- Academic Editors
†These authors contributed equally.
This is an open access article under the CC BY 4.0 license.
Electroacupuncture pretreatment (EA-pre) has been shown to help reduce myocardial ischemia-reperfusion injury (MIRI), but the underlying mechanism remains unclear. Our previous studies indicated that EA activates the cerebellar cortex, specifically the Crus Ⅰ. However, whether activation of the Crus Ⅰ contributes to the attenuation of MIRI induced by EA-pre remains unclear. This study investigated the possible relationship between EA-induced relief of MIRI and the activation of Crus Ⅰ.
Electrocardiogram recording, echocardiography, and cardiac histology staining were used to assess the heart’s functional status. In vivo electrophysiological recordings, Fos-targeted recombination in active populations (Fos-TRAP) gene-labeling technology and chemogenetic viral modulation were used to explore the effects of Crus Ⅰ activation in EA-pre on MIRI.
In vivo electrophysiological recordings demonstrated that Crus Ⅰ plays a crucial role in EA-pre by modulating sympathetic activity to alleviate MIRI. Subsequent Fos-TRAP studies showed that EA stimulation primarily induces changes in the neuronal activity of Crus Ⅰ Purkinje cells (Crus ⅠPC). Chemogenetic viral manipulations further verified that EA-pre suppresses PC activity in MIRI.
EA-pre mitigated cardiac sympathetic nerve dysfunction during MIRI by regulating Crus ⅠPC activity.
Acupuncture is widely used to treat various diseases by stimulating specific areas on the muscle surface [1]. The sensory input generated by acupuncture activates somatic-autonomic reflexes via peripheral nerves, primarily those in the dorsal root ganglion or trigeminal ganglion. This sensory information is transmitted from the spinal cord to the brain, leading to the activation of peripheral autonomic pathways and the regulation of physiological functions [2].
Acute myocardial ischemic injury and myocardial infarction remain the leading cause of death and disability worldwide, and the most effective intervention is prompt and effective myocardial perfusion using thrombolytic therapy or percutaneous coronary intervention [3, 4, 5]. However, the process of myocardial reperfusion induces further myocardial cell death, an injury known as myocardial ischemia-reperfusion injury (MIRI) [6, 7, 8]. Pretreatment has been recognized as an effective intervention to mitigate MIRI, including drug, electroacupuncture (EA) and ozone [9, 10, 11, 12]. Within contemporary therapeutic strategies for cardiovascular disorders, electroacupuncture pretreatment (EA-pre) has gained recognition as a non-pharmacological intervention demonstrating dual cardioprotective properties: angina mitigation and cardiac functional enhancement [13, 14]. Nevertheless, the central nervous system mechanism by which EA-pre attenuates MIRI is not yet thoroughly understood.
The cerebellum was traditionally associated only with movement planning and execution. However, recent research has uncovered its role in complex, multi-level information processing, including emotion, cognition, and reward [15, 16, 17, 18, 19]. Studies on pain have shown that the cerebellum and supplementary areas were consistently activated by painful stimuli [20, 21]. The cerebellar cortex region known as Crus Ⅰ receives inputs from the cerebral parietal and somatosensory regions and integrates signals from the primary somatosensory and motor cortices [22, 23, 24]. Crus Ⅰ Purkinje cells (Crus ⅠPC) may coordinate interactions between the cerebellum and non-motor cortical regions, such as the medial prefrontal cortex (mPFC), through phase-difference encoding [25]. Interactions between Crus Ⅰ and higher-order structures-primarily the dorsolateral prefrontal cortex, mPFC, and ventral tegmental area-modulate multiple dimensions of pain processing [26]. Notably, functional magnetic resonance imaging results indicated that acupuncture at the Shenmen (HT 7) point suppressed motor cortex activity and activated the cerebellar Crus Ⅰ [27]. Despite growing interest in the cerebellum’s role in pain and related disorders, its precise functions remain to be elucidated.
In the present study, we probed the mechanism underlying the role of the cerebellar cortex in the modulation of MIRI through EA-pre, building on our previous research involving the cerebellar nuclei [28, 29, 30].
A total of 126 male C57BL/6J wild-type mice weighing 20–25 g and aged 7–8
weeks old were used. The mice were purchased from Hangzhou Ziyuan Experimental
Animal Technology Co., Ltd. (License No. SCXK (Zhe) 2019-0004, Hangzhou, Zhejiang, China). The mice were
housed in the laboratory animal room of Anhui University of Chinese Medicine with
alternating lighting (lights on at 08:00, off at 20:00). The vivarium environment
was maintained at 25
All surgical procedures were conducted under aseptic conditions. The mice were fasted for 12 hours (h) before surgery. Prior to surgical intervention, the mice were anesthetized with 4% isoflurane (1 L/min, R510-22-10, RWD Life Technology Co., Shenzhen, China) in an induction chamber and maintained on 1% isoflurane. The status of the mice was carefully observed throughout the procedure. The mouse body temperature was maintained at 37 °C and subsequently rewarmed postoperatively using a regulated heating pad until full recovery. During virus injections and in vivo electrophysiological procedures, the eyes of the mice were coated with roxithromycin ophthalmic ointment (H20003077, Tiantai Pharmaceutical CO., Ltd, Sanming, Fujian, China) to prevent light-induced damage to the eyes.
After anesthetization, EA was performed by vertically inserting a unipolar
stainless-steel acupuncture needle (0.25
Under anesthesia (4% isoflurane, 1 L/min), the anterior thoracic region of the mouse was depilated using depilatory cream. After this, the muscle tissue was carefully separated to expose the third and fourth intercostal spaces. Mechanical ventilation was maintained throughout the procedure using an ALC-V8S ventilator (Shanghai Alcott Biotech Co., Ltd., Shanghai, China) with the following parameters: respiratory frequency, 120 breaths/min; inspiration-to-expiration ratio, 1:2; tidal volume, 1 mL. The MIRI model was established by ligation of the left anterior descending coronary artery (LAD) using a 10–0 silk suture. Following 30 min of occlusion, the suture was loosened to permit a reperfusion period of 2 h. In the Sham group, a left thoracic incision was made to expose the pericardium without ligating the LAD.
Mice were randomly assigned to the following groups (n = 6/group): Sham, MIRI, MIRI+EA-pre, and MIRI+sEA-pre. All mice underwent isoflurane (4%, 1 L/min) inhalation for identical durations and received thoracotomy under maintained anesthesia. The Sham group underwent needle insertion without electrical stimulation or ischemia induction. The remaining groups were subjected to myocardial ischemia-reperfusion injury.
In the in vivo electrophysiological experiment, the mice were allocated into three groups (n = 6/group): Sham, MIRI, and MIRI+EA-pre.
In the Fos-targeted recombination in active populations (Fos-TRAP) genetic editing experiment designed to label EA-activated neurons in cerebellar Crus I, mice were randomly assigned to the following 5 groups (n = 6/group): Promoter On, Promoter Off, 3-day-after EA, 6-day-after EA, and 6-day-after control. Throughout the experiment, drinking water regimens varied by group: the Promoter On group received standard drinking water for the entire duration; the Promoter Off group was maintained on water containing doxycycline (Dox) throughout; the remaining three groups were supplied with Dox-water for 21 days post viral injection, switched to standard water during the labeling window, and returned to Dox-water after the window closed. With the exception of the 6-day-after control group, the other four groups received electroacupuncture stimulation at HT 5 and HT 7 acupoints during the labeling window.
Mice were randomly divided into five experimental groups (n = 6/group): mCherry+Sham, mCherry+MIRI, mCherry+EA-pre+MIRI, hM4Di+MIRI, and hM3Dq+EA-pre+MIRI. Bilateral cerebellar Crus I regions were injected with 120 nL per side using of the following recombinant adeno-associated viruses (rAAV): rAAV-L7-mCherry, rAAV-L7-hM4Di-mCherry, or rAAV-L7-hM3Dq-mCherry. After 21 days to allow for viral expression, all mice received daily i.p. injections of clozapine N-oxide (CNO, BrainVTA Co., Ltd, Wuhan, Hubei, China) for 7 days, along with 30-min daily anesthesia sessions. EA-pre was administered under anesthesia to the mCherry+EA-pre+MIRI and hM3Dq+EA-pre+MIRI groups. On day 8, MIRI or sham surgery was conducted, with an additional CNO injection given 30 mins before the procedure.
Under anesthesia, mice were secured within the brain stereotaxic apparatus (RWD
Life Technology Co., Shenzhen, Guangzhou, China) using a nose clamp and ear bars.
The fur on the mouse’s head was clipped and the scalp was cut to expose the
skull. The cranial surface was wiped with a cotton swab soaked in saline to keep
the skull clean. Stereotaxic coordinates were based on Paxinos and
Franklin’s the Mouse Brain in Stereotaxic Coordinates [31]. For Crus Ⅰ bilateral
injections, the coordinates from bregma were defined as –6.25 mm anteroposterior
(AP),
Under deep anesthesia, mice were secured in a stereotaxic instrument to perform craniotomy. A fine-wire electrode was implanted into the Crus Ⅰ, and neural signal data were acquired using the Plexon multichannel neural signal recording system in digital form. The raw data were processed using Offline Sorter and Neuro Explorer software for sorting units, firing rates analysis, waveform comparison and spectral analysis.
The real-time raw data were detected after a 300 Hz low-cut filter, which was
three standard deviations above the noise amplitude. Suspected individual units
were isolated off-line by manual cluster cutting of various spike waveform
parameters. Only units with sufficient separation and no inter-spike interval
Mice were bilaterally injected with a virus mixture 200 nL of recombinant adeno-associated virus-cellular immediate-early gene Fos promoter-tetracycline transactivator (rAAV-c-fos-tTA) and 200 nL of the recombinant adeno-associated virus-tetracycline response element-tight promoter–mCherry (rAAV-TRE-tight-mCherry) into Crus Ⅰ. The mice were then housed individually for 3 weeks and fed Dox water (Dox, Spark jade, Cas: 24390-14-5, Jinan, Shandong, China). For neuron activity-dependent labeling, the mice were fed with regular water and received bilateral EA stimulation 20 min/day for 3 days. Dox diets were resumed until the mice were perfused for histological analysis.
Electrocardiography (ECG) signals were recorded in mice using limb-lead electrodes connected a PowerLab Standard Ⅱ leads (PowerLab 8, AD Instruments, Sydney, Australia). ST-segment displacement values and low-frequency to high-frequency ratio (LF/HF) were derived from ECG traces using LabChart V8.1.19 software (AD Instruments, Sydney, Australia). Data segments with at minimum duration of 5 min were included for analysis.
Under anesthesia, cardiac ultrasound was performed on mice using a high-frequency ultrasound with a dedicated small animal imaging system (VINNO6 LAB, Suzhou, Jiangsu, China). Two-dimensional and M-mode echocardiograms were acquired from the parasternal long-axis view. Echocardiographic parameters, including left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS), were measured over three consecutive cardiac cycles. Throughout the procedure, body temperature was regulated within the range of 36–37.5 °C.
The coronary artery was re-occluded at the end of reperfusion. Mice were euthanized via terminal overdose of isoflurane anesthesia (5% concentration for
Under deep anesthesia, mice were perfused through the heart with chilled 0.9% saline and 4% paraformaldehyde (PFA, G1101-500ML, Servicebio, Wuhan, Hubei, China) solution. The mouse brains were carefully dissected from the cranial cavity, post-fixed in 4% PFA for 3 days, and subsequently cryoprotected in a 30% sucrose solution. Using a cryostat microtome (Leica CM1900, Wetzlar, Germany), brains were coronally sectioned at 40-µm and rinsed in phosphate-buffered saline (PBS). Following permeabilization and blocking with 0.5% TritonX-100 (BS084-500ml, Biosharp, Beijing, China) and 5% bovine serum albumin (BSA, ED0017-B, Spark jade, Jinan, Shandong, China) in PBS for 1.5 h, the sections were incubated overnight at 4 ℃ in a base solution containing 0.3% Triton X-100 and 3% BSA, to which the primary antibody (anti-Calbindin, ab108404, Abcam, Waltham, MA, USA; 1:500). The next day, the sections were incubated with the secondary antibody (Donkey anti-rabbit IgG HL, ab150061, Abcam, 1:500) in a base solution containing 0.3% Triton X-100 and 3% BSA for 2 h at room temperature under light-protected conditions. After being washed three times with PBS, the sections were mounted using an antifade medium with 4′,6-diamidino-2-phenylindole (DAPI, EE0011-A, Spark jade, Jinan, Shandong, China). Upon completion of the staining process, three randomly selected images were captured.
Myocardial tissue was harvested after surgery, rinsed with saline, fixed in 4% PFA overnight. Then, cardiac tissues were dehydrated in an ethanol gradient at room temperature, cleared of xylene, embedded in paraffin and cut into 5 µm slices. The slices were stained with hematoxylin-eosin (HE, BA4041, BaSO, Shenzhen, Guangdong, China) for observation of their morphological structure. The slices were stained with Weigert ferrohematoxylin, Ponceau acid fuchsin, Marson blue solution, and Aniline blue in order to observe the degree of myocardial fibrosis.
At the conclusion of the experiment, blood was obtained via the orbital sinus from mice under deep anesthesia. After collection, the blood was incubated at 4 °C for 30 min and centrifuged at 3500 rpm for 15 min. The serum was collected, quantified, and assessed according to assay-kit instructions (AiFang biological, Changsha, Hunan, China). Levels of norepinephrine (NE) were measured to assess sympathetic nerve activity, and levels of creatine kinase isoenzyme MB (CK-MB) and cardiac-specific troponin T (cTnT) were measured to determine the extent of myocardial injury.
All statistical analyses were conducted using GraphPad Software version 8.0 (GraphPad Software, San Diego, CA, USA).
Data are presented as mean
To determine the protective effect of EA-pre on MIRI, cardiac function was compared among four groups: Sham, MIRI, MIRI+EA-pre, and MIRI+sham EA-pre (sEA-pre). The MIRI mouse model was induced by 30 min LAD ligation followed by 120 min reperfusion [33, 34] (Fig. 1A). The ST segment of the ECG was elevated in myocardial ischemia, and partially restored after 2 h of reperfusion (Fig. 1B). LF/HF is often taken to be an indicator of sympathetic-vagal balance [35, 36]. ECG analysis indicated a reduction in ST segment deviation and a lower LF/HF ratio in the MIRI+EA-pre group relative to the MIRI group. Conversely, no significant differences were observed between the MIRI+sEA-pre group and the MIRI group (Fig. 1C,D). In addition, we conducted echocardiographic experiments to evaluate cardiac function (Fig. 1E). The MIRI procedure resulted in significant reduction in LVEF and LVFS, as well as increases in left ventricular end-systolic internal diameters (LVIDs) and left ventricular end-diastolic internal diameters (LVIDd). Cardiac function was markedly improved in the MIRI+EA-pre group compared to the MIRI group, whereas no significant difference was observed between the MIRI+sEA-pre group and MIRI group (Fig. 1F). To evaluate the direct cardiac effects of EA-pre, ECG, and echocardiography were performed on both the Sham group and Sham+EA-pre group. Results revealed no significant differences between the groups, indicating that EA-pre administration did not compromise cardiac function (Supplementary Fig. 1).
 Fig. 1.
Fig. 1.
EA-pre alleviated MIRI in cardiac function. (A) Experimental
timeline. (B) Typical ECG curves were compared across all groups. (C) Comparison
of ST deviation in different groups. (D) Comparison of LF/HF ratios between
groups. (E) Comparison of representative M-mode echocardiograms between groups.
(F) Quantitative analysis of echocardiographic measurements. (C,D,F) Data are
presented as mean
Evans blue/TTC double staining showed similar AAR across all groups. It was interesting that the ratio of IA/AAR was significantly higher in the MIRI group than in the MIRI+EA-pre group, indicating greater myocardial damage in the absence of EA-pre (Fig. 2A,B). HE staining and Masson staining revealed elevated myocardial-tissue damage in the MIRI group relative to the sham group. This damage included uneven arrangement of cardiomyocytes, severe cellular edema, myocardial-fiber breakage, and a large infiltration of inflammatory cells, as a previous study reported [37]. Myocardial tissue damage was notably attenuated in the MIRI+EA-pre group (Fig. 2C,D). Correspondingly, the MIRI procedure markedly increased the levels of myocardial injury biomarkers cTnT and CK-MB [38]. Additionally, NE serves as a sensitive indicator of early myocardial sympathetic excitation, and large amounts of NE are released from sympathetic nerve endings during myocardial ischemia [39]. Consistently, the MIRI+EA-pre group exhibited a significant reduction in the expression of myocardial injury markers (Fig. 2E–G). These findings indicated that EA-pre potently alleviated the severity of symptoms, possibly by modulating sympathetic nerve activity after MIRI.
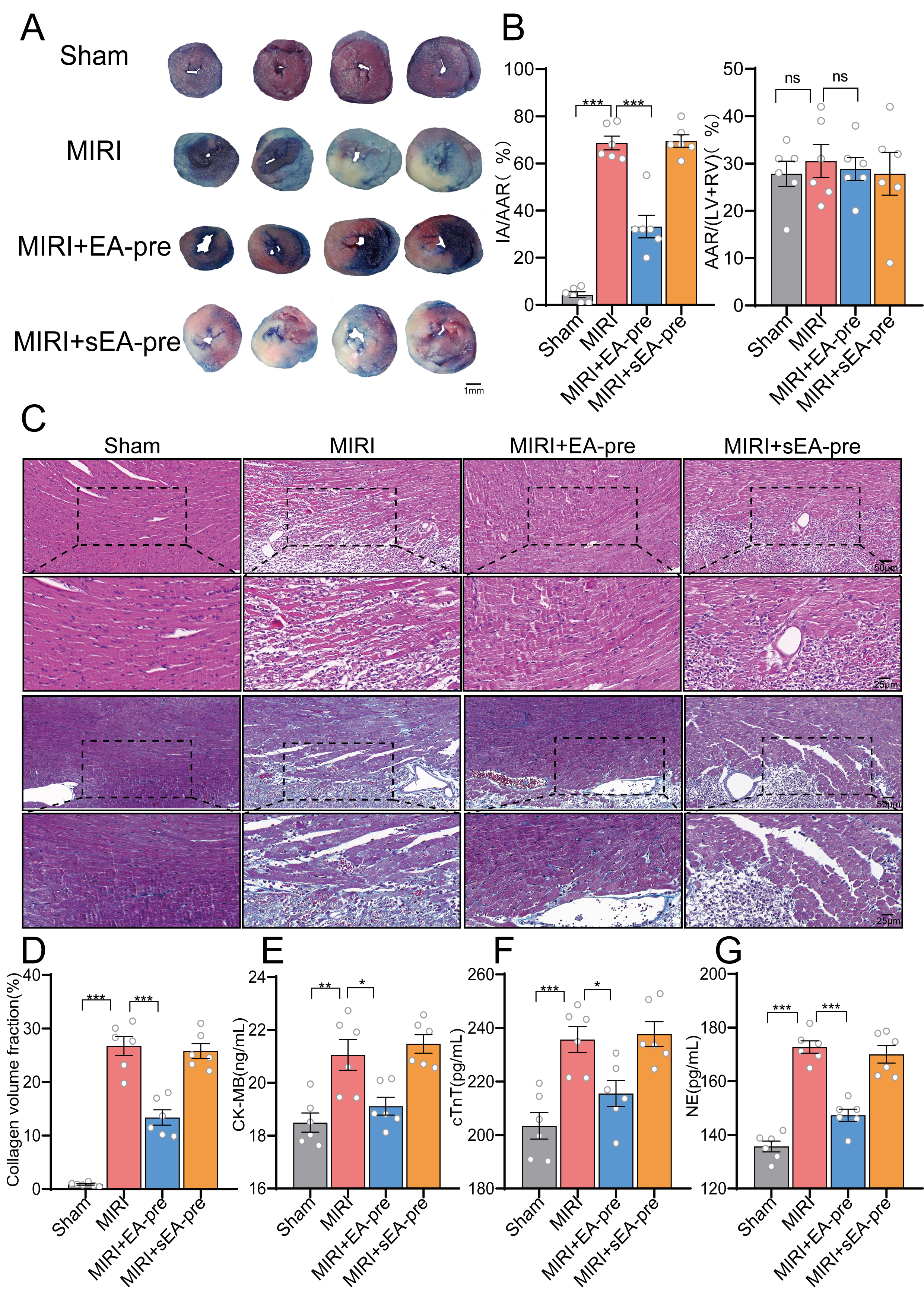 Fig. 2.
Fig. 2.
EA-pre attenuated the extent of cardiac tissue damage in MIRI
mice. (A) Representative images of mouse hearts double-stained with TTC and
Evans blue for determining infarcted areas, scale bar, 1 mm. (B) Ratios of
infarcted area to area at risk and percentage of area at risk. n =
6/group, one-way ANOVA with Tukey’s post-test. (C,D) HE and Masson staining of
myocardial tissue. Scale bar, 50, 25 µm. (E–G) Serum levels of CK-MB,
cTnT, and NE across groups. Data are presented as mean
To investigate changes in Crus Ⅰ neuron activity in MIRI mice, in vivo
electrophysiological recordings of Crus Ⅰ neuron firing were performed (Fig. 3A).
First, well-separated neurons were classified by their spiking characteristics
into WS (trough to peak duration 360.3105
 Fig. 3.
Fig. 3.
Firing characteristics of Crus I neurons in MIRI mice. (A)
Schematic of in vivo mouse electrophysiological experiments with Crus I
electrodes. (B) Classification of recorded Crus I neurons into putative pyramidal
neurons (orange triangles) and F-INs neurons (blue circles) based on peak
duration and firing frequency. (C) Representative spike waveforms from EPs and
F-INs neurons. (D) Comparison of firing frequencies in Crus I EPs and F-INs
neurons across groups. (E) Sample traces of action potentials were recorded from
Crus I EPs and F-INs neurons across various groups. (F) Comparison of Crus I
energy spectrograms across different groups. (D) Data are presented as mean
To examine the impact of EA-pre on Crus Ⅰ, the Fos-TRAP labeling technique was used to identify the specific types of neurons in Crus Ⅰ that contribute to the attenuation of MIRI by EA-pre. Recombinant adeno-associated viruses (rAAV) carrying c-Fos: tetracycline-controlled transactivator (tTA) and tetracycline-responsive element (TRE), were injected into Crus Ⅰ (Fig. 4A). Comparison of mCherry expression/mm2 of neurons across different groups was conducted to validate the Fos-TRAP reliability (Fig. 4B). The fluorescence results indicated that EA-labelled active neurons were regularly distributed in one layer (Fig. 4C). Dox could effectively repress the expression of the TRE promoter (Fig. 4D). Based on the neuronal morphology of the cerebellar cortex, we hypothesized that these EA-labelled active neurons were PC, which received modulation from interneurons [42, 43]. Collectively, these neurons contribute to the modulation of the cerebellar cortex [44].
 Fig. 4.
Fig. 4.
Labeling of Crus Ⅰ neurons activated by electroacupuncture
stimulation. (A,B) Schematic of Fos-TRAP—based labeling of Crus Ⅰ neurons. (C)
Representative mCherry expression in Crus Ⅰ across groups, Scale bar, 100
µm, 50 µm. (D) Comparison of the number of mCherry-expressing neurons in Crus Ⅰ in
each group. Data are presented as mean
To investigate the role of Crus ⅠPC in EA-pre mitigation of MIRI, a recombinase adenovirus carrying an L7-specific promoter (rAAV-L7-mCherry) was injected bilaterally to label PC in Crus Ⅰ. Immunofluorescence analysis revealed that PC were labelled successfully (Fig. 5A). Subsequently, we performed different interventions on Crus ⅠPC in combination with CNO to control the activities of PC (Fig. 5B). ECG analysis indicated significantly elevated S-T segment deviation values and LF/HF ratio in the mCherry+MIRI group during MIRI, which were decreased with EA-pre intervention. Furthermore, the mCherry+MIRI group exhibited elevated levels of both parameters relative to the hM4Di+MIRI group, whereas the mCherry+EA-pre+MIRI group showed reduced values compared to the hM3Dq+EA-pre+MIRI group (Fig. 5C,D). Echocardiographic analysis indicated a pronounced reduction in cardiac function in the mCherry+MIRI group compared with the mCherry+Sham group. By contrast, the mCherry+EA-pre+MIRI group notably improved cardiac performance relative to the mCherry+MIRI group. Cardiac function demonstrated significant enhancement in the hM4Di+MIRI group relative to the mCherry+MIRI group, but substantial impairment was observed in the hM3Dq+EA-pre+MIRI group compared with the mCherry+EA-pre+MIRI group (Fig. 5E,F).
 Fig. 5.
Fig. 5.
Chemogenetic manipulation of Crus Ⅰ𝐏𝐂 neuronal activity in
MIRI. (A) Schematic diagram of protocol and injection site for viral injections. Scale bar, 200
µm. (B) Experimental strategy for chemogenetic regulation of Crus ⅠPC. (C) Group
comparisons of ST deviation. (D) Intergroup differences in LF/HF ratio. (E)
Representative M-mode echocardiograms for each group. (F) Echocardiographic
measurements across groups. Data are shown as mean
Consistent with previous findings, Evans Blue/TTC double staining revealed that EA-pre effectively reduced the IA/AAR ratio in mice subjected to MIRI. Specifically, the hM4Di+MIRI group exhibited a lower IA/AAR ratio than did the mCherry+MIRI group, and the mCherry+EA-pre+MIRI group also showed a lower IA/AAR ratio than did the hM3Dq+EA-pre+MIRI group (Fig. 6A,B). HE staining and Masson staining results also verified these findings (Fig. 6C,D). ELISA results revealed markedly increased levels of CK-MB, cTnT, and NE in the mCherry+MIRI group relative to the mCherry+Sham, mCherry+EA-pre+MIRI, and the hM4Di+MIRI groups. The hM3Dq+EA-pre+MIRI group showed significantly higher CK-MB, CTnT, and NE than the mCherry+EA-pre+MIRI group (Fig. 6E,F,G). In conclusion, our findings indicated that EA-pre enhanced cardiac function in MIRI mice by mitigating sympathetic nerve injury, achieved through the inhibition of Crus ⅠPC.
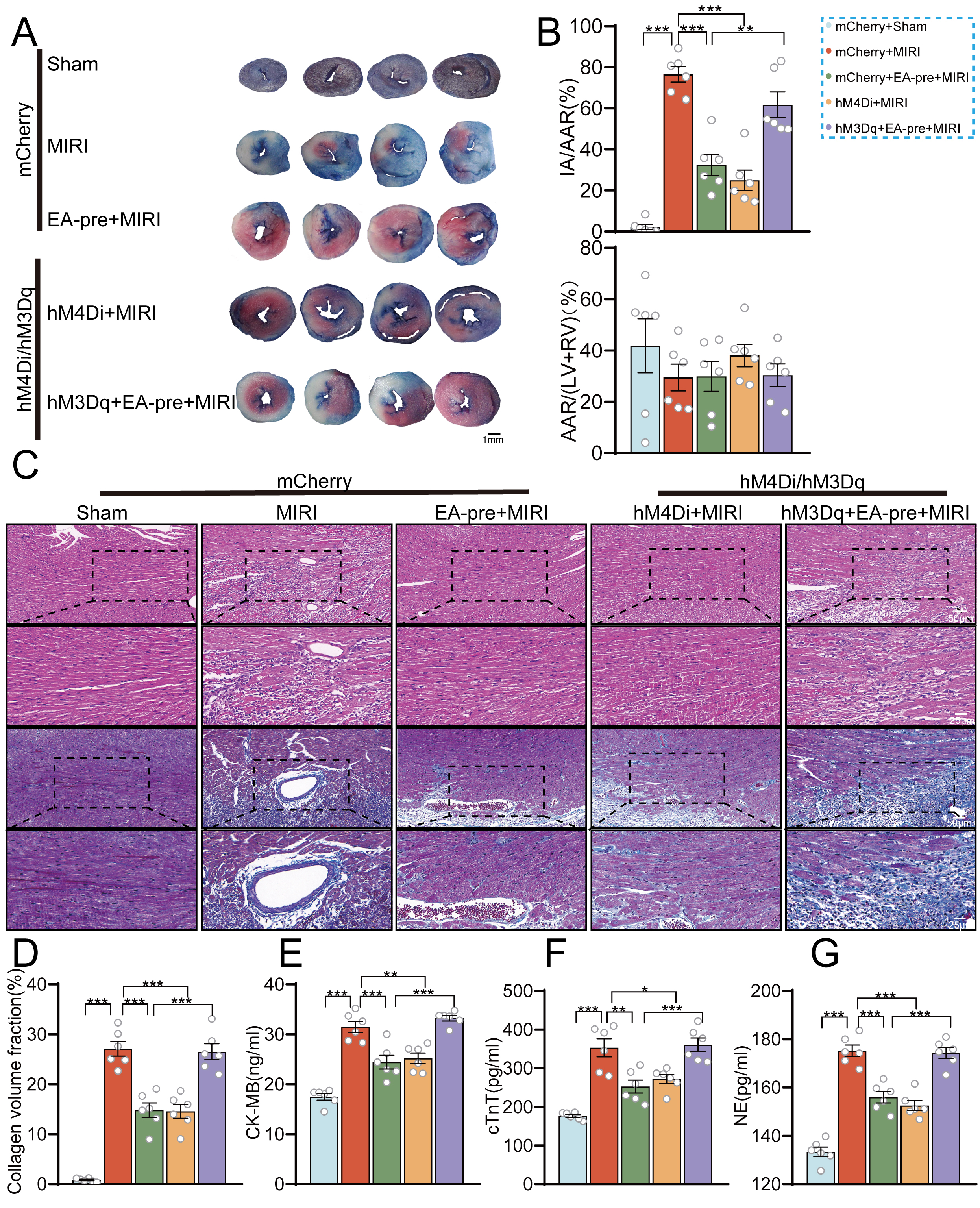 Fig. 6.
Fig. 6.
Effects of chemogenetic manipulation of neuronal activity in
Crus I𝐏𝐂 on cardiac tissue damage in MIRI mice. (A) Representative images
of mouse heart double-stained with TTC and Evans blue for determining infarcted
areas, scale bar, 1 mm. (B) Ratios of infarcted area to area at risk and
percentage of area at risk. n = 6/group, one-way ANOVA with Tukey’s
post-test. (C,D) HE and Masson staining of myocardial tissue. Scale bar, 50, 25
µm. (E,F,G) Serum levels of CK-MB, cTnT, and NE across groups. Data are
presented as mean
The objective of this study was to explore the modulatory role of Crus Ⅰ in the mitigation of MIRI by EA-pre intervention. We confirmed the efficacy of EA-pre in mitigating MIRI with electrocardiography, echocardiography, and myocardial-tissue staining, followed by in vivo electrophysiological recordings of Crus Ⅰ neuron activity. These recordings demonstrated elevated Crus Ⅰ neuron activity in MIRI mice, which was inhibited by EA-pre, reducing the activity of Crus Ⅰ interneurons. Furthermore, Fos-TRAP labeling of neurons responsive to EA stimulation, combined with morphological and distributional analyses of cortical neurons, indicated that Crus ⅠPC plays a crucial role during EA intervention. Last, inhibition of PC activity regulated cardiac sympathetic nerve activity, decreased the NE level in the MIRI mice, and attenuated myocardial injury, thereby protecting cardiac function in MIRI mice.
Previous studies have demonstrated that the cerebral cortex and cerebellum engage in reciprocal interactions [15]. The cerebral cortex transmits information to the cerebellar cortex, and the cerebellar output feeds back to the cerebral cortex via the thalamus [45]. Additionally, sustained cortical activity relies on projections from the cerebellar nuclei to the neocortex [46, 47]. Crus Ⅰ has a key role in cortical brain circuits, especially in depression, cognition and sensation [48, 49]. The PC are the main neurons in the neural network of the cerebellar cortex and have an information integration function [50]. Not only do they receive projections from other neurons, but they are also the only output neurons of the cerebellar cortex [51]. Chronic stress stimulation decreased Crus ⅠPC discharge [52]. Sensory information inputs to the cerebellar cortex were found to encode sensory information in a manner that suspend the discharge of PC, and then send commands to the deep cerebellar nuclei, which in turn regulate the physiological functions of the organism [53, 54]. This was consistent with our findings that PC in Crus Ⅰ served as critical neurons in the therapeutic efficacy of EA. We speculated that inhibition of PC activity may represent a kind of resetting or zeroing of information processing in preparation for the integration of new instructions or sensory information.
Brain-heart interactions have been a focus of scientific research in recent
years. Research has indicated that individuals afflicted with heart failure
exhibit impaired functional responses in the cerebellar cortex and diminished
autonomic regulation [55, 56]. Optogenetic activation of cerebellar PC triggered
synchronized excitation of calcium/calmodulin-dependent kinase II (CaMKII)
The protective role of EA as a peripheral stimulus in the alleviation of cardiovascular disease through the autonomic and central nervous systems has been a subject of study [61, 62]. In addition to MIRI, EA has also been shown to exert adjunctive therapeutic effects on atrial fibrillation, myocardial ischemia, and chronic stable angina pectoris [13, 63, 64, 65]. Concurrently, research findings have indicated that EA-assisted treatment of visceral diseases necessitates central nervous system modulation, encompassing the fastigial nucleus, the lateral hypothalamic area, the paraventricular nucleus of the hypothalamus, and the nucleus of the solitary tract [61, 66]. However, the neuro-modulatory network of sensory stimulation remains to be elucidated. The present study proposed a potential target of neuro-mediated therapy of cardiovascular disease, demonstrating that inhibition of Crus ⅠPC activity by EA partly ameliorated myocardial injury in MIRI.
First, this study used only male C57 mice, and the conditions for expressing the recombinant adenovirus with the L7 promoter are limited. Future studies will consider using L7-cre mice. Second, cerebellar cortex neurons exhibit complex types with distinct morphological features. Their projection patterns, transmitter types, and firing properties require further investigation. Last, the present study tentatively suggested that Crus Ⅰ is involved in the alleviation of MIRI by EA-pre. However, the role of Crus Ⅰ in modulating deep cerebellar nuclei in MIRI was not further investigated. This aspect will receive prioritized attention in our subsequent studies.
In conclusion, EA-pre has been demonstrated to inhibit the activity of PC in Crus Ⅰ, thereby inhibiting sympathetic excitation and exerting a myocardial protective effect, in addition to attenuating MIRI and improving cardiac function. The mechanism highlights the importance of sensory information processing in the cerebellar cortex. Consequently, Crus ⅠPC may represent a viable target for EA therapy in the intervention of cardiovascular diseases.
Further information and requests for resources and reagents could be directed to and will be fulfilled by the Lead Contact, Rong-lin Cai (ronglincai@ahtcm.edu.cn).
YW: data curation, experiments, writing and editing manuscript; WJS: experiments, writing; HMC: experiments; QS, XZ, NXW: project conceptualization and administration; BZ: experiments, writing and editing manuscript; FZ, LH, QY, RLC: funding acquisition, experimental design, project conceptualization and supervision. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Animals were housed in the laboratory animal room of Anhui University of Chinese Medicine with alternating lighting. Experiments followed followed by cervical dislocation as a confirmatory measure per AVMA guidelines and were approved by the Animal Ethics Committee of Anhui University of Chinese Medicine (No. AHUCM-mouse-2022083).
Not applicable.
Rong-lin Cai was supported by the National Natural Science Foundation of China 82575235 and 82074536, Distinguished Young Youth Scientific Research Project in Universities of Anhui Province 2022AH020043, Research Funds of Center for Xin’an Medicine and Modernization of Traditional Chinese Medicine of IHM 2023CXMMTCM019. Qing Yu was supported by National Natural Science Foundation of China 82104999, Excellent Young Youth Scientific Research Project in Universities of Anhui Province 2022AH030062, Open Project of Anhui Province Key Laboratory of Meridian Viscera Correlationship AHMVC2024001.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/JIN44383.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
