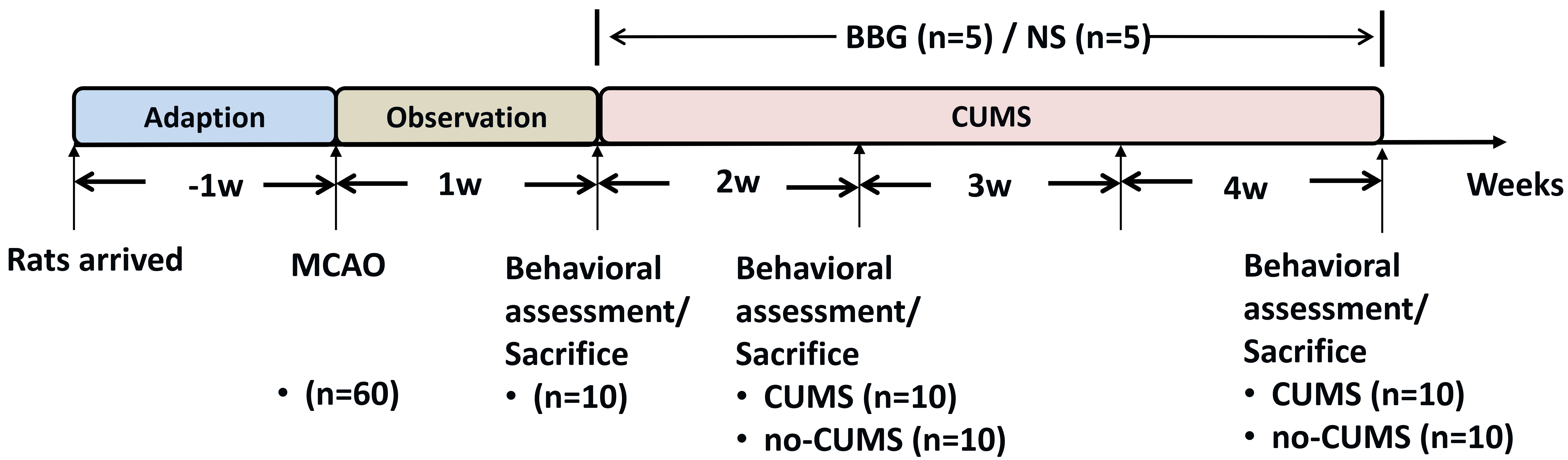 Fig. 1.
Fig. 1.
Diagram of the experimental procedures. BBG, brilliant blue G; NS, normal saline; CUMS, chronic unpredictable mild stress; MCAO, middle cerebral artery occlusion.
MCAO was induced following the protocol outlined in our prior publication [7], with minor adjustments. Briefly, the rats were anesthetized by an intraperitoneal injection of 1% sodium pentobarbital (6 mL/kg) and placed in the supine position on a surgical platform, with their limbs secured. The hair on the right side of the neck was trimmed, and the skin was disinfected with choroidal iodine. The soft tissue was subsequently gently dissected along a longitudinal incision made in the neck skin. The experimenter meticulously ligated the starting point of the right external carotid artery and the proximal end of the common carotid artery. MCAO was induced by inserting a rounded, dull filament through a small oblique incision in the common carotid artery into the internal carotid artery. The ischemia duration was 70 minutes, and cerebral blood flow and rectal temperature were continuously monitored throughout the procedure. The rectal temperature was carefully monitored and maintained at 37 °C during the surgery. The rats that died during or after MCAO were categorized as failed models. In instances of failure, an equivalent number of additional rats were included to maintain a consistent number in the experimental cohort.
PSD was induced by CUMS exposure in conjunction with isolation beginning one week after MCAO. The CUMS paradigm, a very popular and well-developed protocol, involves exposure to various stressors [7]: (1) circadian disruption for 36 hours, (2) water deprivation for 18 hours, (3) fasting for 20 hours, (4) exposure to a humid environment for 12 hours, (5) tilting of the cage at a 45° angle for 17 hours, (6) horizontal oscillation at a frequency of 90–100 cycles/min for 5 minutes, (7) noise stimulation at 80 dB for 10 minutes, (8) tail pinching for 1 minute, (9) forced swimming for 5 minutes in 4 °C water, and (10) space restriction for 2 hours. One of the first five “long” stimuli was randomly paired with one of the last five “short” stimuli daily. Stroke model rats were subjected to two of these stressors daily over a 3-week period.
A 7 mg/mL solution of BBG was prepared in sterile physiological saline and stored in the dark at 4 °C. This solution was injected into the peritoneal cavity of rats at a standard dose of 50 mg/kg. One week after MCAO, the rats in the stroke + CUMS + BBG group received daily injections of BBG solution beginning 30 minutes after the cessation of stress exposure. Physiological saline served as the control in the stroke + CUMS + NS group. The duration of drug injection was 3 weeks.
As previously described [31], neurological deficit scores were assessed 12 hours after MCAO using the Longa method. A score of 0 represented walking in a straight line, indicating no neurological deficit, whereas a score of 4 represented an inability to walk or impairment of consciousness, indicating very severe neurological impairment and possible death. Therefore, rats with scores ranging from 1 to 3 were deemed successful models of ischemic stroke, whereas those with scores of 0 or 4 were excluded from the analysis.
After ~15% of the MCAO model rats were excluded based on the experimental requirements, a total of sixty rats were included in the statistical analysis.
Prior to testing, all the model rats were allowed to become familiar with and adapt to the testing environment. After each experiment, the equipment was thoroughly cleaned with 75% alcohol to eliminate any residual odor from the preceding animal. Behavioral assessments were consistently conducted at predetermined times to mitigate the potential impact of circadian rhythm variations.
Rat body weight measurements were conducted between 8:00 AM and 12:00 PM.
An uncovered box measuring 100 cm length
The novelty-suppressed feeding test (NSFT) was conducted in a different
environment than the OFT. A new box with dimensions of 65 cm length
On the first day, two bottles of 1% sucrose water (200 mL each) were provided.
On the second day, one bottle containing pure water and another containing 1%
sucrose water were provided. Both water and food were withheld for 12 hours prior
to the assessment. During the evaluation, 200 mL of pure water and 200 mL of 1%
sucrose water were administered. The quantity of each liquid consumed by the rats
over a 12-hour period was measured and analyzed. The sucrose preference index
(SPI) was calculated using the following formula: SPI = [(amount of 1% sucrose
water consumed)/(amount of 1% sucrose water consumed + amount of pure water
consumed)]
All behavioral assessments were performed by two evaluators. First, rats with a suspected neurological deficit score of 0 or 1 after MCAO were excluded. Second, the behavior of the rats in the OFT, NSFT, and sucrose preference test (SPT) (in that order) was scored to minimize the influence of the previous tests on subsequent tests. These three tests took three days to complete. The average of the two scores for the OFT was analyzed. Body weight measurements, the NSFT, and the SPT were performed by one person, with another person supervising the procedure. All scorers were blinded to the experimental groups of the rats.
The rats (n = 5 per group) were anesthetized with 1% sodium pentobarbital at 1 week, 2 weeks, and 4 weeks after MCAO. Pentobarbital was administered by intraperitoneal injection into the lower left abdominal quadrant at a dose of 150 mg/kg. The animals were observed for 3–5 minutes. Death was confirmed by loss of consciousness, cessation of respiration and heartbeat, and absence of the pupillary light reflex. The cardiac apex was accessed via a venous indwelling needle, which was then inserted into the left ventricle to the ascending aorta. The right auricle was subsequently cut, and 200 mL of normal saline was rapidly infused. Perfusion with 200 mL of 4% paraformaldehyde was terminated upon observing the stiffness of the tail or extremities. The brains were extracted and immersed in 4% paraformaldehyde overnight in a refrigerator set at 4 °C. Finally, the brain tissue was dehydrated and embedded in paraffin. The embedded tissue was subsequently systematically cut into 5 µm thick slices.
The tissue slices were dewaxed in xylene and subsequently hydrated in graded ethanol solutions. Antigen retrieval was performed by heating the slices in EDTA buffer in a microwave. After deparaffinization with xylene and rehydration in graded ethanol solutions, the slices were subjected to a second round of antigen retrieval with EDTA buffer in a microwave. The slices were subsequently covered completely with 10% goat serum at room temperature to block nonspecific binding. After one hour, the sections were incubated overnight at 4 °C with primary antibodies against P2X7R (1:50), NLRP3 (1:50), or Iba-1 (1:50). After primary antibody incubation, the slices were incubated with 50 µL of secondary antibody (1:100) in an opaque box for 1 hour at 37 °C. Excess antibody was added, and the sections were thoroughly washed with PBS. Finally, the cell nuclei were incubated with DAPI working solution at 37 °C for 15 minutes. An antifluorescence quencher was applied, and the slices were mounted. Staining in the CA1 region was visualized under a fluorescence microscope and imaged in a dark environment. ImageJ software was used to quantify the fluorescence intensity per unit area in the CA1 region. The fluorescence intensities of P2X7R, NLRP3, and Iba-1 were calculated using DAPI as a reference and normalized.
The remaining rats were euthanized with 1% sodium pentobarbital at 1 week, 2
weeks, and 4 weeks after MCAO. Affected hippocampal tissues preserved at –80
°C were fully lysed in RIPA lysis buffer, followed by ultrasonication on
ice and centrifugation (12,000 r/min) for 15 minutes at 4 °C. The
carefully collected supernatant was mixed with 5
Following anesthesia (as described in Section 2.8), blood samples (approximately
1.5 mL) were drawn from the right retroorbital venous plexus of the rats. Samples
(n = 5 per group) were collected at 2 and 4 weeks after MCAO and promptly
subjected to centrifugation at 4 °C. The resulting plasma was aliquoted
into EP tubes and stored at –80 °C. For analysis, the samples were
diluted tenfold and processed in accordance with the manufacturer’s instructions.
The plasma levels of IL-6, IL-4, IL-10, and IL-1
The data were analyzed using SPSS 24.0 software. In this
study, all the data were tested with the Shapiro‒Wilk test and met the criteria
for a normal distribution. The data collected at 1 week after MCAO were used as
the baseline data before chronic stress induction. The data collected at 2 weeks
and 4 weeks after MCAO were standardized by calculating the values for 2 w/1 w
(%) and 4 w/1 w (%) after MCAO, respectively. Differences in behavioral scores,
the relative gray values of the protein bands and immunofluorescence intensity
between the stroke group and the stroke + CUMS group were analyzed using two-way
ANOVA. When an interaction between CUMS exposure and time was observed, simple
effects analysis was performed, with comparisons between groups or between time
points adjusted via Bonferroni correction. When no interaction between CUMS
exposure and time was observed, main effects analysis with Bonferroni’s post hoc
correction was used. Plasma cytokine levels in the stroke group and the stroke +
CUMS group were compared via an independent samples t test. Similarly,
differences in behavioral scores and the relative gray values of protein bands in
the stroke + CUMS + NS group and the stroke + CUMS + BBG group were analyzed via
an independent samples t test. Statistical graphs were produced via
GraphPad Prism 9.5.0 (Dotmatics, San Diego, CA, USA). A p value
Body weight is a sensitive indicator of long-term mood disorders; however,
conflicting results have been observed in clinical practice. In this study, no
interaction effect between CUMS and time on body weight [F(1, 36) = 0.126,
p = 0.724] was detected. Main effect analysis revealed that CUMS had a
significant effect on body weight [F(1, 36) = 10.889, p = 0.002].
Specifically, the body weight of the stroke + CUMS group was lower than that of
the stroke group (t = 3.344, p = 0.002). Main effect analysis
also revealed that time had a significant effect on body weight [F(1, 36) =
14.757, p
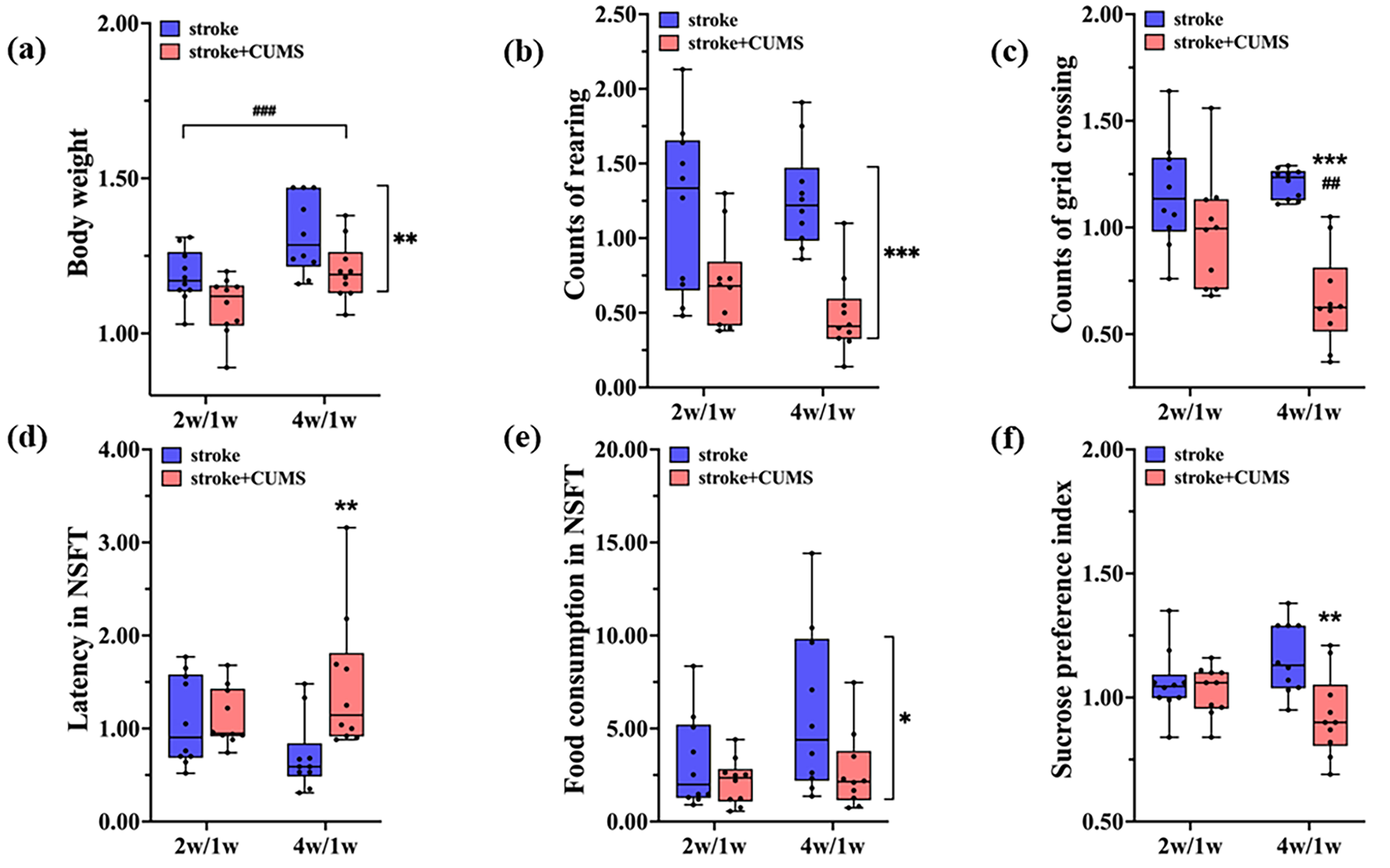 Fig. 2.
Fig. 2.
Effects of CUMS and time on the body weight and behavior of rats
subjected to stroke. Effects of CUMS and time on body weight (a) and performance
in the OFT (b,c), NSFT (d,e) and SPT (f) in rats with stroke. The data are
presented as the means
In this study, no interaction effect was detected between CUMS and time on the
number of rearings [F(1, 36) = 1.256, p = 0.270]. Main effect
analysis revealed that CUMS had a significant effect on the number of rearings
[F(1, 36) = 27.282, p
There was an interaction effect between CUMS and time on the number of grid
crossings [F(1, 36) = 6.857, p = 0.013]. Simple effect analysis
revealed that in the stroke + CUMS group [F(1, 36) = 10.494, p =
0.003], the number of grid crossings at 4 weeks after MCAO was significantly
lower than that at 2 weeks after MCAO (t = 3.247, p = 0.003).
However, in the stroke group [F(1, 36) = 0.215, p = 0.646], there
was no obvious difference. Simple effect analysis revealed that at 4 weeks after
MCAO [F(1, 36) = 31.356, p
Performance in the OFT can reflect the locomotor activity of rats. These findings indicate that stroke model rats exhibit a gradual decrease in their ability to engage in physical activity as the duration of stress increases.
Increased latency to feeding and little change in food intake in the NSFT are two important indicators of depressive-like behavior. There was an interaction effect between CUMS and time on the latency in the NSFT [F(1, 36) = 5.150, p = 0.029]. Simple effect analysis revealed no significant differences in latency between 2 and 4 weeks after MCAO in the stroke group [F(1, 36) = 2.760, p = 0.105] or the stroke + CUMS group [F(1, 36) = 2.396, p = 0.130]. Simple effect analysis revealed that at 4 weeks after MCAO [F(1, 36) = 11.326, p = 0.002], the latency of the stroke + CUMS group was longer than that of the stroke group (t = 3.367, p = 0.002), whereas at 2 weeks after MCAO [F(1, 36) = 0.024, p = 0.877], there was no significant difference in latency between the two groups.
There was no interaction effect between CUMS and time on the amount of food consumed in the NSFT [F(1, 36) = 1.470, p = 0.233]. Main effect analysis revealed that CUMS had a significant effect on the amount of food consumed [F(1, 36) = 5.596, p = 0.024]. The amount of food consumed in the stroke + CUMS group was lower than that in the stroke group (t = 2.366, p = 0.024). Main effect analysis also revealed that time had no significant effect on the amount of food consumed [F(1, 36) = 3.280, p = 0.078]. Therefore, there was no significant difference in food intake between 2 and 4 weeks after MCAO (Fig. 2d,e; Supplementary Table 1).
The sensation of sweet taste is associated with pleasure, and the SPT is used to determine whether a depressed animal is experiencing anhedonia. There was an interaction effect between CUMS and time on the SPI [F(1, 36) = 5.319, p = 0.027]. Simple effect analysis revealed no significant difference in the SPI between 2 and 4 weeks after MCAO in the stroke group [F(1, 36) = 2.660, p = 0.112] or the stroke + CUMS group [F(1, 36) = 2.659, p = 0.112]. Simple effect analysis revealed that at 4 weeks after MCAO [F(1, 36) = 13.802, p = 0.001], the SPI of the stroke + CUMS group was significantly lower than that of the stroke group (t = 3.726, p = 0.001). However, this effect was not obvious at 2 weeks after MCAO [F(1, 36) = 0.206, p = 0.653] (Fig. 2f, Supplementary Table 1). These findings suggest the successful establishment of a chronic stress-induced depression model.
There was no interaction effect between CUMS and time on the expression of Iba-1
[F(1, 16) = 3.716, p = 0.072], caspase-1 [F(1, 16) = 0.007,
p = 0.934], IL-18 [F(1, 16) = 1.915, p = 0.185], P2X7R
[F(1, 16) = 0.402, p = 0.535] or NLRP3 [F(1, 16) = 0.458,
p = 0.508]. Main effect analysis revealed that CUMS had a significant
effect on Iba-1 [F(1, 16) = 37.824, p
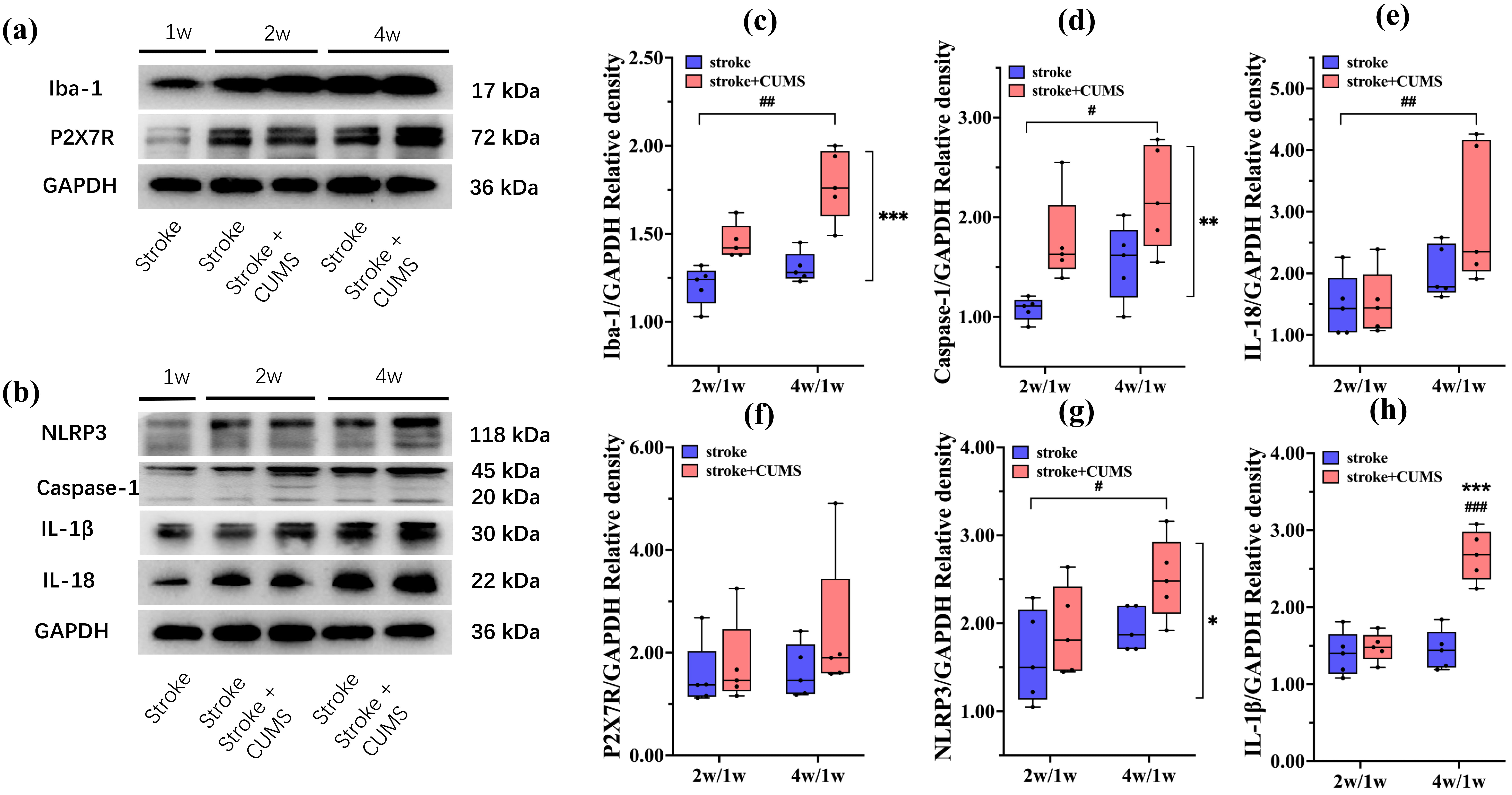 Fig. 3.
Fig. 3.
Relative expression of Iba-1 and P2X7R/NLRP3 signaling
pathway-related proteins in the affected hippocampus in the stroke and stroke +
CUMS groups. Representative blots showing the levels of Iba-1 and proteins
related to the P2X7R/NLRP3 signaling pathway (a,b). Graphs showing the relative
protein expression levels of Iba-1 (c), caspase-1 (d), IL-18 (e), P2X7R (f),
NLRP3 (g), and IL-1
Moreover, main effect analysis revealed that time significantly affected the expression of Iba-1 [F(1, 16) = 13.127, p = 0.002], caspase-1 [F(1, 16) = 6.442, p = 0.022], and IL-18 [F(1, 16) = 9.914, p = 0.006] and NLRP3 [F(1, 16) = 5.194, p = 0.037]. Iba-1 (t = 3.610, p = 0.002), caspase-1 (t = 2.539, p = 0.022), IL-18 (t = 3.150, p = 0.006) and NLRP3 (t = 2.279, p = 0.037) expression was increased at 4 weeks after MCAO compared with 2 weeks after MCAO. However, the same results were not obtained for P2X7R [F(1, 16) = 0.754, p = 0.398] (Fig. 3a–g; Supplementary Table 2; The original western blot images for Fig. 3a and Fig. 3b are provided in the Supplementary Figures).
There was an interaction effect between CUMS and time on the expression of
IL-1
Ischemia can induce an inflammatory response in the hippocampus, and chronic stress can exacerbate structural damage, which is involved in mood disorders [33, 34]. We observed that the levels of some inflammatory proteins increased with prolonged stress, which may have been at least partially related to increased Iba-1 expression or microglial activation. These results suggest that the levels of some inflammatory markers gradually increase over time, as expected, at least in part.
The CA1 region, which is closely associated with the depressive phenotype, has structural and functional importance [35]. Our focus was on the distribution and fluorescence signals of three core proteins within this region. Sections were specifically stained for P2X7R (red fluorescence), NLRP3 (red fluorescence), Iba-1 (green fluorescence) and DAPI (blue fluorescence).
We found no interaction effect between CUMS and time on the fluorescence intensity of Iba-1 [F(1, 16) = 0.789, p = 0.388], NLRP3 [F(1, 16) = 1.440; p = 0.248], or P2X7R [F(1, 16) = 1.967, p = 0.180]. These findings are consistent with the results observed for the whole hippocampus. Main effect analysis revealed that CUMS had a significant effect on the fluorescence intensity of Iba-1 [F(1, 16) = 12.490, p = 0.003], NLRP3 [F(1, 16) = 8.590, p = 0.010] and P2X7R [F(1, 16) = 6.113, p = 0.025]. Specifically, the fluorescence intensities of Iba-1 (t = 3.532, p = 0.003), NLRP3 (t = 2.930, p = 0.010) and P2X7R (t = 2.471, p = 0.025) were significantly higher in the stroke + CUMS group than in the stroke group (Fig. 4; Supplementary Table 3).
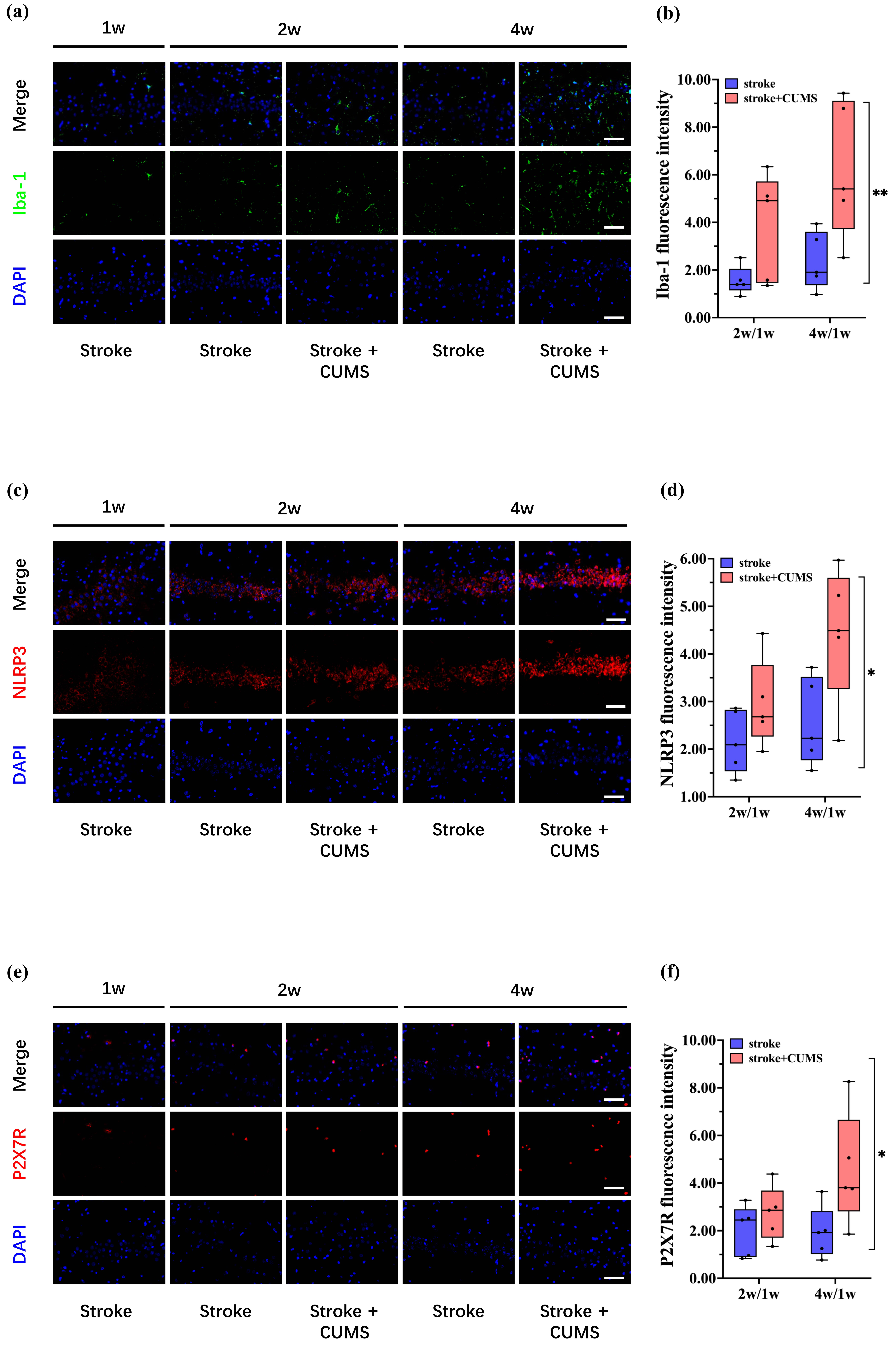 Fig. 4.
Fig. 4.
Comparison of P2X7R, NLRP3 and Iba-1 staining in the affected
CA1 region between the stroke and stroke + CUMS groups (400
However, main effect analysis also revealed that time did not significantly affect the fluorescence intensity of Iba-1 [F(1, 16) = 3.332, p = 0.087], NLRP3 [F(1, 16) = 4.312, p = 0.054] or P2X7R [F(1, 16) = 1.616, p = 0.222] (Fig. 4; Supplementary Table 3).
Systemic inflammatory responses occur in somatic lesions caused by chronic
stress, indicating that peripheral inflammatory markers are potentially valuable
for diagnosing or predicting mood disorders. Since the venous blood of rats could
not be obtained or did not meet the requirements for effective analysis at 1 week
after MCAO, we compared plasma levels of inflammatory factors between only 2 and
4 weeks after MCAO. Compared with the stroke group, the plasma levels of IL-4
(t = 2.519, p = 0.036) in the stroke + CUMS group was lower 4
weeks after MCAO, but the IL-10 level (t = 2.688, p = 0.028)
was increased. However, the plasma levels of IL-6 in the stroke + CUMS group were
lower than those in the stroke group at both 2 (t = 7.176, p
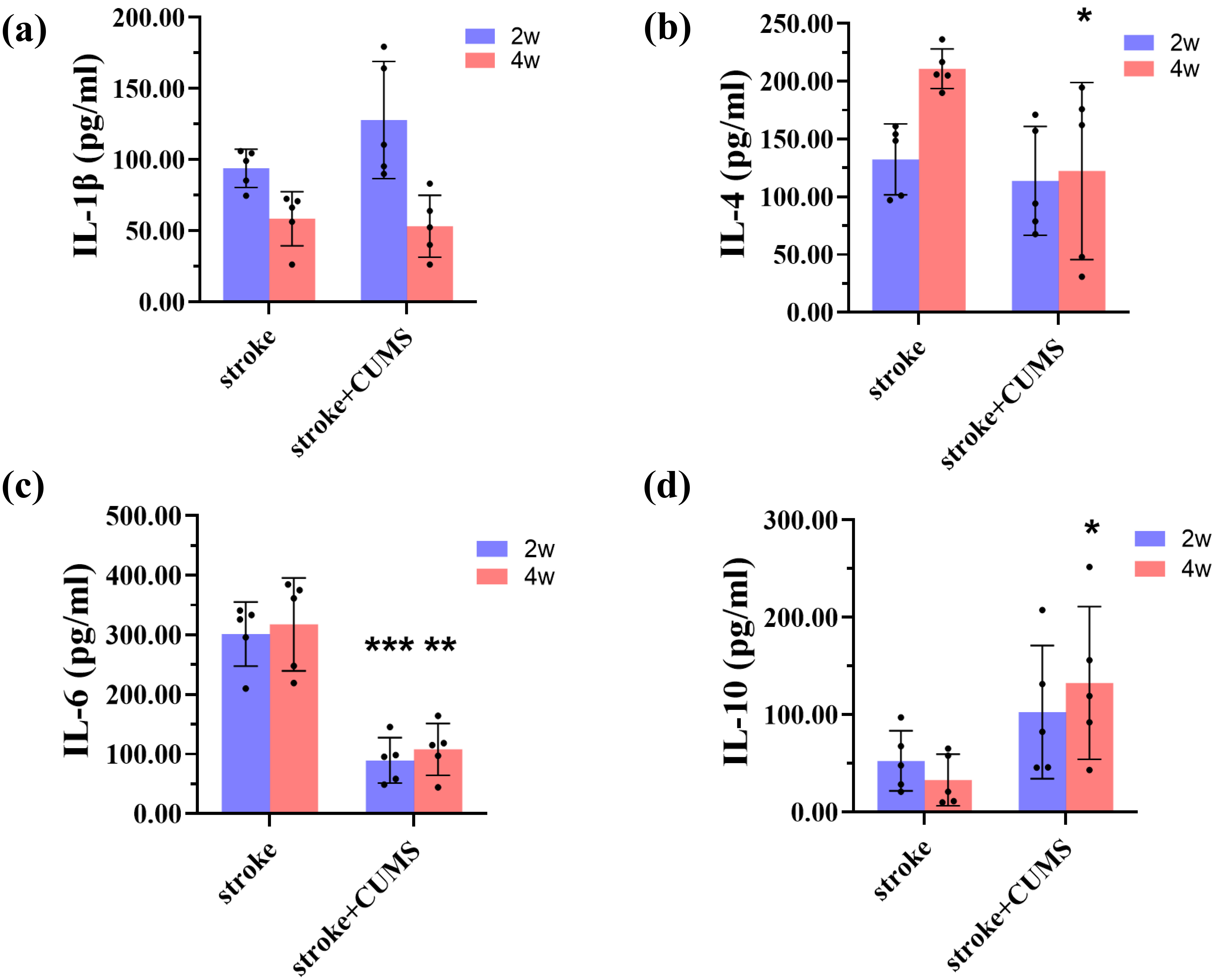 Fig. 5.
Fig. 5.
Comparison of peripheral cytokine levels between the stroke and
stroke + CUMS groups. (a–d) ELISA results for IL-1
To further investigate the role of the P2X7R/NLRP3 signaling pathway in PSD, we
inhibited P2X7R on the cell membrane using BBG, and alterations in downstream
inflammatory protein expression were assessed. At 4 weeks after MCAO, compared
with the stroke + CUMS + NS group, the stroke + CUMS + BBG group presented
increased numbers of rearings (t = 4.706, p = 0.006) and grid
crossings (t = 3.243, p = 0.012) in the OFT, an elevated SPI
(t = 2.447, p = 0.040), and a shortened latency in the NSFT
(t = 3.772, p = 0.005), with no change in food intake in the
NSFT (t = 0.175, p = 0.866) (Fig. 6a–d). In addition to
reversing the changes in behavioral scores, BBG decreased the expression of
IL-1
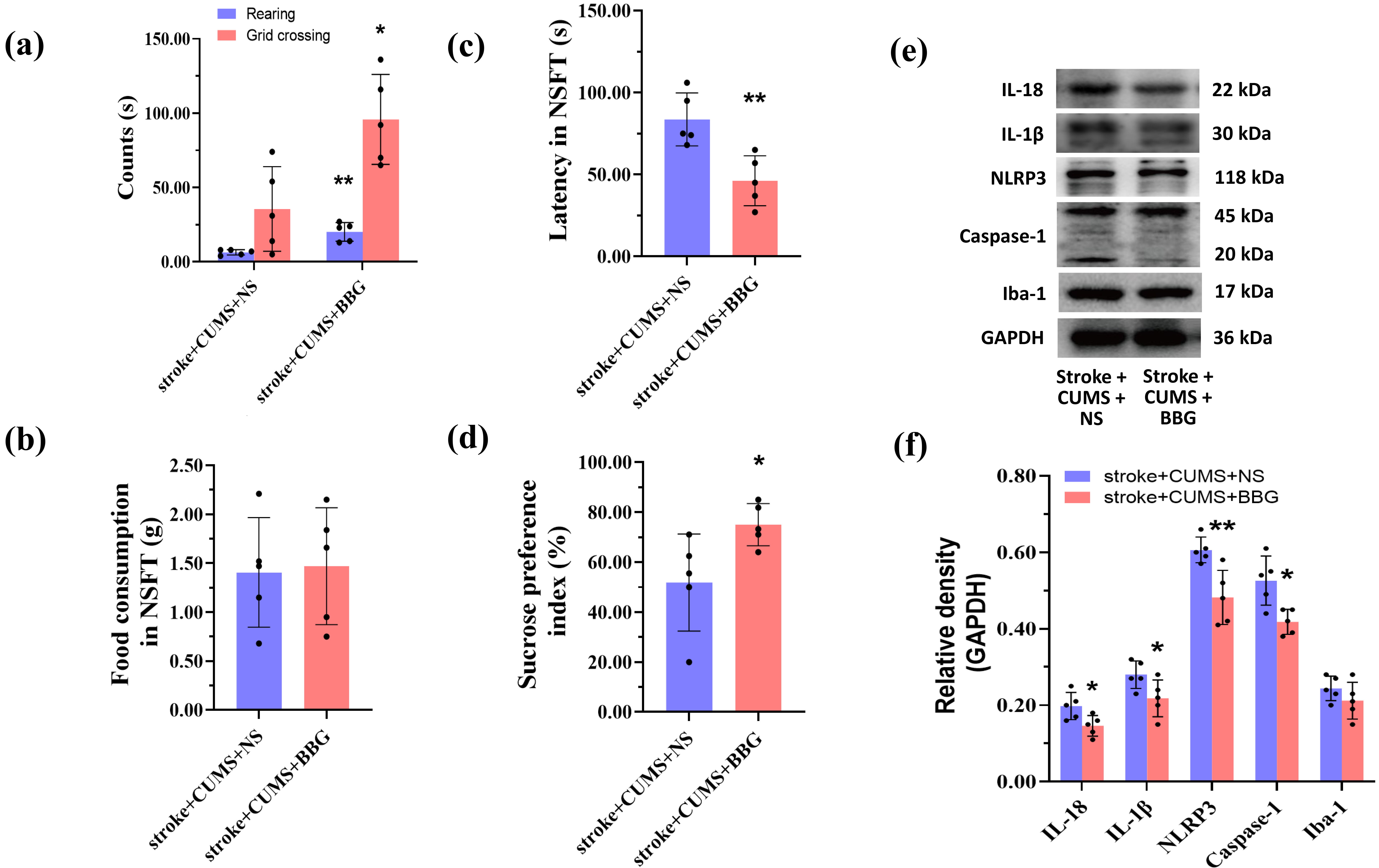 Fig. 6.
Fig. 6.
BBG alleviates depression-like behaviors and mitigates
hippocampal neuroinflammation in rats with stroke. Statistical analysis of the
behavioral scores of rats subjected to stroke in the OFT (a), NSFT (b,c), and SPT
(d). Western blot bands for inflammatory markers and statistical analysis of the
relative gray values of the bands are displayed in (e) and (f), respectively. The
data are presented as the means
The novel insights from our study are as follows. (1) The hippocampal P2X7R/NLRP3-mediated inflammatory pathway is involved in chronic stress-induced depressive behavior in rats subjected to stroke. (2) A significant CUMS-by-time interaction effect on depression-like behavior was observed in rats subjected to stroke.
The CUMS paradigm is a protocol that is widely used in basic research to induce depression-like behavior in rats subjected to stroke [36]. Our investigation confirmed that 3 weeks of exposure to chronic stress induced depression-like behaviors in rats subjected to stroke. These behaviors included a reduced SPI, a prolonged latency and diminished food intake in the NSFT, and decreased locomotor activity in the OFT and were accompanied by a decline in body weight. These outcomes are consistent with findings from previous studies [7, 37]. In this study, the OFT was conducted under dim lighting [38], and the reduction in locomotor activity may have been related to the systemic inflammatory response induced by CUMS [39]. In contrast to previous studies, we assessed behavioral scores at three time points. Specifically, the score at one week after MCAO served as the baseline level before CUMS exposure. We report for the first time that behavioral scores and protein expression at two and four weeks after MCAO are affected by the interaction between CUMS and time. This study fills a gap in this field.
We analyzed the expression of P2X7R and various classic pyroptosis
pathway-related proteins, including NLRP3, caspase-1, IL-1
In addition to investigating the P2X7R/NLRP3 signaling pathway, we investigated hippocampal microglia and detected an increase in Iba-1 expression after 3 weeks of CUMS. Pathologically, a substantial increase in the number of microglia was observed in the CA1 region, and this alteration was predominantly evident in the group subjected to stroke and 3 weeks of CUMS exposure. As crucial immunocytes in the central nervous system, microglia play a critical role in the phagocytosis of cellular fragments and harmful metabolites, thereby mitigating the adverse effects of intrinsic and extrinsic factors on neurons [40]. However, overactivation of microglia leads to the continuous production of cytotoxic products, such as reactive oxygen species and proinflammatory cytokines, exacerbating local damage within the microenvironment. This study highlights the pivotal involvement of hippocampal microglia in depressive-like behaviors induced by CUMS in rats subjected to stroke, which is consistent with findings from previous study [41].
In contrast to previous studies, in this study, behavioral scores and the
expression of related proteins were investigated at different times before and
after CUMS exposure. CUMS and time had interaction effects on performance in the
OFT (number of grid crossings), NSFT (latency) and SPT (SPI). Thus, we conclude
that fundamental indices of depressive behavior are not impacted solely by CUMS
but are intricately linked to the duration of stress exposure. By reviewing the
literature, we found that 4 to 12 weeks of chronic stress exposure is required to
induce depressive-like behavior in stroke-free rats [8, 9, 10, 11]. Notably, stroke model
rats exhibited depressive-like behavior after CUMS exposure for a significantly
shorter timeframe of 3 weeks. These findings may be related to the important role
that different stress protocols play in this process. However, the results of the
present study preliminarily verified the interaction between CUMS and the
duration of stress exposure, resulting in the development of depressive-like
behavior in stroke model rats over a relatively short period. Additionally, at
the mechanistic level, our study reveals that CUMS and time have an interaction
effect only on hippocampal IL-1
Inflammatory responses and impaired immune function constitute the
pathophysiological underpinnings of stress-related injury. Many studies have
confirmed the activation of the host immune system by both acute and chronic
stressors [45, 46, 47]. Stress-related injuries primarily manifest as alterations in
cellular and/or humoral immunity [47]. Adaptive changes in immune function,
driven by the secretion of substantial amounts of inflammatory cytokines, are
characteristic responses to stress [45]. In our investigation, we determined the
plasma levels of four cytokines known for their anti- or proinflammatory effects:
IL-6, IL-4, IL-10, and IL-1
Moreover, this study has the following limitations: (1) The duration of chronic stress was limited to either 1 or 3 weeks, and data from other time points were unavailable. (2) Only rats that were not exposed to CUMS served as controls. (3) Phenotypic alterations in microglia were not investigated. The potential infiltration of brain tissue by circulating macrophages, which also express Iba-1, could not be definitively ruled out. (4) The slightly insufficient sample size in some groups may have affected the statistical power. (5) Underlying mechanisms, such as hippocampal neuronal apoptosis, synaptic plasticity, blood-brain barrier disruption, and peripheral immune cell infiltration, were not validated. (6) Female rats were not included, as significant sex differences in depression susceptibility and stress tolerance have been observed. To gain deeper insight into the biological mechanisms underlying the development of chronic stress-induced PSD-like behavior, more reasonable classification methods or gene knockout could be used in future studies to observe phenotypic changes in microglia in the hippocampus or frontal cortex.
These findings are consistent with the involvement of P2X7R/NLRP3 signaling in the affected hippocampus and the dysregulation of inflammatory/immune responses in the manifestation of depression-like behaviors induced by chronic stress in rats subjected to stroke. In particular, a significant interaction effect of CUMS and time on depressive-like behavior was observed in stroke model rats.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
XT, SW and WT conceived and designed the study. WT, SW, YZ and CY performed the study. YZ, YY, and JH performed data analysis, graphics, and interpretation. XT, YZ, YY and JH wrote the paper. XT, YZ, YY, JH and SW revised the paper for intellectual content. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This research received approval from the Animal Ethics Committee of the People’s Hospital of Hunan Province, Hunan Normal University (Approval No. 202418). All procedures were conducted in accordance with the Regulations for the Administration of Laboratory Animals approved by the State Council of the People’s Republic of China.
Not applicable.
This work was supported by the Hunan Provincial Natural Science Foundation (2023JJ30349) and Scientific Research Project of Hunan Education Department (23A0078) to XT, and Scientific Research Project of Hunan Health Committee (202203102866) to JH.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/JIN40005.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
