- Academic Editor
†These authors contributed equally.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with strong genetic and environmental components. Despite progress made over the past decades, no effective therapies targeting the core symptoms of ASD are currently available. More research is required to explore the underlying mechanisms of ASD and discover potential therapeutic targets. Chromodomain helicase DNA-binding protein 8 (CHD8) is one of the most significant high-confidence ASD risk genes identified to date. However, the precise roles and mechanisms of CHD8 in neurodevelopment and behaviors remain incompletely understood. Zebrafish represent an emerging model organism for ASD research. While several zebrafish models with Chd8 disruption have been established, behavioral consequences have not been thoroughly characterized.
Leveraging the high survival rate of homozygous Chd8 mutant males, we comprehensively assessed their behaviors.
The mutants exhibited social deficits across multiple assays, including shoaling, social interaction and three-chamber social preference test. Additionally, anxiety-like behavior, locomotor coordination deficits, and macrocephaly were observed. These phenotypes closely resemble the symptoms in patients carrying disruptive CHD8 mutations.
Our findings establish this Chd8 mutant zebrafish line as a robust model for investigating ASD pathological mechanisms and screening for potential therapies.
Autism spectrum disorder (ASD) is one of the most prevalent heritable neurodevelopmental disorders, currently affecting approximately one in 31 children in the United States [1]. Key diagnostic features include persistent deficits in social interaction and communication, alongside restricted interests and repetitive behaviors as well as other deficits, such as sensorymotor defects [2, 3, 4]. Diagnosis of ASD still relies primarily on behavioral observations and caregiver reports, as no definitive biological markers have been identified [5]. Despite being described over eighty years ago, no treatments effectively target ASD’s core symptoms [6]. ASD is a multifactorial disorder with strong genetic and environmental associations [7]. To gain insights into its biological mechanisms, various model systems have been established, including induced pluripotent stem cells, brain organoids, nonhuman primates, and rodents [8, 9]. However, each model system has inherent limitations.
Zebrafish (Danio rerio) is emerging as a valuable model organism in translational neuroscience and behavioral research, offering significant promise for understanding complex neuropsychiatric disorders like ASD and discovering novel therapeutics [10, 11]. The popularity of zebrafish stems from its relatively low cost, rapid development, ease of genetic manipulation and potential for high-throughput screening. Social deficits are a core feature of ASD. Zebrafish are naturally social animals and perform well in a variety of social behaviors including social dominance, social affiliation and social cognition, making them relevant for studying social dysfunction [12]. Crucially, the neural circuits underlying social behavior are evolutionarily conserved among vertebrates [13, 14]. Numerous zebrafish ASD models have been established based on known genetic risk factors and environmental exposures [15, 16, 17], yet the field is still nascent in unraveling the precise pathophysiological mechanisms. Diverse models remain essential to reflect ASD heterogeneity and uncover core etiological pathways.
Over 100 high-confidence ASD risk genes have been identified, many involved in chromatin modification and synapse function [18]. Among these, Chromodomain helicase DNA-binding factor 8 (CHD8), an ATP-dependent chromatin remodeler (CHD family member CHD1~CHD9), has emerged as one of the most significant ASD risk genes in a series of large-scale exome sequencing studies [19, 20, 21]. Disruptions in CHD8 appear to define a distinct ASD subtype [20, 22]. Individuals carrying pathogenic CHD8 variants exhibit a broad range of clinical spectrum [23], likely reflecting CHD8’s multifaceted roles in neurodevelopment. Animal studies confirm these diverse functions; CHD8-deficient mice and nonhuman primates display a range of phenotypes [22, 24, 25]. However, reported behavioral outcomes, particularly concerning sociability, show inconsistencies, ranging from normal to significantly impaired [24, 26, 27, 28, 29, 30]. More consistent animal models are needed to elucidate the core mechanisms of CHD8-regulated biological and pathological pathways. Although zebrafish studies have begun exploring Chd8’s role in ASD etiology [22, 31, 32, 33], potential behavioral deficits, especially in social domains, remain uninvestigated.
In the present study, we examined the development and behaviors of the Chd8 mutant zebrafish generated by Clustered Regularly Interspaced Short Palindromic Repeats-associated protein 9 (CRISPR-Cas9) [34]. Unlike Chd8-disrupted mice, which typically die embryonically, homozygous mutant zebrafish could survive to adulthood, particularly the males. Mutants also displayed macrocephaly at early stages, mirroring a key human symptom. Capitalizing on the high survival rate of homozygous mutant males, we conducted comprehensive behavioral analyses. We identified consistent social deficits across multiple tests, such as shoaling, social interaction and three-chamber social preference. Additionally, anxiety-like behaviors and locomotor coordination deficits were observed. These results establish Chd8 mutant zebrafish as a powerful model for studying ASD etiology.
Adult wildtype zebrafish (Danio rerio) of AB type were obtained from China Zebrafish Resource Center (Wuhan, Hubei, China) and maintained and bred at standard temperature (26–28 °C) in circulating water system under pH 7.2–7.6. The fish were kept on a 14 h/10 h light/dark cycle and fed twice daily with artemia. The animals were handled according to the guidelines from the Institutional Animal Care and Use Committee (IACUC) of Nanchang University. The fish were anesthetized with 0.003% tricaine (Cat. No. A-5040, Sigma, Louis, MO, USA).
The Chd8 mutant line (M1) was obtained from Dr. Jing (Shanghai Jiao Tong University, Shanghai, China) [34], which was achieved by CRISPR-Cas9 genome-editing with sgRNA (5′-GGTGTGTCTGCTTCAGGATG-3′) targeting the exon 12 of Chd8 (Fig. 1A, Ref. [34]). Tail-fins clips were collected for genomic DNA extraction and genotyping. The Chd8 M1 mutant fish were identified by polymerase chain reaction (PCR) using the following primers: 5′-TCCGAAAATCCAAAAAGAGCCAC-3′ (forward), 5′-CTGAGTCTGTGGTTGATAAATGCC-3′ (reverse). The PCR products of matching length were sent to Sangon Biotech (Shanghai, China) for sequencing. Five bases were found to be deleted in exon 12 of the Chd8-/- homozygotes (Fig. 1B).
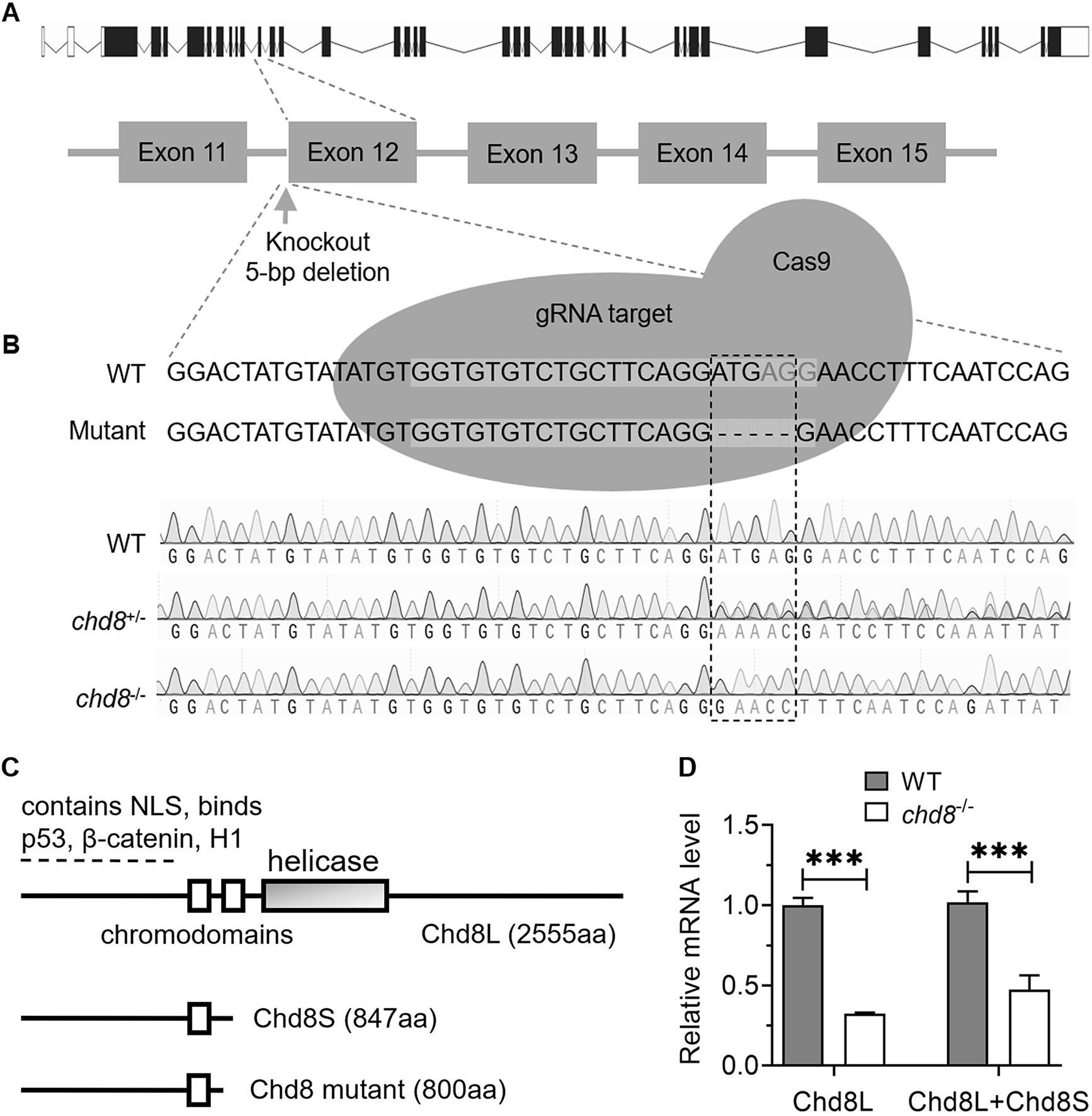 Fig. 1.
Fig. 1.
Characterization of the Chd8 mutant line. (A)
Schematic depicting CRISPR-Cas9 generation of the Chd8 mutant line
(According to Zhong et al. [34]). (B) Genomic DNA sequencing
chromatograms confirming genotypes: wild type (WT), heterozygous
(Chd8+/-) and homozygous (Chd8-/-) mutants. (C)
Primary structures of zebrafish long (Chd8L) and short (Chd8S)
isoforms due to alternative splicing [45], alongside the predicted truncated
protein resulting from the CRISPR-induced mutation. NLS, nuclear localization
signal; H1, histone H1. (D) Relative expression levels of Chd8 isoforms
in Chd8-/- embryos at 4 days post fertilization (dpf). Data
represent mean
Total RNA was extracted from zebrafish embryos using Takara RNAiso Plus (Total RNA extraction reagent, Cat. No. 9109, Takara, Beijing, China) in accordance with the manufacturer’s instructions, followed by phenol-chloroform purification to ensure RNA integrity. First-strand cDNA was synthesized using M-MLV Reverse Transcriptase (Takara, Cat. No. 639524) with Oligo(dT)₁₈ primers to selectively target polyadenylated mRNAs.
Quantitative PCR (qPCR) was performed using the TB Green® Premix
Ex Taq™ II (Tli RNaseH Plus, Takara, No. RR820A) on
a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster
City, CA, USA). Each 20 µL qPCR reaction mixture contained 0.2 µL of
the synthesized cDNA, 10 µL of TB Green Premix Ex Taq II, 0.4 µL of
ROX Reference Dye (50
| Genes | Forward primer | Reverse primer |
|---|---|---|
| eEF1a1a | 5′-CTGCCAGTGTTGCCTTCGT-3′ | 5′-CCTTGCGCTCAATCTTCCA-3′ |
| chd8L | 5′-AGGCGGTGAAGGTGAGATGCTAG-3′ | 5′-ATAGGCTGGAGTGAGGAAGTGGTG-3′ |
| chd8L+S | 5′-ACAGGTGGTGCAGATTTCTCA-3′ | 5′-TACGGGCTCCTGGATCTTTG-3′ |
| vglut1 | 5′-GCCAGTGTATGCCATCATCGTAGCC-3′ | 5′-TCCACAGTTCATGAGTTTACGGACGTT-3′ |
| vglut2a | 5′-CCCCTCCACTGGAAAGAAGTCGTC-3′ | 5′-TACTCTCCTCAATGTAGCAGCGTTC-3′ |
| vglut2b | 5′-CGGCCTCGGATTCTGCATATCGTTC-3′ | 5′-GATCGCAGCACCAAACACCCT-3′ |
| vglut3 | 5′-AAGTCGTCTGGCTACTACCTCGTTC-3′ | 5′-TGGCTCCTTCCCCAATAGAGGTT-3′ |
| slc1a2a | 5′-TTTCTTTGTGGCATTTGGGATTTGCAT-3′ | 5′-ATGAGTCCAACGATGACCGTGACCA-3′ |
| slc1a4 | 5′-GCTTTCCGCTCATATGCCACT-3′ | 5′-ACATGATCCAGGACACAAGCACCA-3′ |
| slc1a7b | 5′-TCTTTATTGCCCAGGTCAACAACTACGAG-3′ | 5′-AGAGCGTCTCCCATCACGTTGACCA-3′ |
| grin1a | 5′-CCAGCTCAACTTGTCGGACCCGTCT-3′ | 5′-GTCATGCCATCCCCTGCAGTCCACA-3′ |
| grin1b | 5′-TGTTTCACCACATTAGGTCGACA-3′ | 5′-TCCAAAGATATGTTCGGTGTTTGCT-3′ |
| gad1b | 5′-AACTCAGGCGATTGTTGCAT-3′ | 5′-TGAGGACATTTCCAGCCTTC-3′ |
| gad2 | 5′-CAAAGCCCGAACACGCCTA-3′ | 5′-TTTCTCAGCCTCCCCGACAT-3′ |
| gabra1 | 5′-CAGGCAGAGCTGGAAGGAT-3′ | 5′-TGCCGTTGTGGAAGAACGT-3′ |
| gabra6 | 5′-TCTTGTGTTGGACAGGTGGTG-3′ | 5′-AGGTCGGAGTCTGTTGTCATATC-3′ |
| glyt1 | 5′-GCTACCCCAGATAGTCAAGCAATGTGA-3′ | 5′-AACGTCAACCCCTACCCCAACCGTA-3′ |
| glyt2 | 5′-TTGTACTACCTGTTCGCTTCGTTG-3′ | 5′-GGCTGACATAGGTTTTATTGTCCACT-3′ |
| glrba | 5′-CTGGCTCTACAACTCTTTCCTATGGAC-3′ | 5′-GCCTCCTCAGAGTGAAGATGACCT-3′ |
| glrbb | 5′-CATGAAAGCTGTTTAACGACCGAT-3′ | 5′-TCTTCTGATGACAGCTGCGAA-3′ |
Male fish (3–6 months post fertilization, mpf) of wild type (WT) and Chd8-/- homozygous mutants were selected for behavioral assays. After being transferred to the testing tank, fish were allowed to settle down for 1 min before behavioral assays started. The fish were filmed with Point Grey camera FlyCapture2 (Version 2.13.3.61, Point Grey, Richmond, BC, Canada) for 10 minutes. The swimming behaviors were tracked and analyzed with Noldus video tracking software EthoVision XT11.5 (Noldus Information Technology, Wageningen, the Netherlands). All behavioral tests were performed during the daytime between 10:00 AM and 4:00 PM.
Open field test was adapted to measure the anxiety behavior of zebrafish. Thirty males of WT or Chd8-/- mutants were selected for open field test. In each test, a single fish was placed in a round tank filled with water to 4 cm in height and 18 cm in diameter. The shallow water depth promotes horizontal movements and limits vertical movements [35]. In the open field test, zebrafish, like rodents, exhibit thigmotaxis (“wall hugging”)—a tendency to explore near the tank walls while avoiding the central zone (designated as an 8 cm diameter circle). Recognized as an indicator of anxiety-like behavior [36, 37], thigmotaxis was operationally defined in this study as swimming within 2 cm of the wall of the tank. Preliminary behavioral analysis revealed two distinct patterns: nimble thigmotaxis, featuring rapid S-shaped swimming with touch-and-go wall interactions, and stiff thigmotaxis, characterized by inflexible C-shaped swimming at a largely constant speed. Fish showing nimble thigmotaxis demonstrated superior body control, facilitating flexible and elegant swimming. In contrast, fish exhibiting stiff thigmotaxis showed weaker body control, indicating impaired coordination, and consequently swam with rigid, inflexible movements. To compare behavioral characteristics between WT and Chd8-/- fish, we determined the proportion of nimble versus stiff thigmotactic behavior relative to all observed thigmotactic behaviors.
Social interactions were measured following a similar dyadic paradigm reported recently [38]. Male zebrafish pairs of the same genotype (WT or Chd8-/-) were introduced into a round tank (18 cm diameter, 4 cm water depth). Dyadic interactions in zebrafish frequently involve aggression [39], a feature also common in ASD [40]. However, these interactions also capture broader social behaviors like communication and play [38], making this assay suitable for measuring social interactions. We quantified the following specific behaviors based on established criteria [41]: repel (mutual encounter and contact followed by directional change), circle (mutual circling, often initiating or terminating a chase), bite (targeting lateral/tail fins, eliciting a stronger reaction than repel) and chase (pursuit of one fish by the other). Repel and circle, relatively subtle, are considered display behaviors while bite and chase are more aggressive.
Oliveira et al. (2011) [39] described zebrafish fights as comprising two stages: mutual assessment and chase/flee. Mutual assessment involves interactive behaviors such as repel, circle, bite and chase [39]. In our experiments, we defined the chase stage specifically as interactive chase. The subsequent flee stage was identified when no further interactive behaviors occurred following a chase [42].
Shoaling assays were adapted from Miller and Gerlai [43]. Trials involved groups
of six fish per replicate, with six replicates per genotype (WT and
Chd8-/-). Experiments were conducted in a 40
Social preference test was performed according to Engeszer et al. [44].
The test tank (50
Behavioral data were acquired and processed using EthoVision XT11.5 (Noldus
Information Technology, Wageningen, the Netherlands) from video recordings of all
assays. The Shapiro-Wilk test and Levene’s test were used to assess the
assumptions of normality and homogeneity of variances, respectively, for all
variables. GraphPad Prism 8.0.1 (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis, and Student’s
t-test was adopted for parametric data analysis. The data were presented
as mean
Similar to mammals, zebrafish Chd8 exists in at least two alternative splicing isoforms: long (Chd8L, 2555 amino acids (aa)) and short (Chd8S, 847 aa) [45]. To investigate the role of Chd8 in zebrafish, we utilized a CRISPR-Cas9 induced Chd8 mutant line obtained from the Jing lab (Shanghai Jiao Tong University, China) [34]. Sequencing confirmed that this mutant carries a 5-base pair deletion in exon 12 (Fig. 1B). The deletion is predicted to introduce a premature stop codon, resulting in a truncated protein of approximately 800 aa. This truncated protein would consist of the N-terminal 790 aa of Chd8 followed by an additional 10 aa due to a reading frame shift. Importantly, the mutation is predicted to disrupt the reading frame of both Chd8L and Chd8S transcripts (Fig. 1C, Ref. [45]).
To assess Chd8 expression levels, we designed two primer pairs for quantitative PCR (qPCR): one specific for Chd8L and recognizing both Chd8L and Chd8S isoforms. At 4 days post fertilization (dpf), qPCR revealed a significant reduction in Chd8L mRNA levels in homozygous Chd8 mutants (Chd8-/-) compared to wild-type (WT) controls. Similarly, transcript levels detected by the common primer pair (presumably representing total Chd8 mRNA) were also markedly lower in mutants (Fig. 1D). These results indicate that the mutation significantly downregulates Chd8 expression, though further experiments are required to determine if this reduction is caused by nonsense-mediated mRNA decay (NMD) or other mechanisms. Collectively, these findings demonstrate that the Chd8 mutation disrupts transcription and likely prevents translation of both Chd8L and Chd8S isoforms.
To assess the impact of Chd8 dosage on development, we crossed heterozygous (Chd8+/-) males and females, then quantified and measured the offspring. Inter-eye distance, a proxy for brain growth, was significantly wider in Chd8-/- embryos at 5 dpf (Fig. 2A,B), suggesting brain overgrowth reminiscent of the macrocephaly commonly observed in patients with CHD8 mutations.
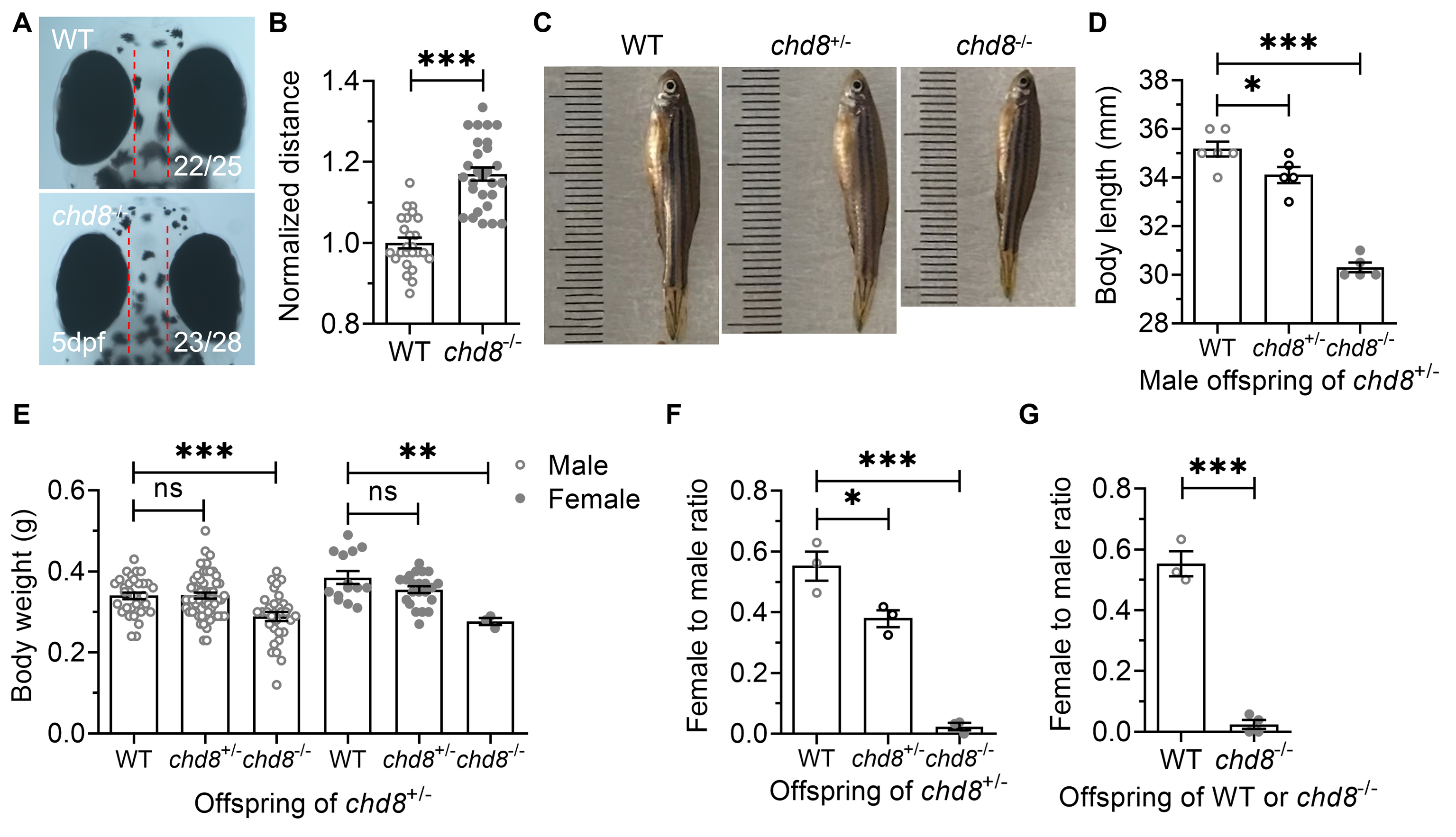 Fig. 2.
Fig. 2.
Developmental phenotypes in WT, Chd8+/-
and Chd8-/- zebrafish. (A,B) Inter-eye distance (a proxy for brain
size). (C,D) Body length and (E) Body weight of male and female offspring derived
from Chd8+/- crosses. (F) Female-to-male ratio in offspring
from Chd8+/-crosses. (G) Female-to-male ratio in offspring from WT
or Chd8-/- crosses. Data are presented as mean
Unlike Chd8-disrupted mice, which exhibit embryonic [24, 28, 29, 46] or postnatal lethality in conditional knockouts [47, 48], homozygous mutant zebrafish were viable, with some surviving to adulthood. However, under identical rearing conditions, the Chd8-/- offspring exhibited slower growth, reduced body size, and delayed sexual maturity compared to their WT and Chd8+/- siblings (Fig. 2C,D). Chd8-/- mutants also exhibited significantly lower body weight than WT controls, irrespective of sex (Fig. 2E).
The offspring of Chd8+/- parents showed a significantly reduced proportion of females among Chd8+/- and Chd8-/- mutants compared to WT (Fig. 2F). Although viable and fertile, homozygous mutants produced fewer and less frequent spawns than WT and heterozygous fish. This female deficiency was even more pronounced in the offspring derived from Chd8-/- crosses (Fig. 2G).
The observed macrocephaly in Chd8-/- zebrafish suggests underlying neurodevelopmental defects. Given that altered neurotransmission is a common feature in ASD patients and animal models, we examined the expression of key genes involved in major neurotransmitter systems: glutamate, gamma-aminobutyric acid (GABA) and glycine.
qPCR analysis at 4 dpf revealed significant dysregulation of genes critical for glutamatergic transmission in Chd8 mutants. This included altered expression of vesicular glutamate transporters (markers of glutamatergic neurons, Fig. 3A), solute carrier family 1 members (slc1a2a, slc1a4, slc1a7b; glutamate/neutral amino acid transporters, Fig. 3B), and grin1 (encoding an essential N-methyl-D-aspartate (NMDA) receptor subunit, Fig. 3B). Similarly, genes related to GABAergic transmission were affected, including those encoding enzymes for GABA synthesis and GABA receptors (Fig. 3C). Expression of glycine transporters and receptors was also affected in mutants (Fig. 3D).
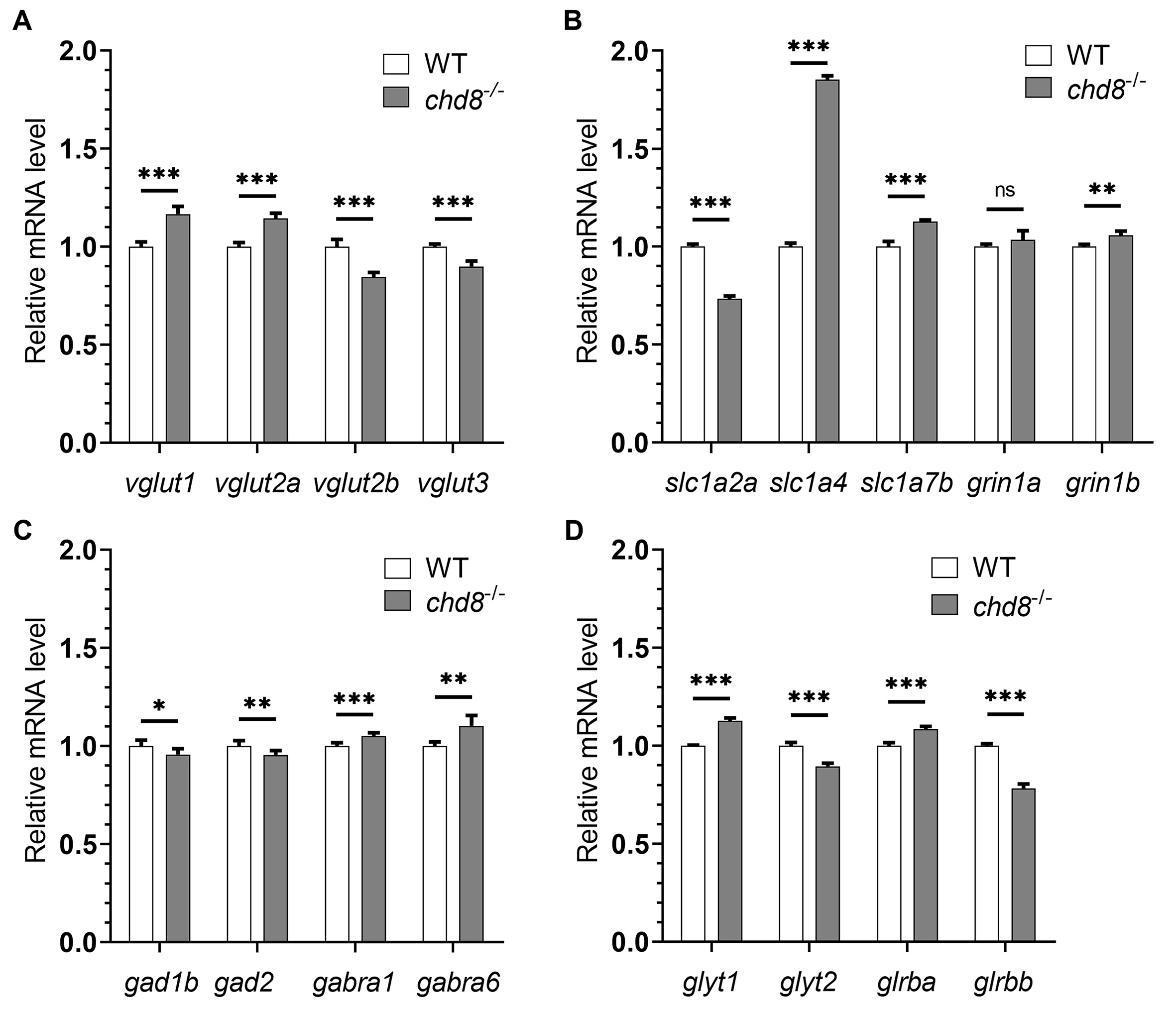 Fig. 3.
Fig. 3.
Dysregulated neurotransmission gene expression in
Chd8-/- embryos. qPCR analysis of neurotransmission markers in
4 dpf embryos. (A,B) The expression levels of genes involved in glutamatergic
transmission. (C) The expression levels of GABAergic transmission genes. (D) The
expression levels of glycinergic transmission genes. WT mRNA expression levels
were set to 1 and Chd8-/- expression is shown relative to WT. Data
are presented as mean
Collectively, the dysregulation across these major neurotransmitter pathways indicates compromised development of neural circuit function in Chd8 embryos—a finding consistent with alterations observed in ASD.
Patients with CHD8 mutations exhibit variable autism symptoms and some of these symptoms, especially sociability, can be recapitulated inconsistently in mouse models [24, 26, 27, 28, 29, 30]. Leveraging the high survival rate of Chd8 homozygous mutant zebrafish, we assessed behavioral phenotypes using an open field test, which is a validated assay for locomotor activity and anxiety-like behaviors in zebrafish and rodents [49].
Individual fish were placed in a round basin and recorded for 10 min (Fig. 4A). Chd8-/- mutants showed no significant differences from WT in total distance travelled or average velocity (Fig. 4B,C), indicating intact baseline locomotion. Time spent in the central zone also did not differ (Fig. 4D).
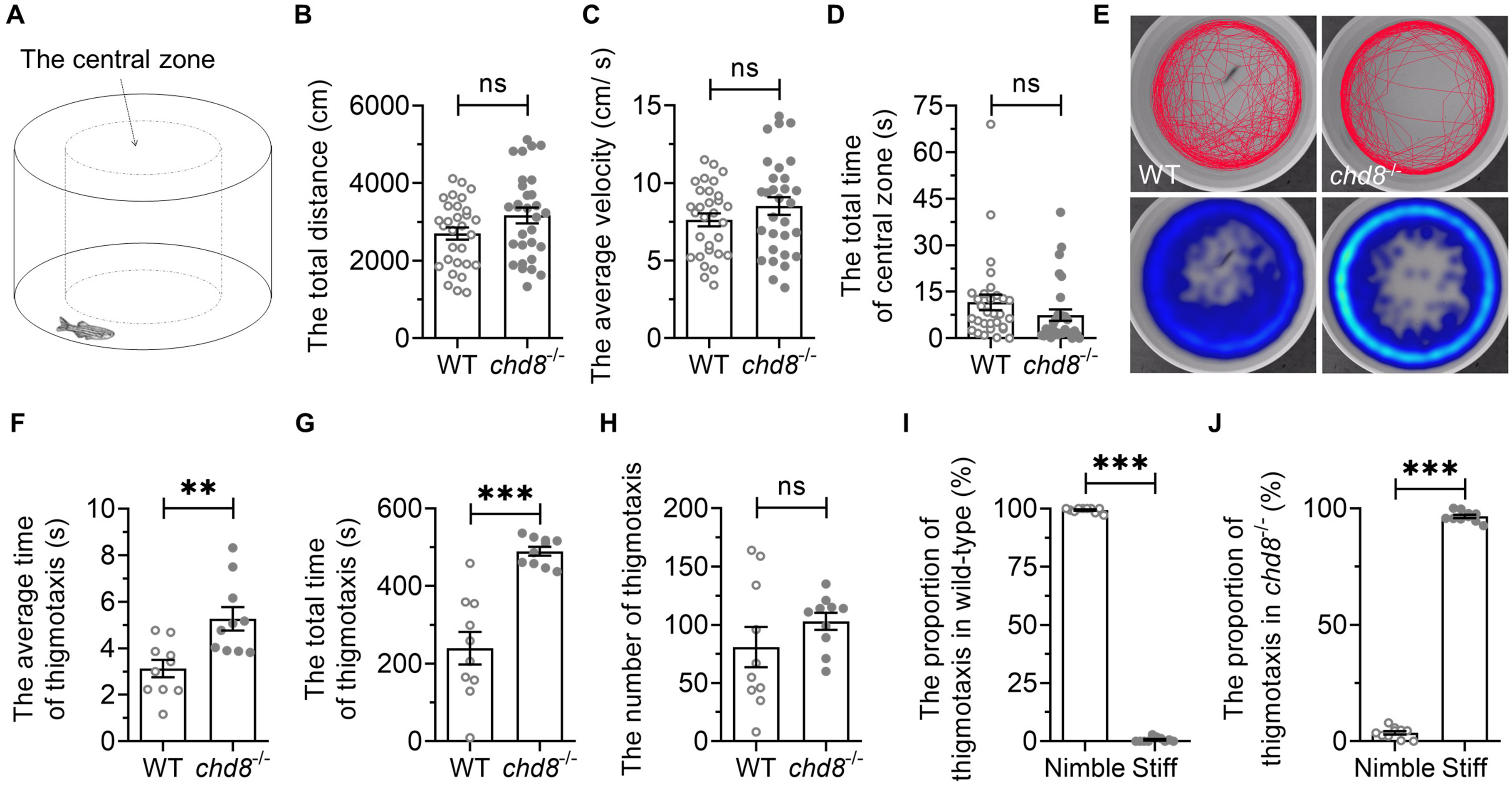 Fig. 4.
Fig. 4.
Open field behavior in WT and Chd8-/-
zebrafish. (A) Illustration of open field test. (B) Total distance traveled and
(C) average velocity in 10 min. (D) Time spent in central zone. (E)
Representative heatmaps showing spatial distribution over 6 minutes. (F) Average
thigmotaxis episode duration. (G) Total thigmotaxis time. (H) Number of
thigmotaxis episodes. (I,J) Proportion of thigmotaxis events classified as nimble
(S-shaped) versus stiff (C-shaped) swimming postures. Data are presented as mean
We further analyzed thigmotaxis (also called “wall hugging” or “wall following”), an evolutionarily conserved response to novel environments that facilitates the search for shelters, protection and/or escape routes [49, 50]. Therefore, thigmotactic behavior is generally regarded as anxiety-like behavior, and reflects the level of anxiety to some extent [49, 50]. When the fish swam within 2 cm of the wall, we designated it as thigmotaxis (Fig. 4E). The duration of each episode of thigmotaxis was measured from wall-entry to exit. While the number of thigmotactic episodes was comparable between genotypes (Fig. 4H), the average duration of thigmotactic episodes was much longer in Chd8-/- mutants (Fig. 4F). The mutants exhibited increased total time in thigmotaxis (Fig. 4G). These findings suggest heightened anxiety-like states in mutants.
We noticed qualitative differences in swimming posture during thigmotaxis. WT fish exhibited nimble, flexible and elegant S-shaped swimming, whereas mutants frequently adopted stiff, inflexible and rigid C-shaped postures with minimal tail movement. The proportion of either type of the thigmotactic behaviors was calculated and it was significantly different between WT and Chd8-/- mutants (Fig. 4I,J). This kinematic abnormality suggests underlying locomotor coordination deficits.
Zebrafish tend to swim collectively in groups, forming social aggregations (shoals). To assess group behavior, we recorded shoaling dynamics in groups of six fish for 10 minutes (Fig. 5A,E). While total distance travelled and average velocity showed no significant differences between genotypes (Fig. 5B,C), Chd8-/- mutants exhibited significantly larger average inter-fish distances within shoals compared to WT (Fig. 5D). This indicates impaired shoal cohesion and coordination in mutant fish.
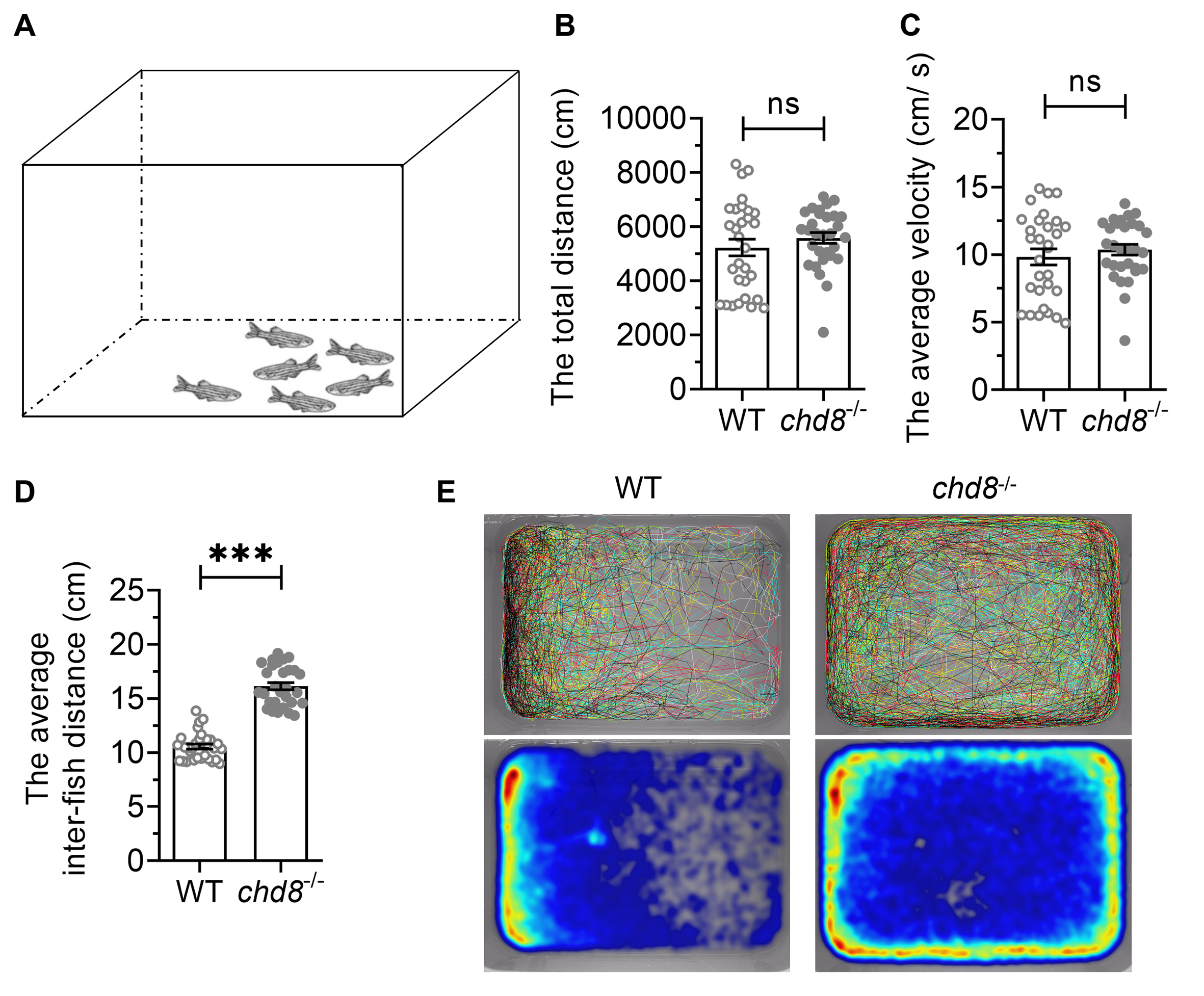 Fig. 5.
Fig. 5.
Shoaling behavior in WT and Chd8-/-
zebrafish. (A) Schematic illustration of shoaling test. (B) The total distance
travelled and (C) Average velocity of the shoal over 10 minutes. (D) Mean
inter-fish distance within shoals. (E) Representative swimming trajectories (top)
and heatmaps (bottom) showing spatial distribution over 5 minutes. Data are
presented as mean
Given that impaired social communication is an ASD core syndrome, we adapted an aggression-testing paradigm to quantify dyadic social interaction. A pair of zebrafish with the same genotype was placed in a round basin and their behaviors were recorded for 10 minutes (Fig. 6A). The interaction between the two individuals was categorized into different types, including repel, circle, bite and chase (Fig. 6B). Chd8-/- exhibited significantly fewer repels, circles and bites than WT pairs (Fig. 6C–E). While total chase duration was comparable, Chd8-/- displayed significantly longer average chase duration and fewer number of chase episodes (Fig. 6F–H). The chasing accompanied by interaction was distinguished as interactive chasing while chasing without interaction as following. Chd8-/- exhibited strikingly reduced proportion of interactive chases (Fig. 6I,J).
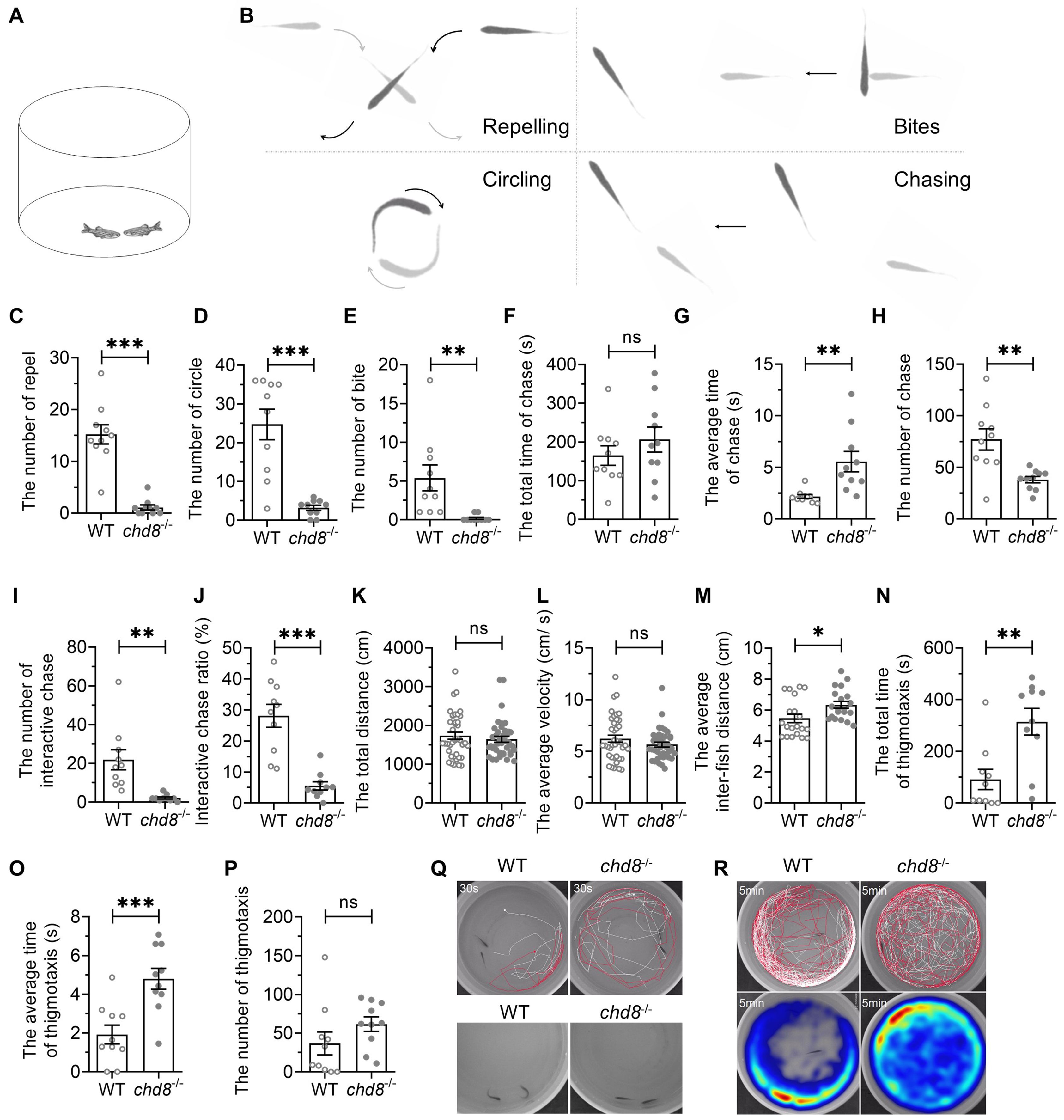 Fig. 6.
Fig. 6.
Social interaction deficits in Chd8-/-
dyads. (A) Illustration of social interaction test of dyads. (B) Schematic
diagram of different social interaction in the dyads. (C–E) Frequency of repel,
circle and bite events. (F) Total chase duration. (G) Mean chase duration. (H)
Chase frequency. (I) Interactive chase frequency. (J) Proportion of interactive
chases. (K,L) Total distance traveled and average velocity. (M) Mean inter-fish
distance. (N–P) Thigmotaxis parameters. (Q,R) Representative trajectories (30
sec) and heatmaps (5 min) showing spatial distribution. Data are presented as
mean
Locomotor activity (distance, velocity) showed no genotypic differences (Fig. 6K,L). However, Chd8-/- dyads maintained larger inter-fish distances than closely interacting WT pairs (Fig. 6M), indicating loose cohesion.
Thigmotaxis was also assessed in the dyads (Fig. 6Q,R). The time spent by both fish within 2 cm of the wall was designated as an episode of thigmotaxis. While the episode number was comparable between the genotypes (Fig. 6P), Chd8-/- mutants exhibited increased total thigmotaxis time (Fig. 6N) and longer average episode duration (Fig. 6O). The results indicate that the mutant dyads also exhibited anxiety-like behavior, like that found in open field test.
These results demonstrated that Chd8-/- dyads showed social interaction deficits as well as anxiety-like behavior.
We assessed social preference using a three-chamber test (Fig. 7A). The tank was divided into three chambers by transparent nets with wholes, allowing visual and olfactory perception of social stimulus fish. A social stimulus fish was randomly placed in one side chamber. The middle chamber (approximately 3 times longer than side chambers) contained three zones: social zone (next to the chamber with social stimulus zebrafish), central zone, non-social zone (the side without stimulus fish) (Fig. 7A). The subject fish was placed in the central zone of the middle chamber separated by two separators and allowed for a 30-s acclimation period. Then the two separators were removed and the subject fish was allowed to swim freely in the middle chamber (Fig. 7A,B). In the entire test, there was no difference in the distance travelled and the average velocity of WT and Chd8-/- (Fig. 7C,D). WT spent more time in the social zone, ~400 s in social zone vs. ~200 s in both central zone and non-social zone, indicating a strong social preference (Fig. 7E). Chd8-/- mutants spent much less time in the social zone than WT (less than 250 s vs. ~400 s) (Fig. 7E) while they spent much more time in central zone (Fig. 7F). There was no difference of the time spent in the non-social zone between WT and Chd8-/- mutants (Fig. 7G). Consistent with these findings, the social preference index was significantly lower in Chd8-/- mutants (Fig. 7H, Ref [51]), confirming disrupted social behavior.
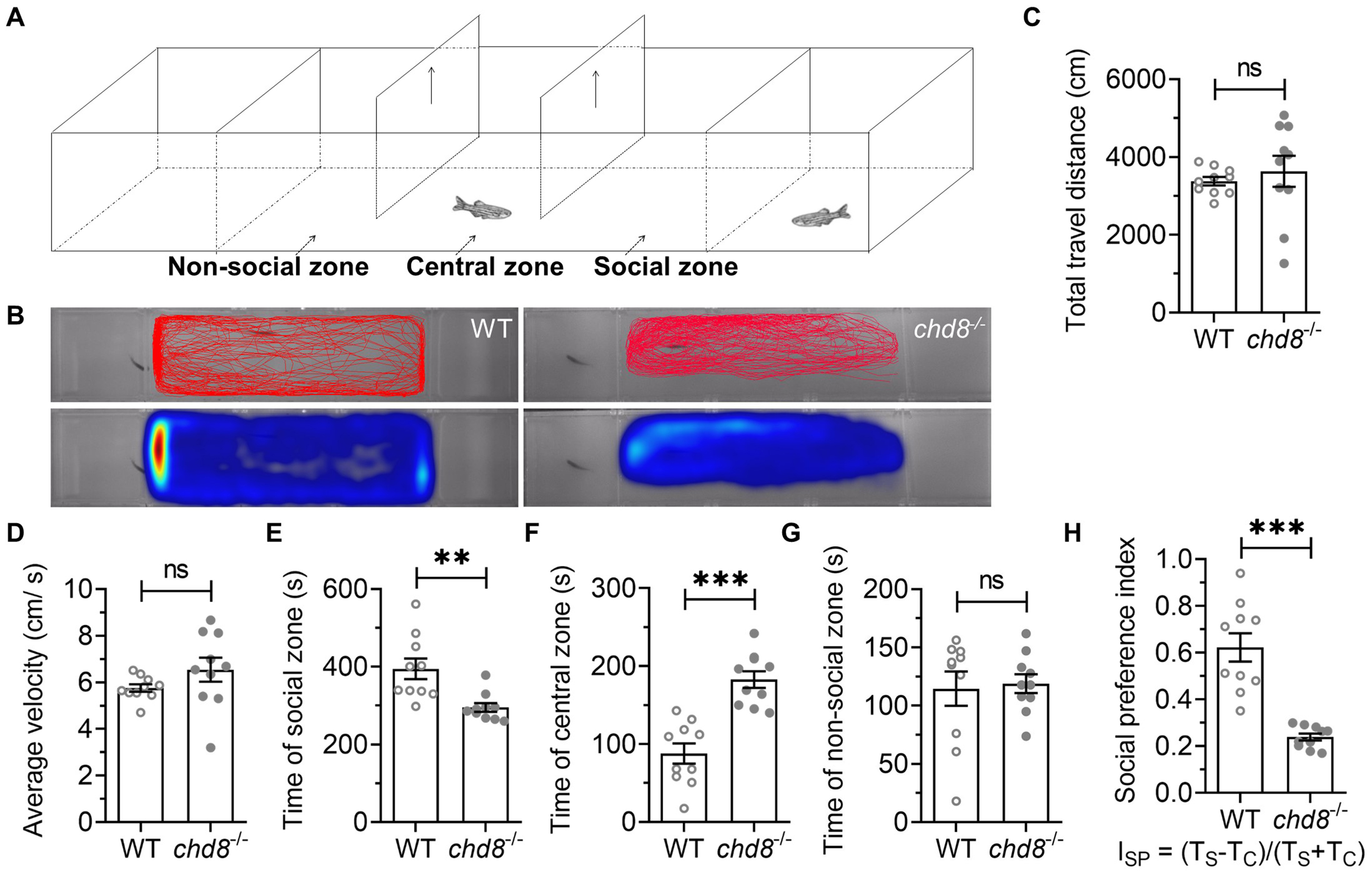 Fig. 7.
Fig. 7.
Social preference deficits in Chd8-/-
zebrafish. (A) Three-chamber social test schematic. (B) Representative heatmap
showing spatial distribution over 10 minutes. (C) Total distance traveled and (D)
average velocity. Time spent in (E) social zone (F) central zone, and (G)
non-social zone. (H) Social preference index (ISP = [TS –
TC]/[TS + TC]); TS: time in social zone, TC: time in
central zone (according to Rein et al. [51]). Data are presented as
mean
This study demonstrates that Chd8 homozygous mutant zebrafish, unlike embryonically lethal mouse counterparts [46], survive to adulthood. While qPCR confirmed reduced expression of both Chd8L and Chd8S, we were unable to assess Chd8 protein levels due to the lack of commercially available antibodies. The viability of these mutants may exhibit greater tolerance to major organ damage caused by Chd8 deficiency. This survival advantage enables investigation into Chd8’s roles in neurodevelopment and behavior relevant to ASD.
Chd8 exhibits dosage-sensitive functions in early nervous system development [52, 53]. Brain growth responds non-linearly to reduced Chd8 levels: heterozygotes and mild hypomorphs display overgrowth, while severe hypomorphs show hypoplasia [53]. Notably, socio-communicative deficits or repetitive behaviors, were absent in either heterozygotes or mild hypomorphs [53]. Given inconsistent behavioral phenotypes reported in Chd8 mutant mice [24, 26, 27, 28, 29, 30], we focus on homozygous zebrafish mutants.
Our Chd8 mutants recapitulate key features of patients with CHD8 mutations. Firstly, Macrocephaly was observed in our Chd8 mutants with high penetration, mirroring human patients [22, 54, 55] and findings in Chd8 morphant zebrafish [22, 31] and knockout mice [24], underscoring its evolutionarily conserved neurodevelopment role. Secondly, gastrointestinal defects were also reported in zebrafish and mice Chd8 models [32, 56], further supporting functional conservation. Finally, and most importantly, we consistently detected anxiety-like behaviors (Fig. 4) and especially social deficits through multiple assays, including shoaling, social interaction and social preference (Figs. 5,6,7). Collectively, these data establish Chd8 mutant zebrafish as a valuable model for dissecting CHD8’s pathological mechanisms in neurodevelopment and ASD.
The abnormal dyadic interactions in Chd8 mutants were particularly revealing. Mutants exhibited prolonged passive following but significantly reduced interactive chasing compared to WT (Fig. 6). In WT pairs, chased individuals typically respond reciprocally, culminating in interactive chasing. Mutants, however, rarely responded, leading to prolonged chasing episodes. These chases were often interrupted, potentially due to distraction, exhaustion or sensory deficits warranting further study. The reduced repelling, circling and biting suggest these prolonged chases are not primarily aggressive. They may instead represent impaired social reciprocity or an aberrant drive for social approach. The lack of interactive chasing likely reflects a failure in mutual responsiveness, possibly due to deficient social skills, impaired cognitive processing or dysregulated sensorimotor integration, which need further studies [57].
Beyond social impairment, mutants displayed increased anxiety-like behavior, a common ASD comorbidity [58] also reported in CHD8 mutation carriers [59] and Chd8 mutant mice [24, 30, 60]. Intriguingly, Chd8 duplication in mice reduces anxiety-like behavior [61], highlighting the critical importance of precise Chd8 dosage for normal neurodevelopment and behaviors.
Mutants also exhibited strikingly abnormal thigmotactic locomotion alongside prolonged thigmotaxis episodes. Their rigid, inflexible swimming postures (Fig. 4I,J) suggest underlying motor coordination deficits. This aligns with dystonic movement disorders in patients with CHD8 variants [62, 63] and Chd8-deficient mice [64, 65]. Conditional knockout of Chd8 in cerebellar granule neuron progenitors causes hypoplasia of cerebellum and impairments in proliferation, differentiation, and synaptogenesis [64, 65], implicating cerebellar dysfunction in these motor deficits. We cannot exclude the possibility that locomotor abnormalities contribute to impaired social behaviors, a relationship requiring further investigation.
As a high confidence ASD risk gene (Simons Foundation Autism Research Initiative, SFARI), CHD8 has been extensively studied in mice, yet reported social behaviors range from deficient to normal [24, 26, 27, 28, 29, 30, 66]. Zebrafish studies of Chd8 focused on developmental roles; behavioral phenotypes were unexplored due to transient knockdown effects [22, 31, 67]. Chd8 knockout zebrafish were established, but no neural behavior was investigated either [32, 34]. Our study provides consistent evidence of social deficits in Chd8 mutant zebrafish across multiple paradigms, reinforcing the highly conserved biological functions of CHD8 from fish to humans.
Sample sizes for growth measurement were kept small to minimize handling stress. Behavioral tests were used only homozygous males due to the scarcity of adult female homozygotes. Core ASD symptoms like repetitive behaviors and sensory deficits remain to be assessed. While brain overgrowth and neurotransmitter dysregulation were detected early, their persistence in adults and correlation with behavioral phenotypes require future study.
Leveraging the high survival rate of a Chd8 mutant zebrafish, we identified developmental and behavioral deficits mirroring symptoms in patients with CHD8 mutations. These findings underscore the conserved roles of CHD8 across vertebrates. This zebrafish model could serve as a powerful tool for investigating ASD pathological mechanisms and discovering potential therapies.
The data that support the finding of this study are available on request from the corresponding author, HAX.
HTW: Investigation, methodology, visualization, review. XTF: Formal analysis, visualization, validation, original draft, review. YFW: Validation, review. LYL: Review, resources, validation. ZZL: Validation, review, resources. HAX: Conceptualization, original draft, review & editing, supervision, funding acquisition. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was conducted following the ethics approval from the Institutional Animal Care and Use Committee (IACUC) of Nanchang University. The approval date was March 4th 2019.
The authors thank the Jing’s lab in Shanghai Jiao Tong University for providing the zebrafish Chd8 mutant line.
This work is supported by grants from the National Natural Science Foundation of China (Grant No. 82260279 and 31960169), Jiangxi Provincial Natural Science Foundation (Grant No. 20202ACB206002 and 20213BCJ22057) and School of Basic Medical Sciences, Nanchang University.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
