- Academic Editor
†These authors contributed equally.
Objectives: The majority of neuromyelitis optica spectrum disorders (NMOSD) patients are seropositive for aquaporin-4 (AQP4)-specific antibodies [also named neuromyelitis optica immunoglobulin G antibodies (NMO-IgG)]. Although NMO-IgG can induce pathological changes in the central nervous system (CNS), the immunological changes in the CNS and peripheral tissue remain largely unknown. We investigated whether NMO-IgG binds to tissue expressing AQP4 and induces immunological changes in the peripheral tissue and CNS. Methods: C57BL/6 female mice were assigned into an NMOSD or control group. Pathological and immunological changes in peripheral tissue and CNS were measured by immunostaining and flow cytometry, respectively. Motor impairment was measured by open-field test. Results: We found that NMO-IgG did bind to astrocyte- and AQP4-expressing peripheral tissue, but induced glial fibrillary acidic protein and AQP4 loss only in the CNS. NMO-IgG induced the activation of microglia and modulated microglia polarization toward the classical (M1) phenotype, but did not affect innate or adaptive immune cells in the peripheral immune system, such as macrophages, neutrophils, Th17/Th1, or IL-10-producing B cells. In addition, NMOSD mice showed significantly less total distance traveled and higher immobility time in the open field. Conclusions: We found that injection of human NMO-IgG led to astrocytopathic lesions with microglial activation in the CNS. However, there were no significant pathological or immunological changes in the peripheral tissues.
Neuromyelitis optica spectrum disorders (NMOSD) are inflammatory demyelinating disorders of the central nervous system (CNS) [1]. Although the incidence of NMOSD is relatively low, severe NMOSD attacks can lead to disability and mortality. Aquaporin-4 (AQP4) is highly expressed in astrocytes and peripheral tissue such as kidney collecting duct and skeletal muscle [2]. Most (70%) NMOSD patients are seropositive for AQP4-specific antibodies, which are also referred to as neuromyelitis optica immunoglobulin G antibodies (NMO-IgG) [3, 4].
Current evidence supports the idea that NMO-IgG is pathogenic in NMOSD. For example, the levels of serum NMO-IgG are correlated with the severity of the disease in NMOSD patients, and plasma exchange that depletes NMO-IgG is clinically beneficial [5]. In the past decades, efforts have been made to develop animal models of NMOSD that mimic clinical and pathological features of NMOSD to varying degrees. Most of the reported NMOSD models involve systemic or direct injection of NMO-IgG into experimental animals, which induces neuroinflammation with astrocytopathic lesions and demyelination [6]. Recently, Hillebrand et al. [7] reported that systemic injection of NMO-IgG alone could breach the blood-brain barrier (BBB) and induce pathological changes in the CNS. Taken together, the studies have provided compelling evidence for the pathogenicity of NMO-IgG.
Previous studies concerning the NMOSD model mainly focused on pathological effects in the CNS; the immunological changes in CNS and peripheral tissue induced by NMO-IgG were seldom studied. In the present study, we investigated whether systemically administered NMO-IgG would bind to tissue that expressed AQP4 and induce pathological changes. In addition, the immunological changes induced by NMO-IgG in peripheral tissue and the CNS were examined; we also tested for motor impairment in the treated subjects.
Plasma was obtained from healthy controls and NMOSD patients. AQP4-IgG was assessed by a cell-based indirect immunofluorescence assay. The plasma was obtained from acute NMOSD patients before the administration of any corticosteroids or other immunosuppressive therapy. NMO-IgG and control-IgG were purified using Melon Gel IgG Purification Kit (Thermo Fisher Scientific, Waltham, MA, USA), and further concentrated using Amicon Ultra-15 centrifugal filters (Merck Millipore, KGaA, Darmstadt, Germany). The protein concentration was determined by bicinchoninic acid assay (BCA) (Thermo Fisher Scientific), and the final concentration of IgG was adjusted to 20 mg/mL.
C57BL/6 female mice (18–20 g, 6–8 weeks old, Shanghai Model Organisms Center, Shanghai, China) were used. The NMOSD mouse model was constructed as previously reported [8, 9]. Mice were injected (s.c.) with an emulsion containing 4 mg/mL complete Freund’s adjuvant (BD Difco, Franklin Lakes, NJ, USA) at 4 sites. Then, they were injected (i.p.) with 200 ng pertussis toxin (List Biological Laboratories, Campbell, CA, USA) on days 4 and 7 after immunization. Mice started to receive injections (i.p.) of NMO-IgG on day 0 for 8 consecutive days (5 mg NMO-IgG/day). The control group received injections of adjuvant, pertussis toxin, and the same amount of control-IgG.
Motor impairment was graded by experimental allergic encephalomyelitis (EAE)
score as previously described: stiff tail, 0.5; tail paralysis, 1; limp tail and
waddling gait, 1.5; paralysis of one limb, 2; paralysis of one limb and weakness
of another limb, 2.5; bilateral hind limb paralysis, 3; hind limb paralysis with
forelimb weakness, 4; death, 5 [10]. Subtle locomotor abnormalities were assessed
in the open field test (OFT). The OFT apparatus consisted of a plastic cage (420
Mice were euthanized and perfused with phosphate buffer saline (PBS) and 4%
paraformaldehyde. The spinal cords, optic nerves and brains were harvested. The
tissue was cryosectioned and incubated with primary antibodies overnight at 4 °C:
mouse anti-glial fibrillary acidic protein (Cell Signaling Technology, Danvers,
MA, USA), rabbit anti-AQP4 (Sigma-Aldrich, St. Louis, MO, USA), rabbit anti-Iba-1
(Wako, Osaka, Japan). Sections were then washed with PBS and incubated with
appropriate secondary antibody (Thermo Fisher Scientific) for 1 h at room
temperature. Sections were stained with 4
Mice were anesthetized with tribromoethanol (M2940, Aibei biotechnology,
Nanjing, Jiangsu, China) and perfused with cold PBS. The spinal cord and spleen
were isolated and stored in hanks’ balanced salt solution (HBSS) on ice until
processing. The spinal cord was cut with sharp scissors and digested in a water
bath at 37 °C for 30 min. The tissue was then homogenized with a plunger in
fluorescence-activating cell sorter (FACS) buffer and centrifuged at 600
Variables were checked for normality before analysis. Differences between 2
groups were analyzed by a 2-tailed unpaired Student’s t test or
Mann-Whitney U test. Data were presented as mean
To determine the effects of NMO-IgG on motor function, the EAE scoring system
was used. All NMOSD mice showed stiff tails on day 8, with only one NMOSD mouse
showing a waddling gait on day 5, but the motor dysfunction resolved on day 6.
Subtle locomotor abnormalities in NMOSD mice were measured in the OFT. As shown
in Fig. 1, NMOSD mice had significantly less total distance traveled (p
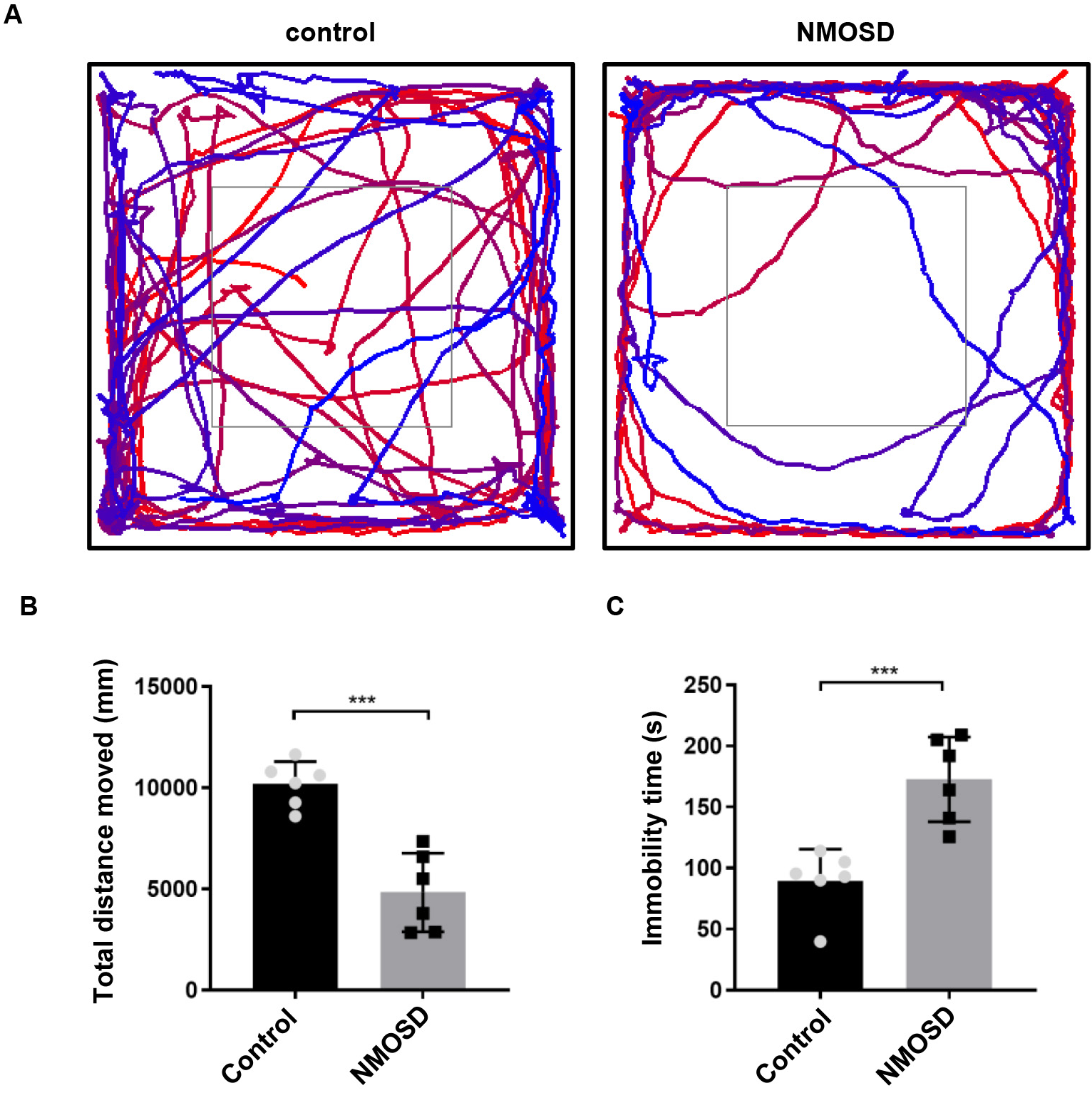 Fig. 1.
Fig. 1.Subtle motor impairments of NMOSD mice detected by open field
test. (A) Graphical representation of the walking paths of control and NMOSD
mice. (B,C) Total distance traveled and immobility time of mice on OFT.
***p
Human IgG staining revealed that NMO-IgG infiltrated the spinal cord parenchyma; the deposition of IgG was mainly restricted to the perivascular space, pericentral canal, and subpial space (Fig. 2A). Furthermore, IgG/GFAP double staining showed that the NMO-IgG bound to astrocytes in the spinal cord parenchyma (Fig. 2B). These results confirmed that systemically administrated NMO-IgG infiltrated the spinal cord parenchyma via the breached BBB.
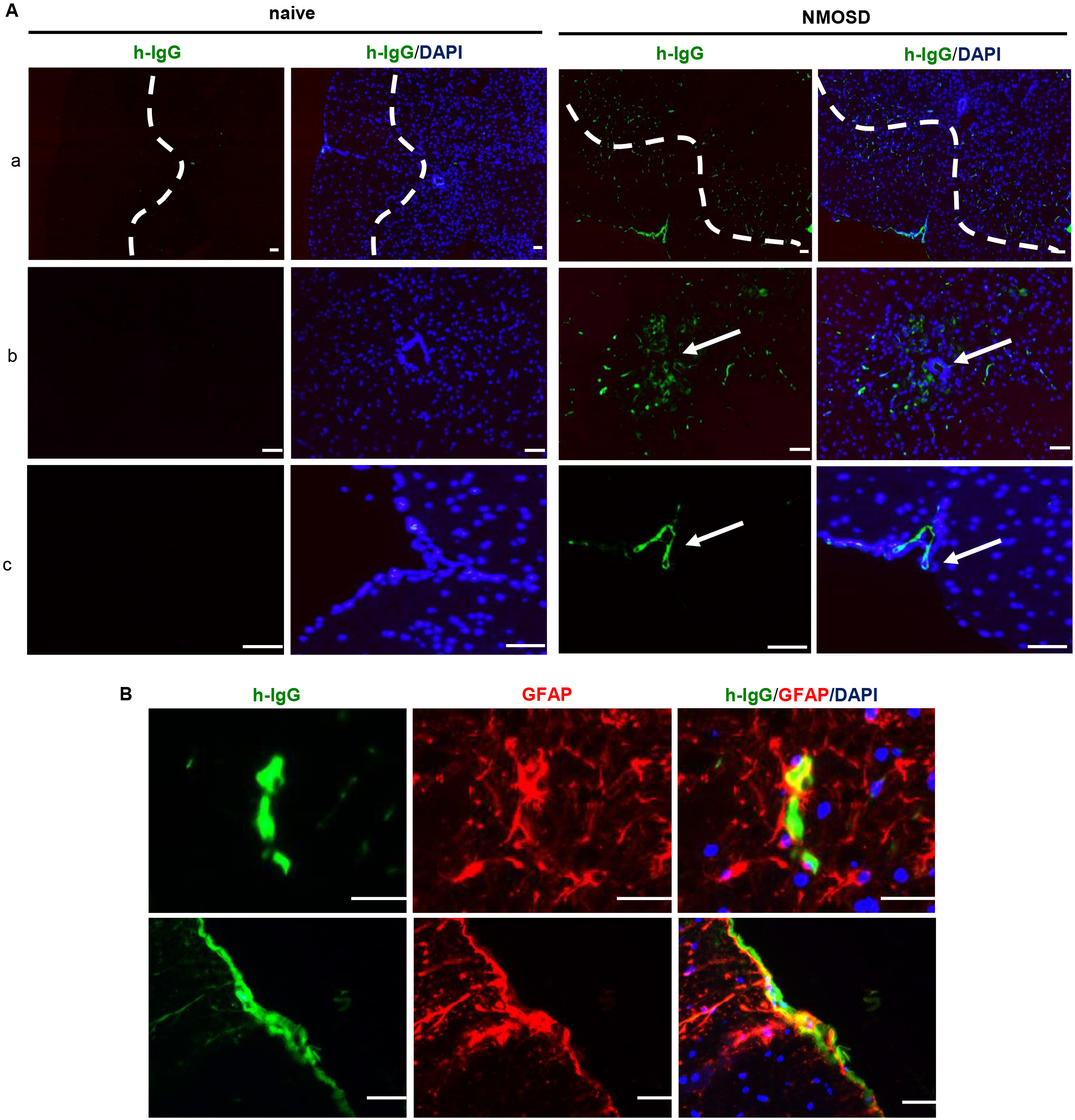 Fig. 2.
Fig. 2.NMO-IgG infiltrated the mouse spinal cord and bound to
astrocytes. (A) Representative photomicrographs of NMO-IgG-infiltrated spinal
cord parenchyma. (a) Deposition of NMO-IgG in the spinal cord parenchyma around
perivascular space; (b) deposition of NMO-IgG in the pericentral canal; (c)
deposition of NMO-IgG in the subpial space. (B) NMO-IgG bound to astrocytes in
NMOSD mice. Scale bar = 50 µm. NMO-IgG, neuromyelitis optica immunoglobulin
G antibodies; DAPI, 4
To examine the effect of NMO-IgG on the expression of AQP4 and GFAP,
immunofluorescence analyses were performed. Results showed that GFAP (p
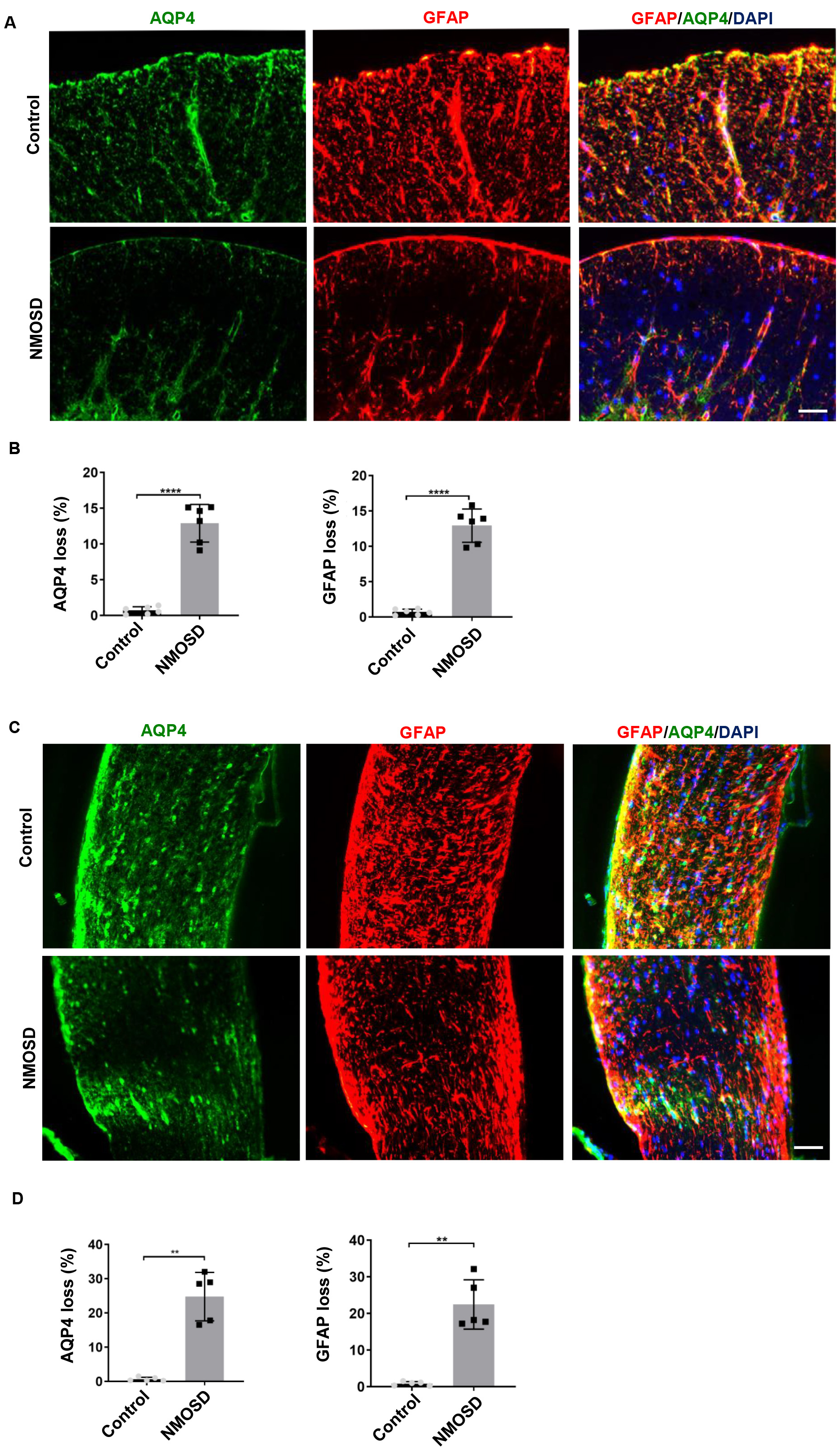 Fig. 3.
Fig. 3.NMO-IgG caused AQP4 and GFAP loss. (A,C) Loss of AQP4 and GFAP
in the spinal cord and optic nerve of NMOSD mice and control mice; (B,D) The
statistical results of the AQP4 and GFAP loss between the two groups, n
= 5–6/gp. **p
Immunostaining revealed markedly higher numbers of Iba-1
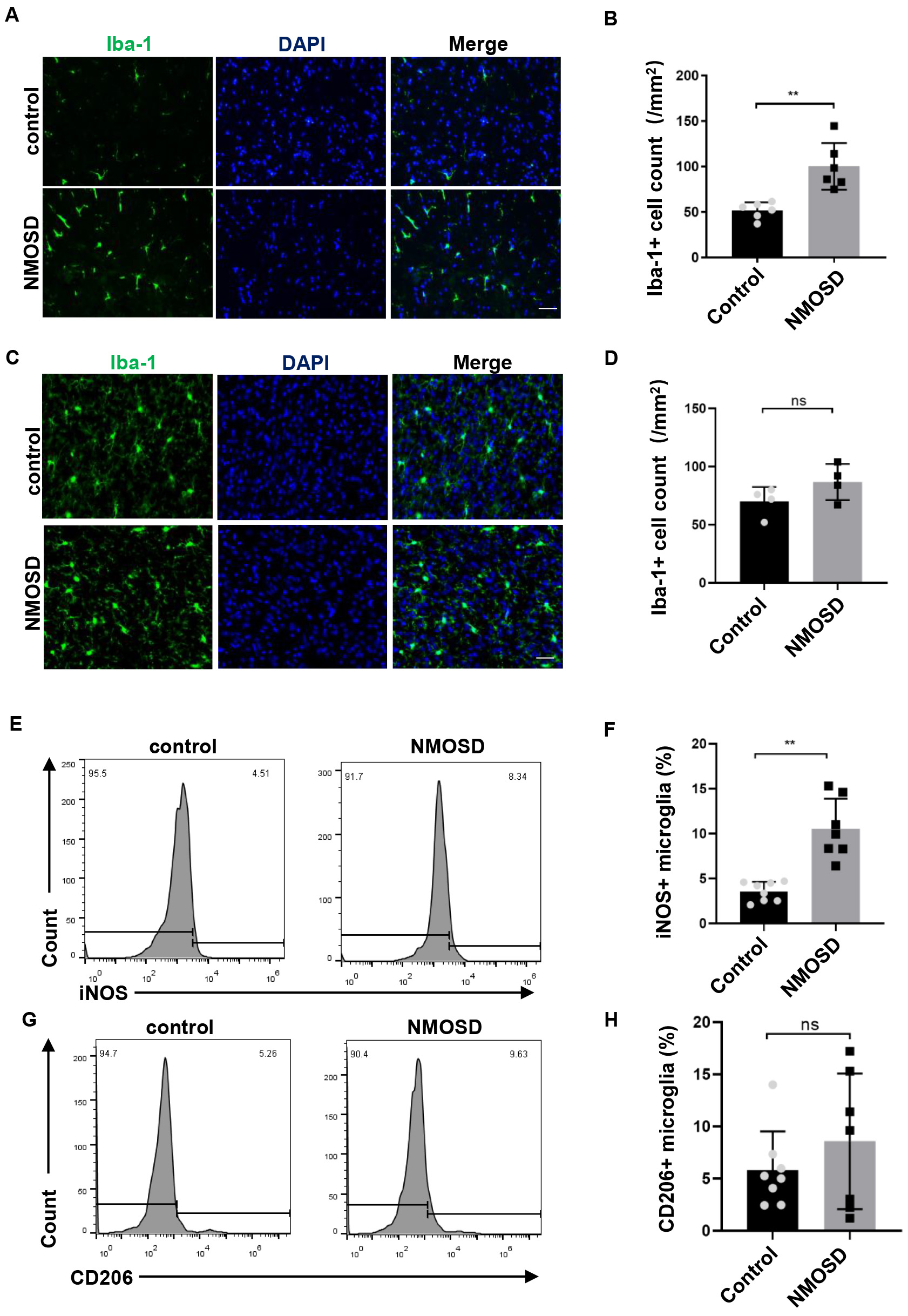 Fig. 4.
Fig. 4.NMO-IgG modulated microglia polarization toward M1. (A,C)
Immunofluorescence staining for Iba-1 in the spinal cord and brain of NMOSD and
control mice, Scale bar = 50 µm; (B,D) The statistical results of the
number of Iba-1
Peripheral distribution of NMO-IgG was evaluated after 8 days of i.p. injections. We examined whether NMO-IgG bound to the peripheral organs that expressed AQP4 and induced changes. As shown in Fig. 5, AQP4 was expressed on the basolateral membrane of the kidney collecting duct and on the plasmalemma of skeletal muscle of naïve mice. IgG/AQP4 double immunostaining revealed that NMO-IgG bound to the basal side of kidney-collecting-duct cells and to the plasmalemma of skeletal muscle in NMOSD mice. However, there was no difference in the AQP4 immunoreactivities between the NMOSD and naïve mice, which indicated that tissue damage was not induced by NMO-IgG.
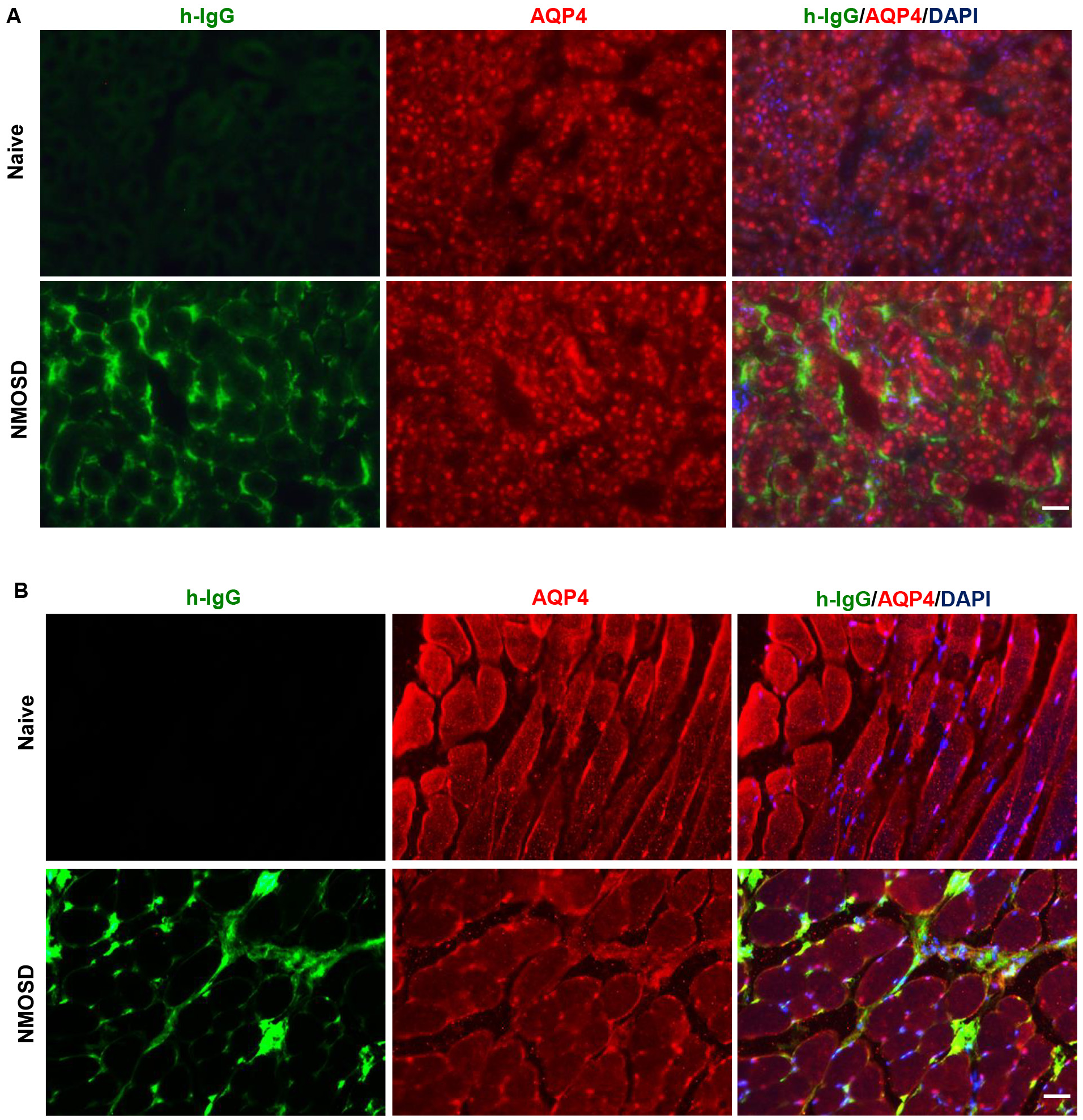 Fig. 5.
Fig. 5.NMO-IgG localization to peripheral tissues that expressed AQP4. (A) IgG/AQP4 double immunostaining revealed NMO-IgG bound to the basal side of kidney collecting duct cells. (B) NMO-IgG localization to plasmalemma of skeletal muscle in NMOSD mice. Scale bar = 50 µm.
To determine the effect of NMO-IgG on peripheral immune cells, we performed flow
cytometry on the spleen. As shown in Fig. 6, no significant differences were
found in the percentage of macrophages/neutrophils of NMOSD and control mice
(p = 0.3446 and p = 0.2128, respectively). Splenocytes were
also stimulated ex vivo for 4 h, and stained with antibodies for the
flow cytometric analysis of Th17/Th1 and IL-10-producing B cells.
We found no significant differences in the percentage of IL-17 (p =
0.4990) and IFN-
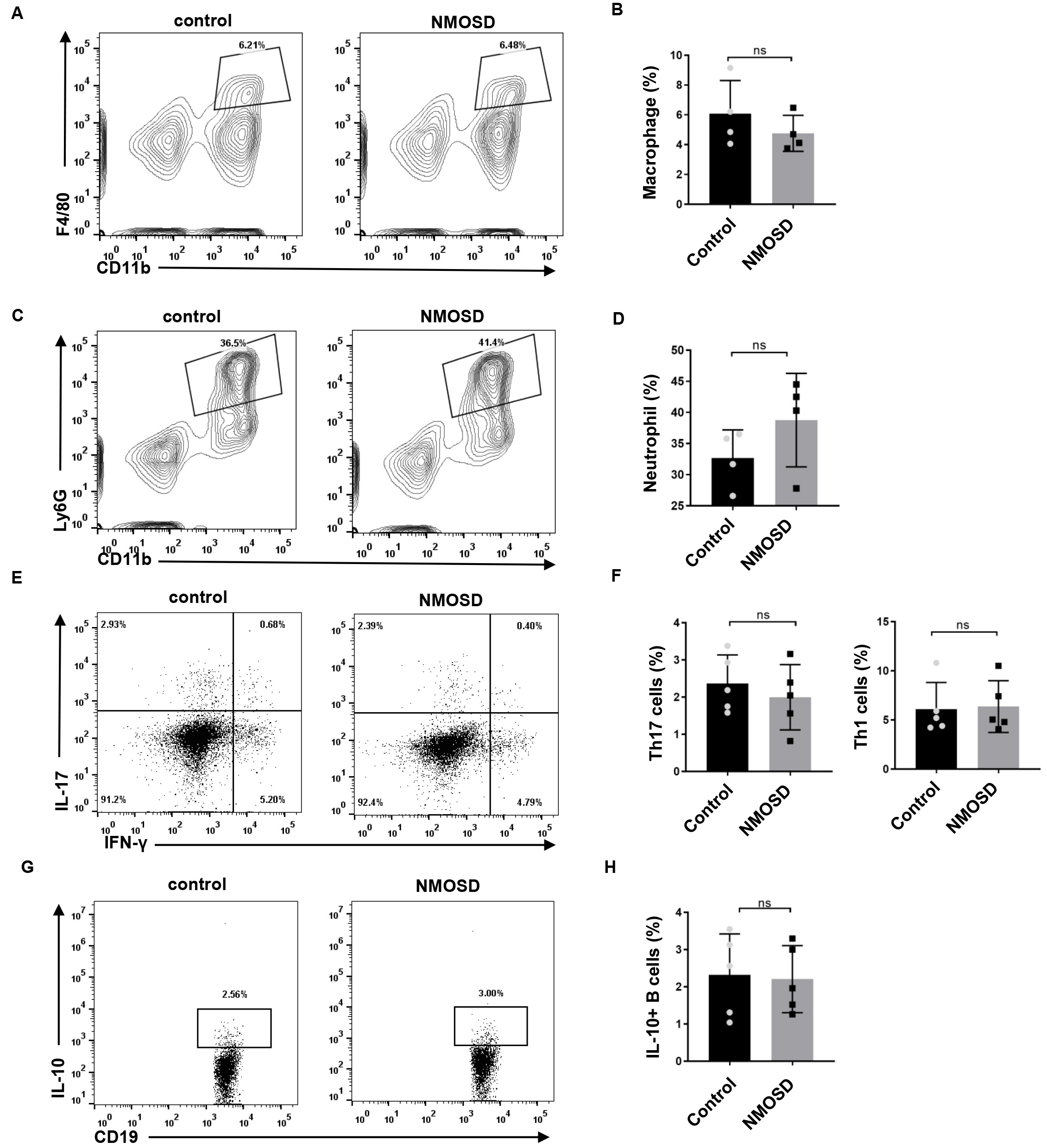 Fig. 6.
Fig. 6.The effect of NMO-IgG on peripheral immune cells. (A–D) The
percentage of CD11b
Current animal models of NMOSD can be divided into two types: systemic-injection models and direct-injection models [6]. In the present study, by using a systemic injection NMOSD model, we revealed that injected NMO-IgG infiltrated the CNS parenchyma and induced GFAP and AQP4 loss. Although NMO-IgG did bind to the peripheral tissue that expressed AQP4, such as kidney and skeletal muscle, it had no effect on AQP4 immunoreactivity in those peripheral tissues. In addition, NMO-IgG triggered activation of microglia and modulated microglia polarization toward M1 in the CNS, but did not affect the percentage of peripheral immune cells.
Our results showed that all NMOSD mice had tiff tails. Consistently, OFT revealed impaired locomotive activity in NMOSD mice; they showed significantly less total distance traveled and greater immobility time than did the control mice. As NMO-IgG did not induce any detectable pathological changes in peripheral AQP4-expressing tissues, the motor abnormalities can be ascribed to the observed astrocytopathic lesions in the spinal cord. Together, the manifestations of NMOSD in the experimental mice recapitulated the mild myelitis observed in some NMOSD patients.
NMO-IgG is rarely detected exclusively in cerebrospinal fluid of NMOSD patients, and NMO-IgG in the CNS was generally thought to originate from circulating peripheral NMO-IgG via a disrupted BBB [11]. Previous studies demonstrated that 24 h after systemic injection, NMO-IgG could be found in the area postrema of mice [12, 13]. In the present study, we found a deposition of NMO-IgG in the spinal cord parenchyma around the perivascular space, pericentral canal, and subpial space. Hillebrand et al. [7] also reported diffused infiltration of NMO-IgG in the CNS. They speculated that NMO-IgG may use three different avenues of entry into the CNS, including meningeal vessels, parenchymal vessels, and circumventricular organs.
Because AQP4 is also highly expressed outside the CNS, we studied NMO-IgG localization in peripheral tissues [2]. Tissue-distribution studies showed that NMO-IgG had bound to the basal side of kidney-collecting-duct cells and to the plasmalemma of skeletal muscle in the NMOSD mice, without affecting the AQP4 immunoreactivities. Consistent with our study, Hillebrand et al. [7] systemically administered highly pathogenic, monoclonal NMO-IgG to rats and found autoantibodies bound to AQP4-expressing peripheral tissue. The difference though, was that they found decreased AQP4 expression in those tissues [7]. This difference may be ascribed to the highly pathogenic, monoclonal NMO-IgG they used, whereas human NMO-IgG is typically polyclonal with different affinities.
Microglia are usually the first responders to pathogen invasion or CNS damage. Emerging evidence has revealed the important role of microglia in NMOSD pathogenesis [14]. There is prominent activation of microglia in the CNS of NMOSD patients, as indicated by the amoeboid morphology and the expression of lysosome marker CD68 [15]. In the present study, there was significantly greater microglial activation in NMOSD mice than in control mice. The observed activation of microglia in NMOSD mice was likely to have been mediated by astrocyte-driven microglial activation. NMO-IgG was reported to have bound to the AQP4 of astrocytes and induced the activation of astrocytes, which in turn drove the activation of microglia; microglial activation was abrogated when NMO-IgG was injected into AQP4 knockout mice [16]. Under different micro-environmental disturbances, the activated microglia could polarize into either the classical (M1) or alternative (M2) phenotype, and exert a pro-inflammatory or anti-inflammatory role, respectively [17]. In the present study, we found that NMO-IgG induced microglia polarization toward M1, which may have participated in the pathological role of NMO-IgG in NMOSD mice.
Autoantibodies can trigger a diverse set of effector responses in peripheral myeloid cells, such as neutrophils and macrophages. In the present study, we examined whether splenic macrophages and neutrophils could be activated in response to human NMO-IgG. We observed no significant difference in the percentage of macrophages or neutrophils between the two groups. Consistently, Pellerin et al. [18] reported that anti-myelin oligodendrocyte glycoprotein antibodies triggered the activation of microglia but not the activation of peripheral macrophages. The frequency of Th17 cells and IL-10-producing B cells in NMOSD patients was reported to be significantly different from that of healthy controls [19, 20, 21, 22]. However, our results showed no statistical differences in these cells between NMOSD and control mice, which may be explained by the fact the NMO-IgG were injected, not self-generated by mice.
In the present study, we found that human NMO-IgG injection led to astrocytopathic lesions with microglial activation in the CNS. However, there were no significant pathological and immunological changes in the peripheral tissues.
The datasets generated during the current study are available from the corresponding author on reasonable request.
WWX: Conceptualization, Methodology, Investigation, Writing - original draft. KW: Conceptualization, Methodology, Investigation. SWB: Conceptualization, Methodology, Investigation. ZW: Visualization, Investigation. LH: Visualization, Investigation. JP: Visualization, Investigation. CX: Conceptualization, Supervision, Writing - review & editing. YTG: Conceptualization, Supervision. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was reviewed and approved by Ethics Committee of the Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (KY2021-137-B). The provided the written informed consents of patients/participants were obtained. All experimental procedures were approved by the Shanghai Model Organisms Center’s Ethical Committee.
Not applicable.
This work was supported by the National Natural Science Foundation of China (81801195), Innovative research team of high-level local universities in Shanghai (SHSMU-ZDCX20211901) and Shanghai “Rising Stars of Medical Talents” Youth Development Program.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
