- Academic Editor
Background: The effects of heat acclimation (HA) on the hypothalamus
after exertional heatstroke (EHS) and the specific mechanism have not been fully
elucidated, and this study aimed to address these questions. Methods: In
the present study, rats were randomly assigned to the control, EHS, HA, or HA +
EHS groups (n = 9). Hematoxylin and eosin (H&E) staining was used to examine
pathology. Tandem mass tag (TMT)-based
proteomic analysis was utilized to explore the impact of HA on the protein
expression profile of the hypothalamus after EHS. Bioinformatics analysis was
used to predict the functions of the differentially expressed proteins. The
differential proteins were validated by western blotting. An enzyme-linked
immunosorbent assay was used to measure the expression levels of inflammatory
cytokines in the serum. Results: The H&E staining (n = 5) results
revealed that there were less structural changes in hypothalamus in the HA + EHS
group compared with the EHS group. Proteomic analysis (n = 4) revealed that
proinflammatory proteins such as argininosuccinate synthetase (ASS1), high
mobility group protein B2 (HMGB2) and vimentin were evidently downregulated in
the HA + EHS group. The levels of interleukin (IL)-1
Heatstroke (HS), a life-threatening condition, is characterized by an
unregulated increase in core body temperature above 40 °C and central
nervous system (CNS) dysfunction manifested as delirium, convulsions or coma [1].
Exertional heatstroke (EHS), the most serious type of heat stroke, usually occurs
when individuals are challenged by intense physical activity with/without
exposure to high temperature. EHS is regarded as one of the leading causes of
death among athletes and military personnel worldwide [2, 3, 4]. Excessive endogenous
heat produced by muscle contraction can overwhelm the temperature regulation
system and cause EHS. A hot and humid environment blunts heat transfer processes
from the skin to the air and can further raise the risk of EHS [5]. Hence, the
combination of physical activity with external environmental factors results in
continuous body heat storage and places tremendous stress on human tissues [6].
When the thermoregulatory system is insufficient to compensate for hyperthermia,
the core body temperature continues to rise, triggering direct cytotoxic effects
and the inflammatory response, eventually causing multiple-organ
dysfunction/injury syndrome (MODS) [7, 8]. The inflammatory response plays an
essential role in MODS induced by heatstroke. Heatstroke induces high levels of
proinflammatory cytokines, especially interleukin (IL)-1 and IL-1
The hypothalamus plays an important role in the CNS and is responsible for thermoregulatory responses [16, 17]. The hypothalamus preoptic area receives thermal sensory information and exits signals to mediate autonomic responses to increase heat loss and thereby activate heat defense mechanisms [18]. However, extreme heat stress contributes to thermoregulatory dysfunction in the hypothalamus, which in turn drives or exacerbates the process of heatstroke. Studies have shown that direct heat stress, neurological inflammation, ischemic and oxidative damage, overload of thermoregulation and increased monoamine neurotransmitter levels are factors leading to hypothalamus impairment during severe heat shock [12, 13, 19, 20]. In addition, some studies utilized proteomics to identify the specific proteins associated with heat stroke-mediated hypothalamic dysfunction and explored the roles of the proteins. These findings provide information on the exact molecular mechanisms underlying heatstroke-induced hypothalamic injury [19, 20]. However, these results were all based on classic heatstroke models, and whether the proteomic approach is applicable for investigating the effects of heatstroke on the hypothalamus needs to be further verified.
Heat acclimatization/acclimation (HA) is one of the most effective methods for reducing HS risk, especially as an achievable preventive measure for EHS, which generally occurs during planned occupational and sporting activities [21, 22]. Exercise-heat acclimatization achieved by repeated heat exposure coupled with physical exercise has been widely practiced among military personnel and athletes [23, 24]. HA has been shown to modulate thermoregulatory activity and thus augment heat tolerance. This process involves an increase in the heat stress response by increasing heat shock protein (HSP) expression and changes in the expression of genes associated with energy metabolism and immune responses [25, 26, 27, 28, 29]. It is well documented that HA training before engaging in strenuous physical exercise lowers the likelihood of acquiring EHS [30]. We successfully developed an HA rat model and verified its beneficial role in preventing EHS-mediated injury to the cerebral cortex. However, the effect of HA on the hypothalamus after EHS and its mechanism of action have rarely been reported. To solve the above problems, we first examined whether HA could mitigate HS-induced hypothalamus damage. Then, from the perspective of proteomics, we analyzed and compared the hypothalamic protein expression profiles of the EHS model rats and the EHS model rats after HA to explore the potential mechanism through which HA improved hypothalamus damage after EHS. The results might offer fresh perspectives and new targets for the development of efficient therapeutic medications and other treatment options for EHS.
In the present study, we hypothesized that HA is a protective agent that can alleviate the negative influence of EHS by modifying the hypothalamic proteome. To test our hypothesis, we established control, EHS, HA, and EHS + HA groups and detected pathological changes in the structure of the hypothalamic tissues in each group. The differentially expressed proteins between the EHS and HA + EHS groups were identified via proteome analysis. Using functional enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, we obtained additional information regarding the mechanism underlying hypothalamic injury in EHS and the protective effects of HA on the hypothalamus after EHS. Finally, based on the proteomic analysis, we performed biological verification of these mechanisms at the tissue and serum levels and confirmed that HA might ameliorate the damage triggered by EHS to the hypothalamus by regulating proinflammatory proteins such as argininosuccinate synthetase (ASS1), high mobility group protein B2 (HMGB2) and vimentin to suppress the inflammatory response. Our study aimed to investigate the protective effects of HA against EHS-related hypothalamic disorders and the related mechanism and provide an experimental basis for therapeutic strategies that can treat organ dysfunction after EHS.
Pathogen-free male Wistar rats approximately 7 weeks of age with an initial
weight of 200–250 g were purchased from Sipeifu (Beijing, China) Biotechnology
Co., Ltd. The rats were kept at an ambient temperature of 24
| The ARRIVE Essential 10 | ||
| Study design | 1 | (a) Control groups were included. |
| (b) The experimental unit: a single animal. | ||
| Sample size | 2 | (a) In 21-day HA regimen, rats were divided into the control group and the HA model group, with 5 rats in each group. After the HA model was successfully established, another 36 rats were randomly divided into a control group, HA group, EHS group and HA + EHS group, with 9 rats in each group. Four hypothalamus samples were taken from each group for proteomic analysis, and the remaining 5 intact brain tissues were taken from each group for H&E staining. |
| (b) The sample size was decided according to sample size calculation, previous researches and practical conditions. G.Power was used to calculate the sample size. The details were described in the statistic paragraph. | ||
| Inclusion and exclusion criteria | 3 | (a) Including criteria: pathogen-free male Wistar rats approximately 7 weeks of age with an initial weight of 200–250 g; excluding criteria: Rats found to have health problems or abnormal behavior and rats that died before or during the experiment. |
| (b) No animals were excluded from this study. | ||
| (c) The value of n in each group were reported exactly. | ||
| Randomisation | 4 | (a) Randomisation was used to allocate experimental units to control and treatment groups. All the experiments were double-blind and randomized using a random number table. |
| (b) To minimize potential confounders, all rats were kept at an ambient temperature of 24 | ||
| Blinding | 5 | All the experiments were double-blind. Third party personnel other than observers and study subjects was aware of the group allocation at the different stages of the experiment (during the allocation, the conduct of the experiment, the outcome assessment, and the data analysis). |
| Outcome measures | 6 | The changes in T |
| Statistical methods | 7 | (a) The details of the statistical methods used for each analysis and the software used were provided in Section 2.7. |
| (b) The Shapiro-Wilk test was used to determine the normal distribution of the data. Levene’s test was used to determine the homogeneity of variance. | ||
| Experimental animals | 8 | The details of the animals used in the study described in Section 2.1. |
| Experimental procedures | 9 | The procedures in enough detail were provided in the Materials and Methods. |
| Results | 10 | Summary statistics for each experimental group presented as the mean |
T
In contrast to the heat acclimation model, which involves only
a high-temperature environment without exercise, we propose a new HA training
scheme in which rats are subjected to exercise simultaneously in a hot
environment and verify the feasibility of this scheme for use with rats. The rats
were randomly divided into a control group and an HA model group (n = 5).
Sterile Body Cape pills
(e-Celsius Performance,
Bodycap company, Paris, France) were inserted into the
abdominal cavity of anesthetized rats at least one week before heat acclimation
training. The rat’s core body temperature (T
The EHS rat model was established according to our previously reported
method [33]. The rats were placed in a prewarmed chamber that was maintained at
39.5
After successfully establishing the 21-day HA model, another 36 rats were randomly and double-blindly divided into 4 groups: the sham
control group (CON; n = 9), exertional heat stroke group (EHS; n = 9), heat
acclimation group (HA; n = 9) and heat acclimation prior to heat stroke group (HA
+ EHS, n = 9). The CON group was exposed to room temperature (24
After the above models were established, hypothalamic tissues were collected (n = 4) under anesthesia, and blood was collected from three of the rats in each group. The rats were anesthetized under 1.5% pentobarbital sodium anesthesia (CAS:57-33-0, Sigma Aldrich, St. Louis, MO, USA) (0.3 mL/100 g) via intraperitoneal injection, the abdominal cavity was opened after disinfection, and the inferior vena cava was exposed. The blood collection needle was subsequently inserted into the blood vessel, after which the blood was collected into coagulant tubes connected to the blood collection needle. Approximately 3 mL of blood was collected per tube. Serum samples were obtained by centrifuging the blood at 3000 rpm for 15 min in a refrigerated centrifuge and then immediately stored at –80 °C. The rats were then decapitated and the brain was quickly harvested and put on ice. A vertical incision (2 mm deep) was made at the optic chiasm close to the mammillary body, enabling the removal of the hypothalamus [26]. Hypothalamic tissues from one side were placed in sterile cryotubes and immediately frozen in liquid nitrogen for Tandem mass tag (TMT)-based proteomic analysis. The hypothalamus specimen from the other side was used for Western blot (WB) analysis. Brain tissues were extracted from another 5 rats and then immersed in 4% paraformaldehyde (PFA) for fixation and subsequent hematoxylin and eosin (H&E) staining.
The brain tissues were fixed with 4% PFA, dehydrated with gradient ethanol and
cleared with xylene, and subsequently embedded in paraffin (Biological Tissue
Embedding Machine, Wuhan Junjie Electronics Co., Ltd., Wuhan, Hubei, China, model
JB-P7). Each brain sample was then sagittally cut (4 µm) using a
rotatory microtome (RM2235, Leica, Witzler, Hesse, Germany). After sectioning,
the paraffin-embedded tissues were dewaxed, rehydrated, stained with hematoxylin
and eosin, dehydrated, cleared and sealed in order [34]. Ten slices of brain
tissue were taken from each rat. The sections were finally observed with an
Olympus optical microscope (Olympus, Shanghai, China). The sections were observed
at 200
The proteomic analysis steps included protein sample pretreatment, enzymolysis, TMT labeling, reversed-phase chromatography, Liquid chromatography-mass spectrometry (LC‒MS) analysis and data analysis; for specific details, refer to the published literature [33].
A tissue grinding machine (HM-24, Huxi Industrial Co., Ltd., Shanghai, China) was used to homogenize the hypothalamus samples from all groups (n = 4) by performing three cycles of 20 seconds each at 6000 rpm. The homogenization was carried out in Radio Immunoprecipitation Assay (RIPA) Lysis buffer (R0020). The lysates were obtained from Solarbio, a biotechnology company located in Beijing, China. Phenylmethanesulfonyl fluoride (PMSF) (P0100, Solarbio, Beijing, China) was added to the lysates. After centrifugation at 12,000 rpm for 15 minutes at 4 °C, the supernatant was collected. The concentration of proteins in the collected supernatant was measured using the Pierce™ Bicinchoninic acid (BCA) Protein Assay Kit (23227, Thermo-Scientific, Waltham, MA, USA).
Protein samples (20 µg) were subjected to electrophoresis on 10% sodium dodecyl sulfate (SDS)‒polyacrylamide gels and subsequently transferred to a Polyvinylidene fluoride (PVDF) membrane with a pore size of 0.22 µm (PR05505, Immobilion-P, Boston, MA, USA). After being blocked with a 5% nonfat milk solution for 2 hours, the membrane was subsequently exposed to the following antibodies: ASS1 (16210-1-AP, rabbit, 1:1000; Proteintech, Wuhan, Hubei, China), HMGB2 (14597-1-AP, rabbit, 1:1000; Proteintech), vimentin (10366-1-AP, rabbit, 1:1000; Proteintech) and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ab8245, mouse, 1:1000; Abcam, Cambridge, UK). Overnight at the following 4 ℃, the membrane underwent incubation in a secondary antibody, which was a conjugate of horse-radish peroxidase (HRP), and specifically targeted rabbit and mouse IgG using goat anti-rabbit and goat anti-mouse IgG (1:10,000, ZB-2301, ZB-2305, ZSGB Biotechnology Co., Ltd., Beijing, China), respectively, in a concentration ratio of 1:10,000, at room temperature for 1 hour and rinsed with triethanolamine buffered saline solution with Tween-20 (TBST) three times. Protein bands were visualized using an enhanced chemiluminescence (ECL) Western Blotting Detection kit (ab133406, Abcam, UK), and X-ray imaging was used visualize the bands. The software ImageJ 5.0 (National Institutes of Health, Bethesda, MD, USA) was used to compute the grayscale values of the bands.
Enzyme-linked immunosorbent assay (ELISA) kits were used to measure the serum
levels of IL-4, IL-1, IL-8, IL-1
All of the data are presented as the mean
The sample size was decided according to sample size
calculation, previous researches and practical conditions. In 21-day HA regimen,
G.Power was used to calculate the sample size, and t tests were used;
the effect sizes were 0.5,
The number of animals chosen for hypothalamus tissue collection and blood
withdrawal were also determined by calculation, reported researches and practical
limitations. G.Power was used to calculate the sample size, and univariate
analysis of variance was used to analyze the data. The results were as follows:
effect size = 0.4,
The results of this section demonstrate the feasibility of our proposed heat
acclimation scheme. The changes in body weight and T
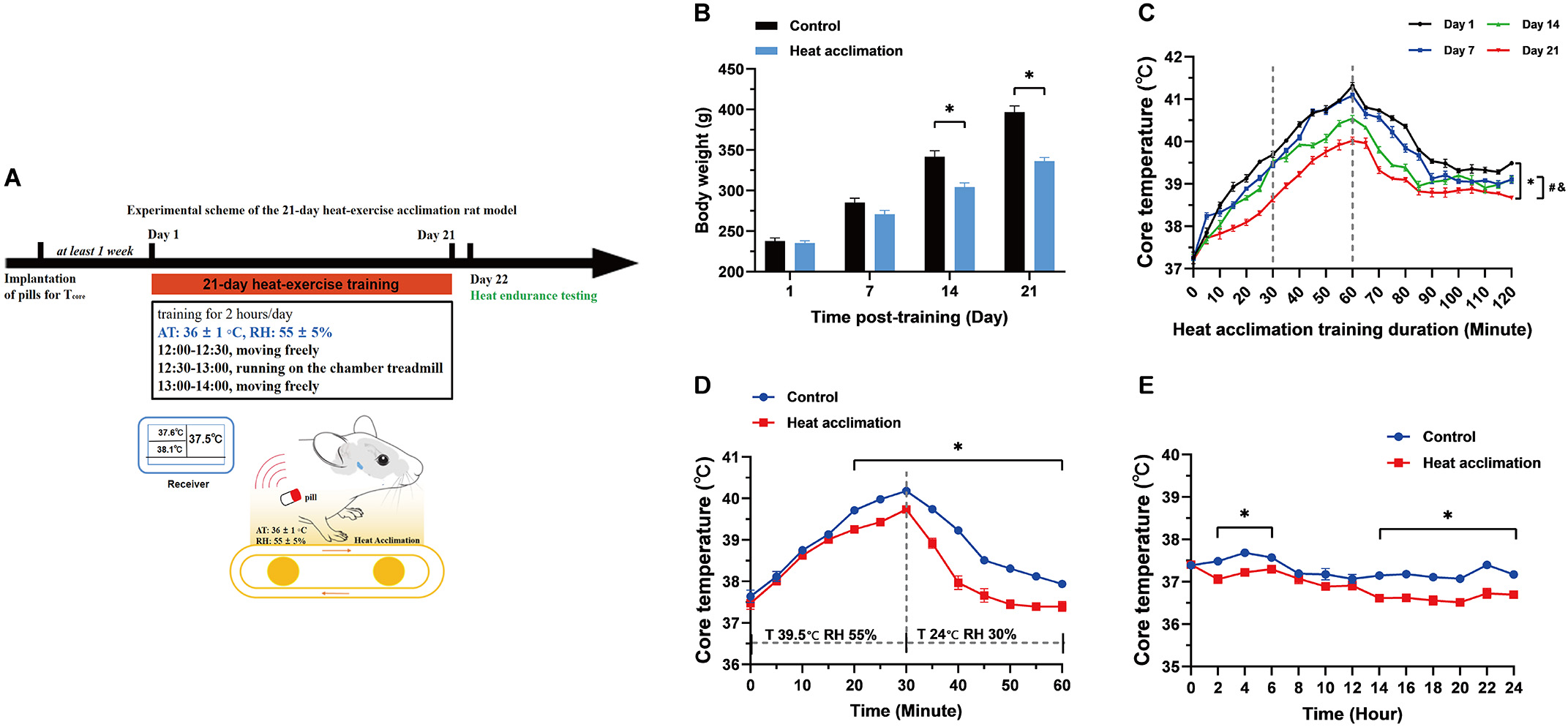 Fig. 1.
Fig. 1.Body weight and core temperature (T
H&E staining was used to visualize the hypothalamic microstructure in each group. Compared with the hypothalamic neurons in the control group (Fig. 2A), those in the EHS group exhibited swelling, loosening of the cytoplasm, widespread vacuolation and nuclear pyknosis (Fig. 2B). In the HA group, nuclear pyknosis and cell swelling were inconspicuous (Fig. 2C). Nuclei exhibiting pyknosis and cellular edema were significantly less severe in the HA + EHS group (Fig. 2D) than in the EHS group. In addition, the number of cells in hypothalamic tissue decreased significantly after EHS (p = 0.000; Fig. 2E), while the number of cells in the HA + EHS group was significantly higher than that in the EHS group (p = 0.006; Fig. 2E).
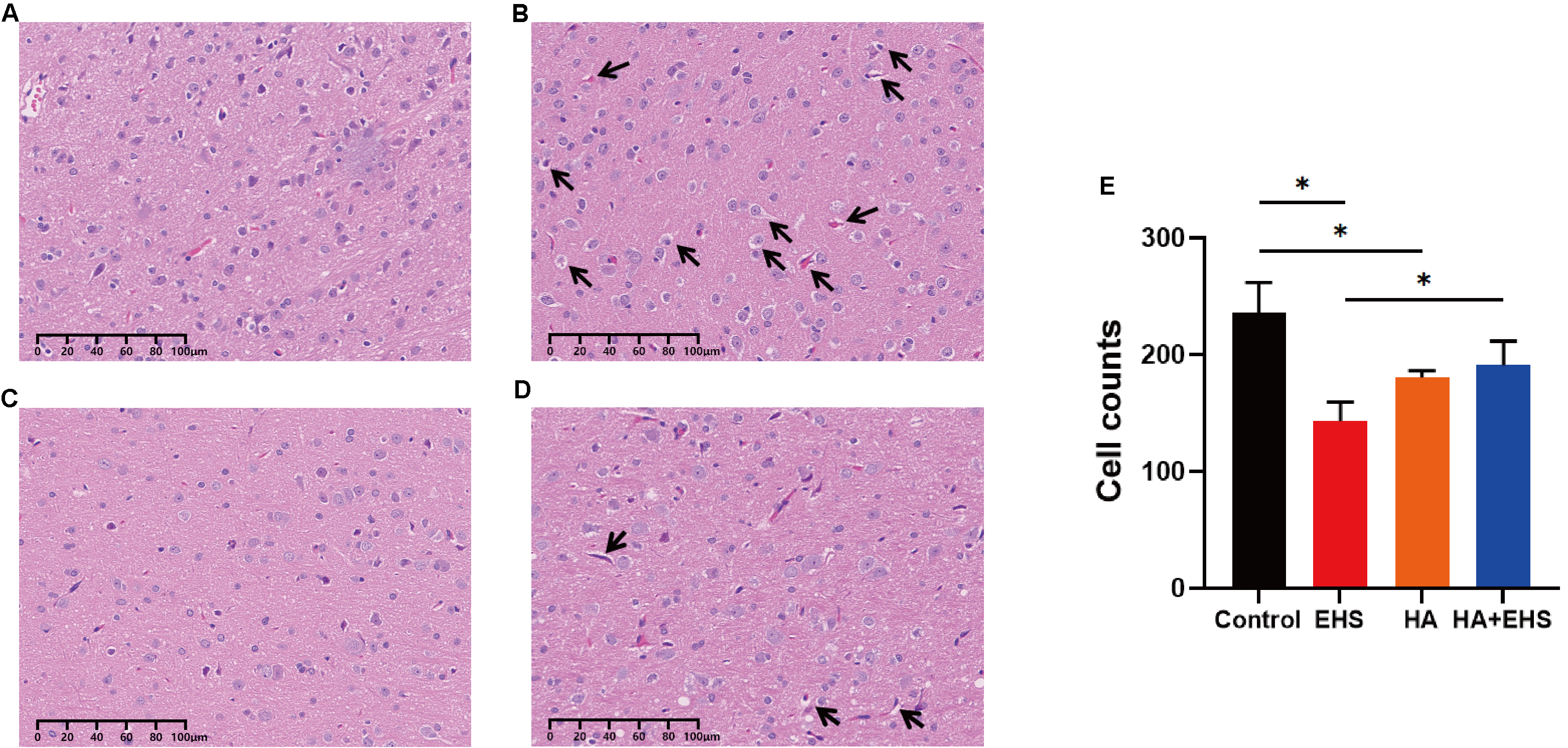 Fig. 2.
Fig. 2.Structural alterations in hypothalamus. Heat acclimation (HA) can improve structural abnormalities in the hypothalamus in rats after exertional heat stroke (EHS). Representative images of hypothalamic hematoxylin and eosin (H&E) staining in the control group (A), exertional heat stroke (EHS) group (B), heat acclimation (HA) group (C) and HA + EHS group (D). Arrows indicate cell swelling and cellular vacuolation. (E) The total number of hypothalamic cells in the control, EHS, HA and HA + EHS groups. *p = 0.000 on EHS vs. Control; *p = 0.006 on EHS vs. HA + EHS; *p = 0.001 on HA vs. Control (n = 5).
The differentially expressed proteins (DEPs) in the hypothalamus from rats in
the EHS and HA + EHS groups were analyzed using TMT-based proteomic analysis. The
threshold fold change (FC) values were above 1.2 or below 0.8, respectively, with
a statistically significant p value
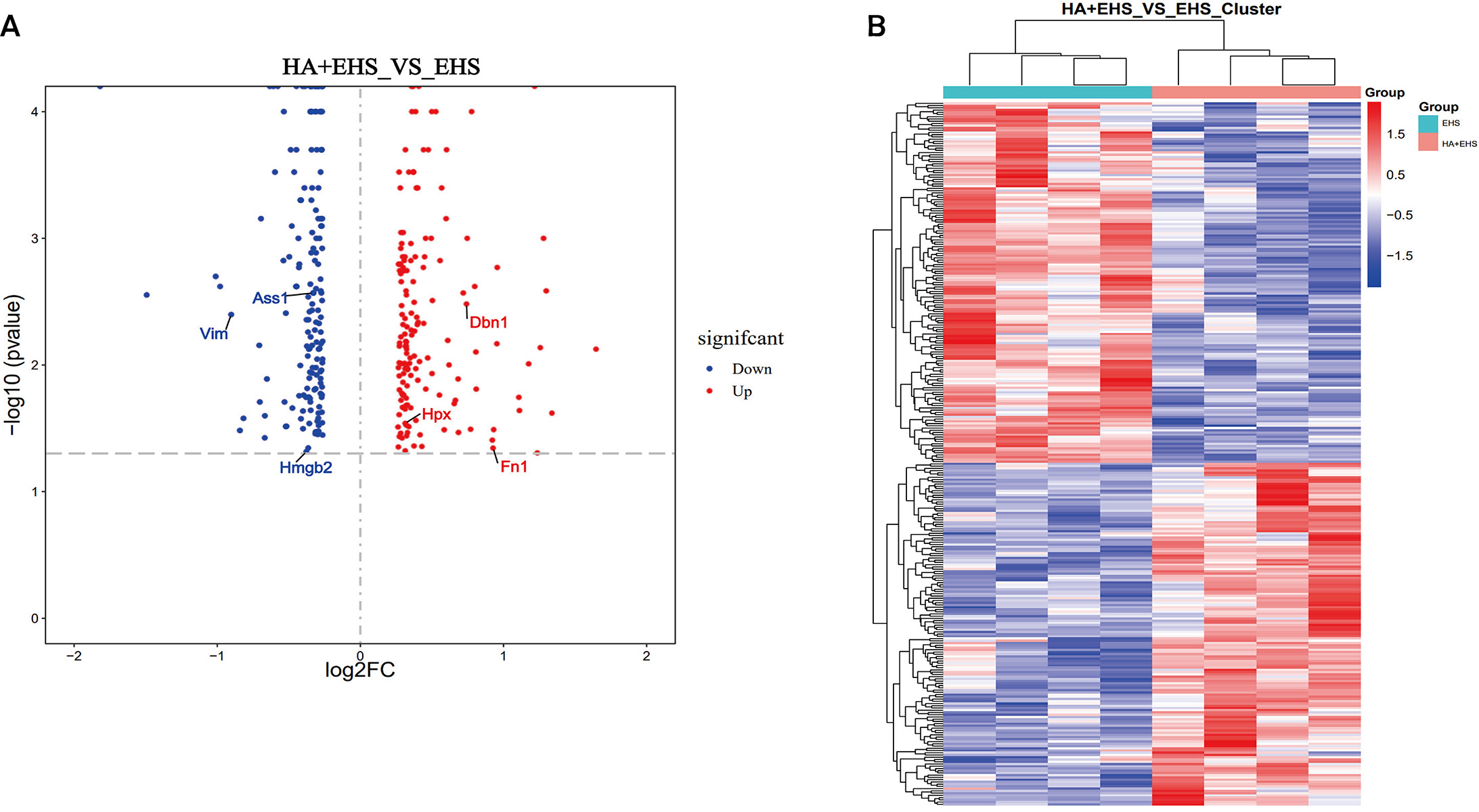 Fig. 3.
Fig. 3.Identification of the differentially regulated proteins. (A)
Volcano plots delineated by using the fold changes (FCs) and p values
between the heat acclimation followed by exertional heat stroke (HA + EHS) group
and the EHS group are presented. Proteins with significantly higher levels are
dotted in red, and those with lower levels are dotted in blue. The differentially
expressed proteins of interest are labeled in the diagram. (B) Heatmaps showing
the hierarchical clustering of differentially expressed proteins between HA + EHS
and EHS (red indicates upregulated proteins, and blue represents downregulated
proteins). Each group contained four individuals (fold change
Subsequently, Gene Ontology (GO) functional annotation clustering and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were conducted on the hypothalamus of the HA + EHS vs. EHS groups to further explore the biological functions of the DEPs. The results of the GO analysis of hypothalamic proteins are shown as follows. Biological process (BP) analysis revealed that the differentially expressed proteins in the HA + EHS and EHS groups were involved in nervous system development, regulation of cell homeostasis, and Cyclic adenosine monophosphate (cAMP) biosynthetic process. The enriched molecular functions (MF) were structural molecule activity, calcium-dependent protein binding, and the receptor for advanced glycation end products (RAGE) receptor binding. The enriched cellular component (CC) terms included cell junctions, cell projections and plasma membrane-bounded cell projections (Fig. 4A; Supplementary Table 2). Notably, the proinflammatory differential proteins are showed in Fig. 5A. ASS1 and HMGB2 mentioned above are involved in BP (Fig. 5B) related to the regulation of the response to cytokine stimuli and inflammatory reactions, and they also participate in MF (Fig. 5C) associated with RAGE receptor binding, cell adhesion molecule binding and integrin binding, which mediate cytokine activities and the immune response process. The CC terms were synapses, cell junctions, and neural projections (Fig. 5D). KEGG pathway analysis revealed that the hypothalamic DEPs in HA + EHS vs. EHS rats were associated with spliceosome pathways (Fig. 4B; Supplementary Table 3). Protein‒protein interaction network analysis (PPI network analysis) (Supplementary Fig. 1) revealed the predicted functional associations between these different proteins and inflammation.
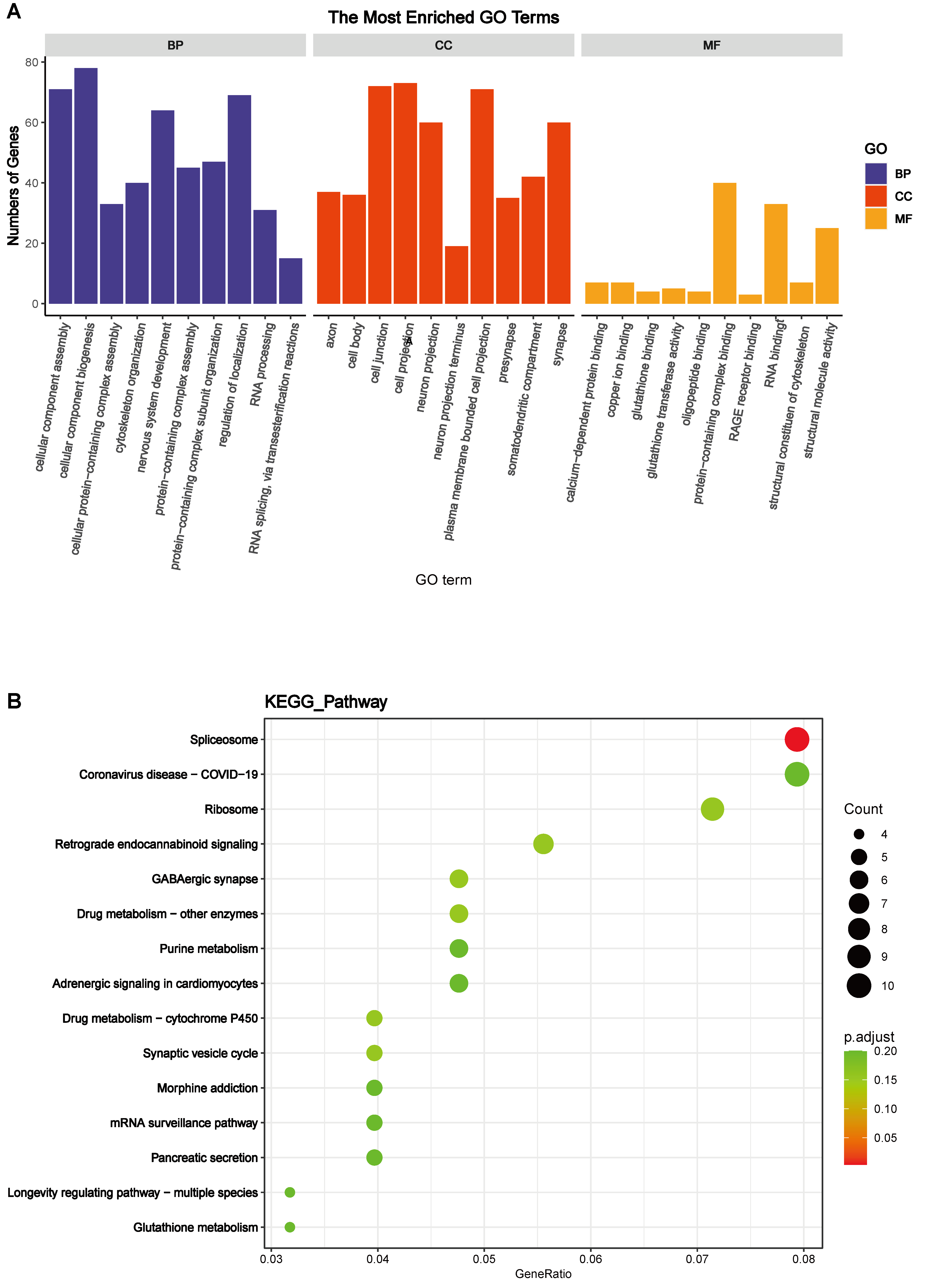 Fig. 4.
Fig. 4.Bioinformatics analyses of the differentially expressed proteins. (A) Gene Ontology (GO) enrichment analysis of biological process (BP), cell component (CC), and molecular function (MF) terms between heat acclimation followed by exertional heat stroke (HA + EHS) group and EHS group are shown. (B) Enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways identified in the HA + EHS vs. Control (CON) comparison are shown.
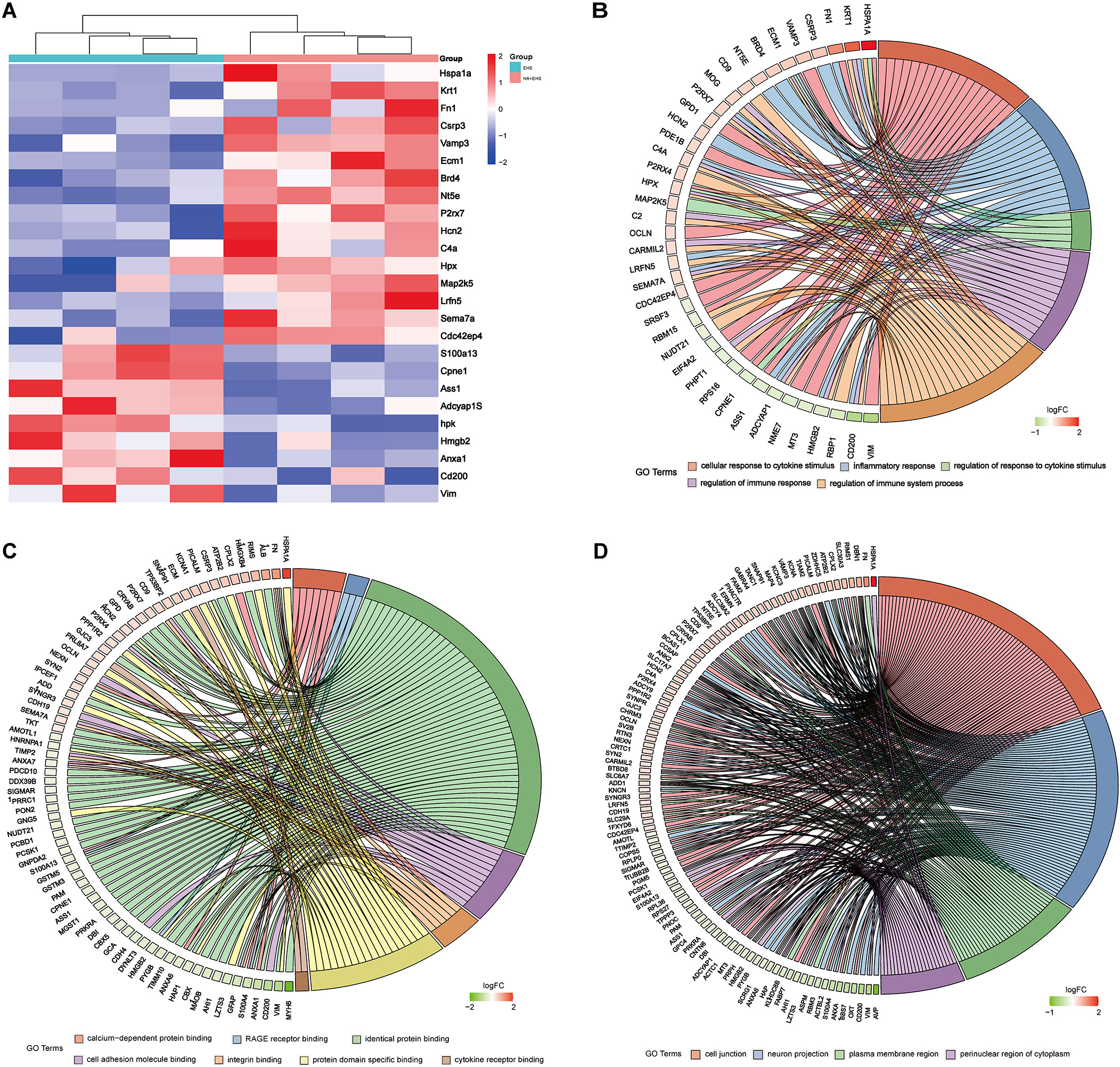 Fig. 5.
Fig. 5.Heatmaps and Gene Ontology (GO) enrichment analysis between exertional heat stroke (EHS) and heat acclimation (HA) + EHS revealed the key differential proteins involved in inflammation. (A) Heatmap of the HA + EHS group vs. the EHS group shows the relative abundances of the differentially expressed proteins involved in inflammation. The downregulated proteins included metalloproteinase inhibitor 2 (TIMP2), programmed cell death protein 10 (PCDP10), S100 calcium-binding protein A13 (S100A13), argininosuccinate synthetase (ASS1), high mobility group protein B2 (HMGB2) and vimentin (VIM). (B) GO enrichment analysis showed that the BPs enriched in these differentially expressed proteins were associated with the response and regulation of cytokines such as interferon-gamma and tumor necrosis factor. (C) The results of the molecular function (MF) analysis are shown. The enriched MFs in the differentially expressed proteins were associated with cell adhesion molecular binding, the receptor for advanced glycation end products (RAGE) binding and integrin binding related to immune inflammation. (D) The cell component (CC) enrichment results are shown. The CCs associated with these differentially expressed proteins were synapses, cell junctions, and neural projections.
Thus, controlling inflammatory conditions could be a key strategy employed by HA to mitigate the hypothalamic dysfunction induced by EHS. ASS1, HMGB2 and VIM may be candidate proteins involved in the mechanism underlying the protective effect of HA against hypothalamic injury after EHS.
To validate the above differential proinflammatory proteins, we performed Western blotting. Compared with those in the CON group, the expression of ASS1 (p = 0.024), HMGB2 (p = 0.009) and VIM (p = 0.009) in the EHS group was significantly greater, and the protein levels of ASS1 and HMGB2 in the HA group were not significantly different, while the expression of VIM in the HA group was significantly greater (p = 0.000). Compared with those in the EHS group, the relative expression levels of ASS1 (p = 0.047), HMGB2 (p = 0.000) and VIM (p = 0.009) were decreased in the HA + EHS group (0.71-, 0.67-, and 0.64-fold, respectively; Fig. 6). Supplementary Figs. 2,3,4,5 present the full-length blots/gels of HMGB2, ASS1, VIM and GAPDH.
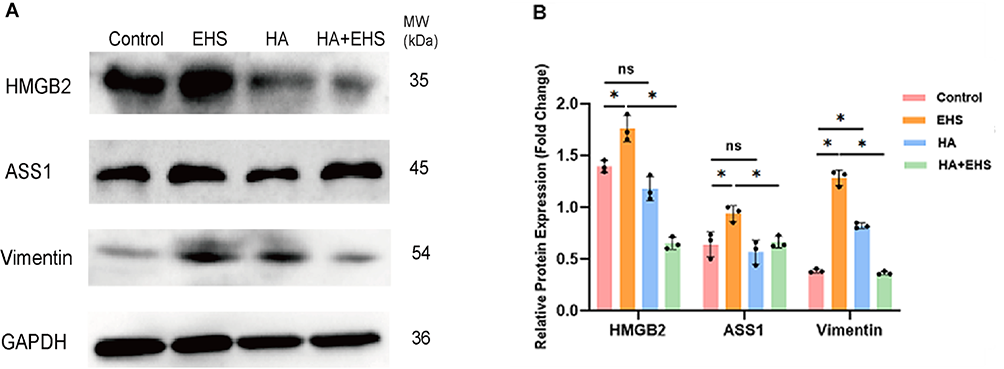 Fig. 6.
Fig. 6.Western blotting identified candidate proteins related to
inflammatory responses. (A) Blots of argininosuccinate synthetase (ASS1), high
mobility group protein B2 (HMGB2), vimentin (VIM) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) are shown. Full-length blots/gels of ASS1, HMGB2, Vim and
GAPDH are presented in the Supplementary Figs. 2,3,4,5. (B) Increases in ASS1,
HMGB2 and VIM expression were detected in the exertional heat
stroke (EHS) group compared to the control group. There was evident upregulation
of VIM and no changes in ASS1 or HMGB2 in the heat acclimation (HA) group
compared to the control group. Similarly, the expression of ASS1, HMGB2 and VIM
was significantly lower in the heat acclimation followed by
exertional heat stroke (HA + EHS) group than in the EHS group. p values
were calculated by one-way analysis of variance (ANOVA); ns, not significant; *, p value; data
are based on one representative experiment from at least 3 independent
experiments (mean
Proinflammatory cytokines such as IL-1 and IL-8 are involved in the inflammatory
response promoted by ASS1, HMGB2 and VIM [43, 44, 45]. Thus, we examined the
expression of IL-1
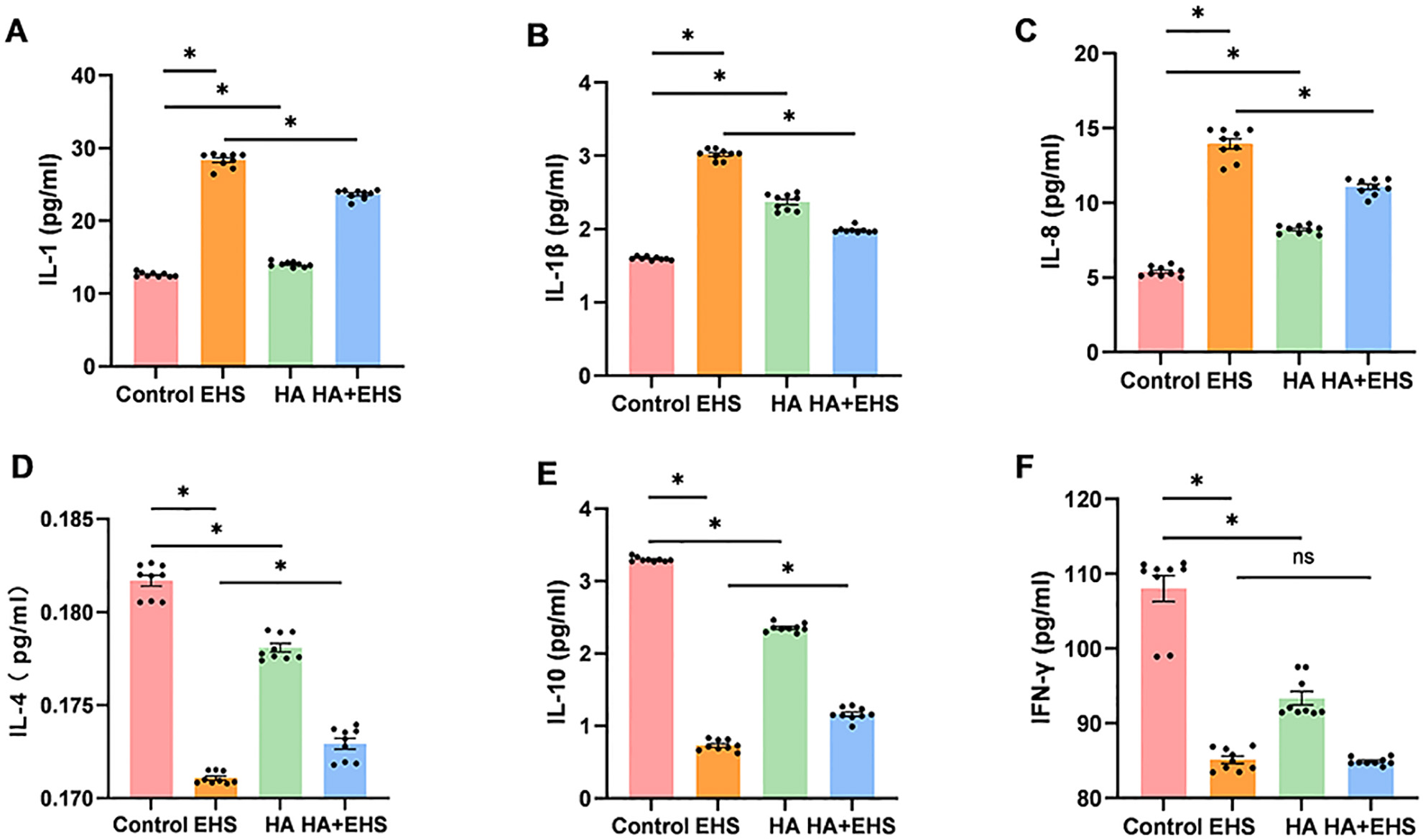 Fig. 7.
Fig. 7.The ability of heat acclimation (HA) to regulate the
inflammatory response in exertional heat stroke (EHS) was validated by measuring
interleukin (IL)-1, IL-1
When the thermoregulatory system is unable to maintain the core body temperature
within a normal range during heat stress, hyperthermia occurs. The cytotoxic
effects of hyperthermia (
Based on the findings of previous studies, the relationship between hypothalamic injury and heatstroke can be summarized as follows: extreme heat exposure leads to decreased mean arterial pressure (MAP), increased intracranial pressure (ICP), and reduced cerebral perfusion pressure (CPP = MAP – ICP), which may trigger hypothalamic ischemia and oxidative stress [49], in turn causing hypothalamic tissue inflammation and neuronal damage [50]. These changes not only result in thermoregulation deficits but also affect the hypothalamic‒pituitary‒adrenal axis (HPA axis) and exacerbate heat intolerance, eventually leading to multiple-organ dysfunction or failure and promoting the occurrence of heatstroke [51, 52]. Systemic inflammatory response syndrome and coagulation abnormalities accompany the progression of heatstroke [1, 53], which further exacerbate hypothalamic inflammatory insult. Therefore, hypothalamic inflammation is closely associated with the occurrence and progression of heatstroke.
In the present study, we first developed a stable HA rat model, providing the basis for establishing a rat model of EHS following HA. Consistent with these earlier findings, our data showed that HA decreased body weight and basal core temperature as well as improved heat tolerance.
Our pathological test results showed that the hypothalamus had evident pathological structural changes after heat shock, which was consistent with the results of some reported studies [19, 20, 54]. The differentially expressed proteins revealed by TMT-based proteomic analysis were related to several cellular activities. In particular, we found that some downregulated proteins between the HA + EHS group and the EHS group were associated with cytokine activity and the inflammatory response. These genes included ASS1, HMGB2 and VIM. Activation of ASS1 plays a central role in mediating host innate immune defense against bacterial infection in vivo by controlling inflammatory macrophage activity [40]. Additionally, as an immunomodulatory molecule, ASS1 modulates the immunological microenvironment and cytokine response via C-X-C motif chemokine ligand 8 (CXCL8) signaling [55]. HMGB2 is known for its proinflammatory role in mobilizing innate immunity and is involved in signaling pathways that are associated with various inflammatory disorders [56]. VIM is critical for nucleotide-binding oligomerization domain (NOD)-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome activation, and its loss leads to decreased levels of proinflammatory cytokines, subsequently protecting against harmful inflammation [57]. Moreover, VIM induces Golgi body stress and neuroinflammation as a component of the NLRP3 pathway [57]. These findings suggested that the harmful effect of EHS on the hypothalamus might be caused by excessive inflammation. Proteomic analysis suggested that HA might ameliorate hypothalamus abnormalities after heat attack by modulating inflammatory responses. Proteomic analysis indicated that proinflammatory proteins such as ASS1, HMGB2 and VIM may be involved in the regulatory effect of HA on hypothalamic inflammation after EHS.
Previous findings have demonstrated that HS induces an inflammatory response in the hypothalamus [19, 20, 54], and it has been shown that HA regulates hypothalamic metabolic and inflammatory processes [26]. However, few studies have examined how HA affects the hypothalamus following the onset of heatstroke. We found that HA could reduce the hypothalamic damage caused by EHS, and this protective effect could be accomplished by reducing inflammation promoted by ASS1, HMGB2, and VIM.
Additionally, proinflammatory cytokines such as IL-1 are engaged in the proinflammatory activities of ASS1, HMGB2, and VIM [43, 44, 45]. We further measured the concentrations of important immuno-inflammatory mediators. Heat acclimation reduced the levels of cytokines, indicating that inflammatory activation was triggered by EHS. Therefore, ASS1, HMGB2, and VIM may be potential targets through which HA improves hypothalamic inflammation after EHS.
Our study has several limitations. First, in addition to controlling body
temperature, the hypothalamus is also responsible for coordinating multiple
physiological homeostasis pathways, such as the stress response, electrolyte and
water balance, and energy metabolism. These results may lack specificity because
we did not conduct additional studies to determine whether these unique proteins
and pathways are involved in the regulation of other processes in the
hypothalamus in addition to body temperature regulation. Additionally, the
molecular mechanism by which HA protects against EHS was not thoroughly
investigated. For example, the particular pathways involved were not
investigated. Third, only serum cytokine levels were examined; the amounts of
cytokines in hypothalamic tissue were not. However, the detection of serum
cytokines is easier and more practical for clinical applications. Fourth, changes
in other classic inflammatory proteins, such as tumor necrosis factor-alpha (TNF-
In conclusion, HA improved the anatomical abnormalities of the hypothalamus caused by EHS. This could be attributed to the fact that HA regulates proinflammatory proteins such ASS1, HMGB2, and VIM, which in turn affects the inflammatory response to EHS.
HA, heat acclimatization/acclimation; EHS, exertional heat stroke; AT, ambient
temperature; RH, relative humidity; T
All data generated or analyzed during this study are included in this article and its Supplementary information files.
WJZ, LFW and QS conceptualized and designed the study. FX, LZM, XL, JBZ and JM performed the research. SYL, HDM and LX provided help and advice on the methods. FX, LZM, XL, JBZ and JM analyzed the data. FX, JBZ and JM wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The experimental procedures were approved by Institutional Animal Care and Use Committee of Beijing Institute of Radiation Medicine (Ethics number: IACUC-DWZX-2022-742) and conducted according to the guidelines and regulations for the use and care of experimental animals in China.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
