- Academic Editors
Background: Long-Covid, characterized by persistent symptoms following acute Covid-19 infection, represents a complex challenge for the scientific community. Among the most common and debilitating manifestations, cognitive fog is a neurological disorder characterized by mental confusion and cognitive difficulties. In this study, we investigated the long-term effects of previous Covid-19 infection on cortical brain activity in patients experiencing cognitive fog symptoms in the medium and long term. Methods: A total of 40 subjects (20 females and 20 males) aged between 45 and 70 years (mean age (M) = 59.78, standard deviation (SD) = 12.93) participated in this study. This sample included individuals with symptoms of cognitive fog, both with and without anosmia, and a control group comprised of healthy subjects. All electroencephalography (EEG) data were collected in two sessions, 1 month and 8 months after recovery from Covid-19, to measure the neurophysiological parameters of P300 and beta band rhythms. Results: The results revealed significant differences in the neurophysiological parameters of P300 and beta band rhythms in subjects affected by cognitive fog, and these alterations persist even 8 months after recovery from Covid-19. Interestingly, no significant differences were observed between the participants with anosmia and without anosmia associated with cognitive fog. Conclusions: These findings provide a significant contribution to understanding the long-term effects of Covid-19 on the brain and have important implications for future interventions aimed at managing and treating brain fog symptoms. The longitudinal assessment of cortical brain activity helps highlight the persistent impact of the virus on the neurological health of Long-Covid patients.
On January 30, 2020, the World Health Organization [1] declared the infectious disease known as coronavirus (Covid-19) a pandemic, raising concerns for international public health [2, 3, 4]. From the first reported case of Covid-19 infection until today, over 450 million cases have been recorded worldwide [5]. Despite global efforts to control the spread of the virus and mitigate its effects, Covid-19 continues to pose a significant challenge for the scientific community [6, 7, 8]. Understanding the long-term aspects of the disease has become a fundamental priority to address its clinical, psychological, and social implications [9, 10, 11]. Following acute SARS-CoV-2 infection, approximately 10–20% of patients experience the condition known as Post-Covid or Long-Covid, characterized by the continuation or manifestation of new symptoms that develop 3 months after the initial infection [12]. The main symptoms may include fatigue, muscle pain, shortness of breath, cough, chest pain, palpitations, anosmia, ageusia, sleep difficulties, and cognitive dysfunction that persist for at least 2 months without other explanation [12]. In addition to numerous physical and neurological symptoms, Long-Covid has been associated with a range of mental health issues, such as post-traumatic stress disorder, anxiety, and particularly depression [13, 14]. Among the main risk factors associated with Long-Covid are the quantity and duration of symptoms experienced during the acute phase of the infection. A study conducted by Sudre et al. [15] found that having more than five symptoms within the first week of infection—including fatigue, headache, breathing difficulties, hoarse voice, and myalgia—increased the likelihood of developing Long-Covid. On the contrary, the severity of Long-Covid symptoms does not appear to correlate with the initial severity of Covid-19 infection [16, 17, 18, 19]. This implies that even patients with mild or moderate acute symptoms could be at risk of developing Long-Covid. Other risk factors include having a high body mass index and being female [20, 21]. Among the most common neurological symptoms associated with Long-Covid, cognitive fog or brain fog is frequently observed [22, 23]. According to a study conducted by Graham et al. [24], 81% of the participants reported experiencing this condition after contracting Covid-19 infection. Cognitive fog does not represent a distinct diagnostic category, but it includes a range of neurological symptoms that may manifest as inattention, short-term memory loss, reduced mental sharpness, and cognitive fatigue [25], negatively affecting people’s daily functioning. The causes of brain fog are not clear. Several etiological hypotheses have been proposed by researchers, including inflammation due to cytokine storm, mitochondrial dysfunction in the brain, reduced tissue oxygenation, and diminished blood flow [26]. Some authors have suggested that cognitive fog could be due to the neuroinvasion of the virus through the vagus nerve, trigeminal nerve, or olfactory tract [27]. Research has shown that SARS-CoV-2 can enter the olfactory mucosa and cause anosmia [28, 29]. Unlike transient anosmia observed in other respiratory infections, persistent anosmia is a Long-Covid symptom that may result from the lasting effects of direct viral damage to the olfactory epithelium [30, 31]. Indeed, imaging studies conducted on patients with persistent anosmia from Covid-19 have demonstrated extensive damage to the olfactory epithelium, primarily characterized by thinning of the tissue [32].
Several studies have suggested that Covid-19 infection may impact brain electrical activity, detectable through electroencephalography (EEG). Changes in EEG have been documented in post-Covid-19 patients, associated with the recovery from psychopathological symptoms, including cognitive impairments [33]. Specifically, a widespread slowing of EEG has been noted, primarily in frontal, central, and temporal regions, accompanied by an increase in theta wave activity and a decrease in alpha wave activity [34, 35]. Regarding beta waves, while some studies have reported increased activity in central and temporal regions, others have indicated a more general reduction [36, 37]. Similar EEG changes have been described in mild cognitive impairment, unrelated to viral infections [38]. Moreover, specific EEG disturbances have been well-documented in patients with severe forms of Covid-19 [39]. Building upon these observations, the hypothesis has been put forth to utilize EEG as a standard diagnostic and monitoring tool to assess persistent neurological symptoms from Long-Covid-19 [34]. Specifically, these symptoms can be assessed through neurophysiological measures such as Event-Related Potentials (ERP) and beta band rhythms [40, 41, 42, 43, 44]. ERPs constitute a powerful analytical tool for measuring and examining brain activity associated with sensory, motor, or cognitive functions triggered by specific stimuli or events. The P300 component emerges as a positive deflection in the ERP waveform, typically occurring around 300 ms after the presentation of a rare or unexpected stimulus. This element is closely linked to cognitive processes involving attention and memory. The onset latency of the P300, the time required for the P300 response to manifest following a stimulus, plays a crucial role as it reflects the speed and efficiency of cognitive processing. The amplitude of the P300 is primarily associated with attention, stimulus relevance, and the ability to discriminate between rare or unexpected stimuli and more common or expected ones. Greater amplitude generally indicates a stronger neural response, reflecting increased cognitive engagement in processing the stimulus. Moreover, the analysis of alpha, beta, and theta band rhythms is crucial because these neural oscillations are frequently associated with higher cognitive processes, including those related to attention, concentration, and information processing. In the context of Long-Covid, the persistence of symptoms such as fatigue, cognitive difficulties, and mental confusion could be linked to alterations in the frequency and organization of these bands’ rhythms.
Based on the previous evidence of EEG changes during Covid-19, we hypothesized that post-Covid-19 brain fog may be characterized by specific EEG alterations. These variations could potentially provide an objective confirmation of the diagnosis in individuals reporting cognitive disturbances. To explore this topic further, we conducted an electrophysiological analysis based on EEG in a sample of 20 patients exhibiting symptoms of post-Covid-19 brain fog, assessing them also 8 months post-recovery.
The main objective of this research was to evaluate the effects induced by a previous Covid-19 infection on brain cortical activity in patients with cognitive fog symptoms in the medium and long term. Specifically, the first aim was to determine if the cognitive symptoms reported by patients as cognitive fog correlated with actual alterations in neurophysiological parameters, namely P300 and alpha, beta, and theta bands. The second objective was to assess whether any neurophysiological alterations remained constant over time, to elucidate the long-term impact of Covid-19 infection on the nervous system and cognitive health. Lastly, the third goal was to investigate if there were significant differences in altered brain cortical activity between subjects experiencing only cognitive fog and those presenting cognitive fog associated with anosmia. In line with the hypothesis of neuroinvasion through the olfactory tract [28, 29], this study postulated that the group of subjects with cognitive fog associated with anosmia would exhibit more intense neurophysiological symptoms compared to the group without anosmia and to the control group.
The subjects were recruited through the Madonna della Consolazione Polyclinic Nursing Home in Reggio Calabria, a region in Southern Italy. All participants who reported cognitive fog had a positive Polymerase Chain Reaction (PCR) test for Covid-19 and had recovered for at least 30 days from the acute phase of the infection, while the health participants did not conduct any PCR tests. The symptoms of cognitive fog were described with statements such as: poor articulation, memory loss, slow reaction times, mental confusion, wandering thoughts, and mental fatigue. The exclusion criteria included the presence of other neurological or psychiatric disorders, a history of previous illnesses, and being in stable clinical conditions. These conditions were confirmed through clinical evaluation by a neurologist and psycho-diagnostic assessment conducted by a clinical psychologist. The presence of reported anosmia was verified through the olfactory test based on Tabert et al. [45]. All participants reported having normal hearing, which was confirmed through evaluation using a Kamplex screening audiometer (PC Werth, London, UK).
A total of 40 subjects (20 females and 20 males) aged between 45 and 70 years (mean age, M = 59.78, standard deviation, SD = 12.93) took part in this study. Specifically, the participants were assigned to three conditions: a group consisting of 10 subjects with cognitive fog symptoms, a comparison group consisting of 10 subjects with cognitive fog and anosmia symptoms, and a control group consisting of 20 healthy subjects. The selection of participants for the control group took into consideration gender, age, and body mass index (BMI) to approximately match the male/female ratio, mean age, and BMI with the characteristics of the cognitive fog and healthy group. Table 1 provides a summary of the descriptive statistics (mean and standard deviation) and the percentages for each variable characterizing the sample.
| Variable | Cognitive fog group | Control group | |
| Gender | Female (%) | 10 (50.0) | 10 (50.0) |
| Male (%) | 10 (50.0) | 10 (50.0) | |
| BMI | Normal weight (range from 18.5 to 24.9) | 20 (50.0) | 14 (35.0) |
| Overweight (range from 25 to 29.9) | 3 (7.5) | 3 (7.5) | |
| Marital status | Single (%) | 4 (10.0) | 2 (5.0) |
| Married (%) | 11 (27.5) | 15 (37.5) | |
| Separated (%) | 2 (5.0) | 0 (0.0) | |
| Divorced (%) | 1 (2.5) | 1 (2.5) | |
| Widowed (%) | 1 (2.5) | 3 (7.5) | |
| Highest educational level | Middle school (%) | 2 (5.0) | 0 (0.0) |
| High school (%) | 4 (10.0) | 6 (15.0) | |
| Bachelor’s degree (%) | 5 (12.5) | 9 (22.5) | |
| Master’s degree (%) | 6 (15.0) | 4 (10.0) | |
| Post-graduate degree (%) | 2 (5.0) | 2 (5.0) | |
| Employment | Unemployed (%) | 1 (2.5) | 3 (7.5) |
| Armed forces (%) | 1 (2.5) | 1 (2.5) | |
| Employee (%) | 6 (15.0) | 6 (15.0) | |
| Entrepreneur (%) | 3 (7.5) | 1 (2.5) | |
| Freelancer (%) | 5 (12.5) | 3 (7.5) | |
| Healthcare personnel (%) | 2 (5.0) | 0 (0.0) | |
| Housekeeper (%) | 2 (5.0) | 4 (10.0) | |
| Retired (%) | 0 (0.0) | 2 (5.0) | |
| Covid-19 therapy | Asymptomatic (%) | 2 (5.0) | |
| Domiciliary (%) | 22 (55.0) | ||
| Ordinary hospitalization (%) | 10 (25.0) | ||
| Intensive care (%) | 6 (15.0) | ||
| Days of hospitalization | M |
4.15 |
|
| (range: 1–118) | |||
| Days of home isolation | M |
29.87 |
|
| (range: 0–120) |
BMI, body mass index; M, means; SD, standard deviation.
The procedures were conducted in accordance with the principles outlined in the Declaration of Helsinki. The study design was approved by the Ethics Committee of the Madonna della Consolazione Polyclinic Nursing Home (protocol code: 2022-269; 27 June 2022), and all participants provided informed consent by signing a consent form before the start of the EEG recording session. EEG data were collected from these subjects during two sessions, one month and eight months after their recovery from Covid-19, as suggested by the relevant literature [46, 47].
In this study, the olfactory test based on Tabert et al. [45], was used to confirm the reported symptoms of anosmia. EEG was used as the measurement tool to investigate neurophysiological parameters. Specifically, the P300 component was studied using an auditory oddball paradigm, and beta frequency band rhythms were analysed.
In the present study, an objective olfactory test based on a previously validated methodology [45] was employed. This test involved presenting 10 distinct olfactory stimuli to participants, including orange, strawberry, coffee, saffron, vanilla, mint, jasmine, cinnamon, citronella, and lavender, with the aim of identifying them. For each scent, participants had to respond to a multiple-choice question with four options, of which only one was correct. Consequently, the obtained scores could range from 0 (complete anosmia) to 10 (absence of olfactory dysfunction).
The EEG data were acquired at a sampling frequency of 500 Hz using a band-pass
filter set between 0.1 Hz and 70 Hz. SCAN software (version 4.3, Neuroscan,
Compumedics, El Paso, TX, USA) along with NuAMP amplifiers (Neuroscan,
Compumedics, El Paso, TX, USA) were employed for recording. Eighteen scalp
electrodes (Ag/AgCl) were positioned according to the standard 10/20 system
described by Jasper & Rasmussen [48]. The reference electrode was placed in
midline derivations such as Fz, FCz, Cz, and Pz, while the ground electrode was
placed between Cz and Pz. All positions were referenced to a common Cz reference
and subsequently offline referenced to the average of the mastoids, using the
left mastoid A1 as a reference. Electrode impedances were kept at or below 10
k
Auditory oddball task consists in exposure to auditory stimuli without the need to provide direct responses. Each participant sat in a partially illuminated and soundproof room, facing a computer monitor positioned approximately 70 cm away. Two speakers were placed next to the monitor for audio playback. Stimuli were presented using specialized software provided by Neurobehavioral Systems Inc, Berkeley, CA, USA. The auditory paradigm consisted of three categories of sounds: frequent pure sinusoidal tones, infrequent pure sinusoidal tones, and new sounds. Specifically, 10% of the stimuli were infrequent tones (2 kHz, duration 200 ms, rise and fall time of 5 ms, sound pressure level of 70 dB SPL). Another 10% consisted of new sounds, while the remaining 80% comprised frequent tones (1.5 kHz, duration 200 ms, rise and fall time of 5 ms, 70 dB SPL). The duration of each tone or noise was 200 milliseconds, with an asynchrony interval of 700 ms between stimuli. The presentation was divided into two blocks, each containing 700 stimuli (560 frequent, 70 infrequent, and 70 new). The intensity of the new sounds was digitally adjusted to ensure they did not exceed 70 dB SPL, measured with a Bruel and Kjaer sound pressure meter. Fourteen distinct new stimuli were used, each repeated a maximum of 5 times. Overall, the time required to complete the task was approximately 20 minutes.
Quantitative EEG analysis was performed using custom-made algorithms developed in Matlab code (The MathWorks Inc., Natick, MA, USA). Power spectral density (PSD) was evaluated by transforming the signal from the time domain to the frequency domain using the Welch method [49]. PSD values were calculated for each epoch, and their averages were computed. To begin, the total absolute power of the signal and the absolute power within each frequency band were calculated for each electrode. In this study, the beta band (14–29 Hz) was considered.
All statistical analyses were performed using SPSS 24.0 software (SPSS Inc.,
Chicago, IL, USA). The measurement parameters were P300 values and beta band
frequency rhythms for each condition (cognitive fog group, cognitive fog and
anosmia group, control group). Descriptive statistics of the parameters of
interest were calculated and examined for both cognitive fog group and the
control group. Firstly, to assess the differences between the two clinical
groups, a repeated measure Multivariate Analysis of Variance (MANOVA) was
conducted 2 (cognitive fog group and cognitive fog and anosmia group)
Table 2 shows descriptive statistics, namely mean and standard deviation, related to P300 latency at 1-month and 8-month assessments in cognitive fog groups and the control group.
| 1-month assessment | 8-month assessment | ||
| P300 latency (ms) | Cognitive Fog without Anosmia | 308.51 ( |
305.90 ( |
| Cognitive Fog with Anosmia | 308.52 ( |
306.11 ( | |
| Control Group | 300.02 ( |
300.01 ( | |
| P300 amplitude (µV) | Cognitive Fog without Anosmia | 4.98 ( |
5.12 ( |
| Cognitive Fog with Anosmia | 4.87 ( |
5.23 ( | |
| Control Group | 6.11 ( |
6.22 ( |
Preliminarily, the factor groups concerning the two clinical cognitive fog
groups (with and without anosmia) does not show significant differences, neither
with latency and amplitude parameters of P300 (respectively F(1, 18) = 0.04,
p = 0.95,
After this, a MANOVA was applied considering as an independent variable the two groups together (cognitive fog group) and the control group. Table 3 presents descriptive statistics, namely mean and standard deviation, related to beta band rhythms at 1-month and 8-month assessments in cognitive fog groups.
| 1-month assessment | 8-month assessment | ||
| Alpha | Cognitive Fog without Anosmia | 9.81 ( |
9.72 ( |
| Cognitive Fog with Anosmia | 9.78 ( |
9.65 ( | |
| Control Group | 11.21 ( |
10.93 ( | |
| Beta | Cognitive Fog without Anosmia | 16.51 ( |
16.02 ( |
| Cognitive Fog with Anosmia | 16.50 ( |
16.41 ( | |
| Control Group | 19.8 ( |
19.7 ( | |
| Theta | Cognitive Fog without Anosmia | 8.08 ( |
8.13 ( |
| Cognitive Fog with Anosmia | 8.03 ( |
8.12 ( | |
| Control Group | 7.38 ( |
7.32 ( |
As shown in Fig. 1, the group with cognitive fog had significantly higher P300
latencies than healthy controls (main effect of groups: F(1, 39) = 16.11,
p
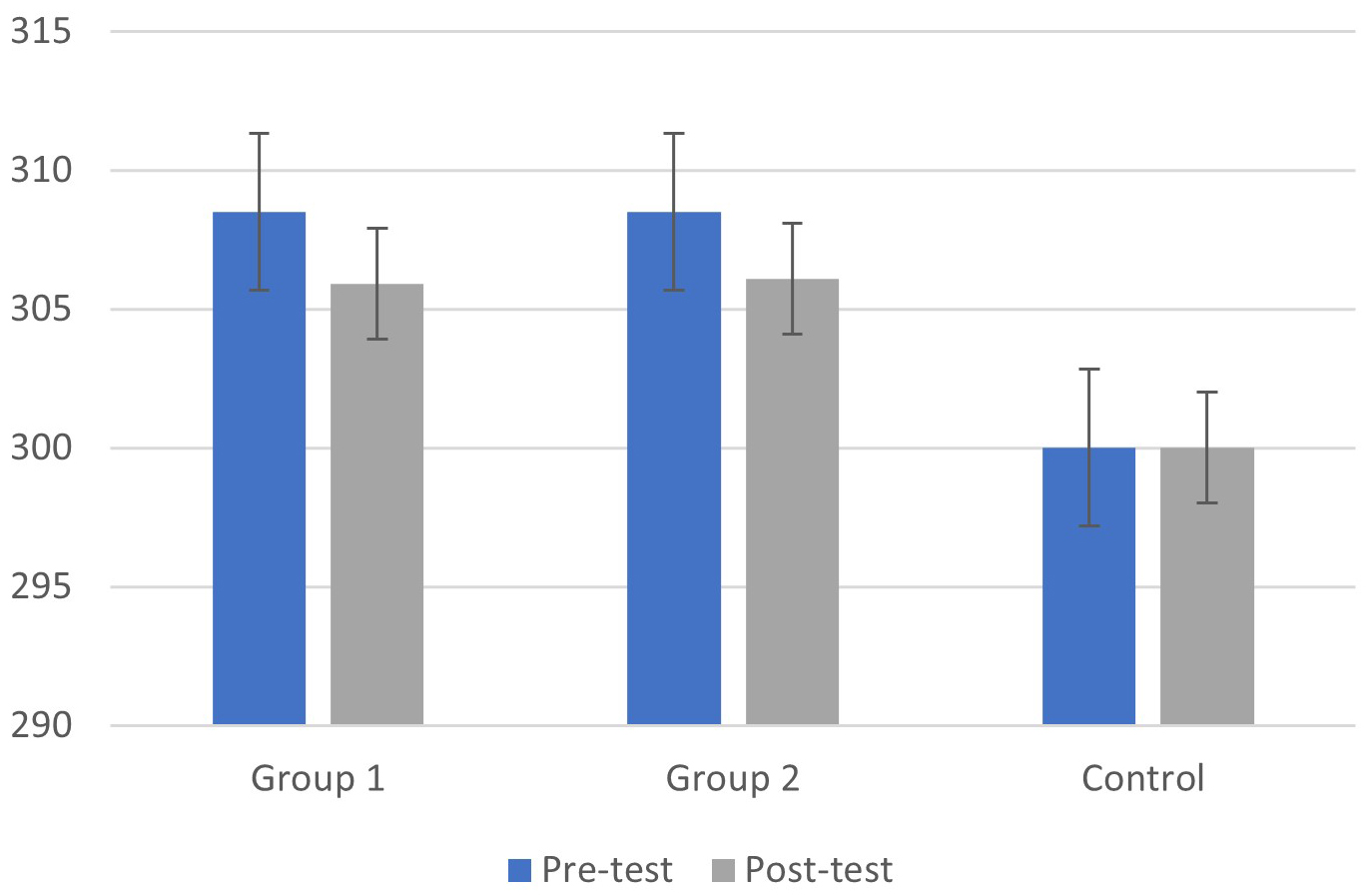 Fig. 1.
Fig. 1.Means (M) and Standard Deviations (SD) of P300 latency in the pre and post-test phases for the cognitive fog and control groups.
As shown in Fig. 2, the group does not exhibit a significant effect (F(1, 39) =
1.08, p = 0.27,
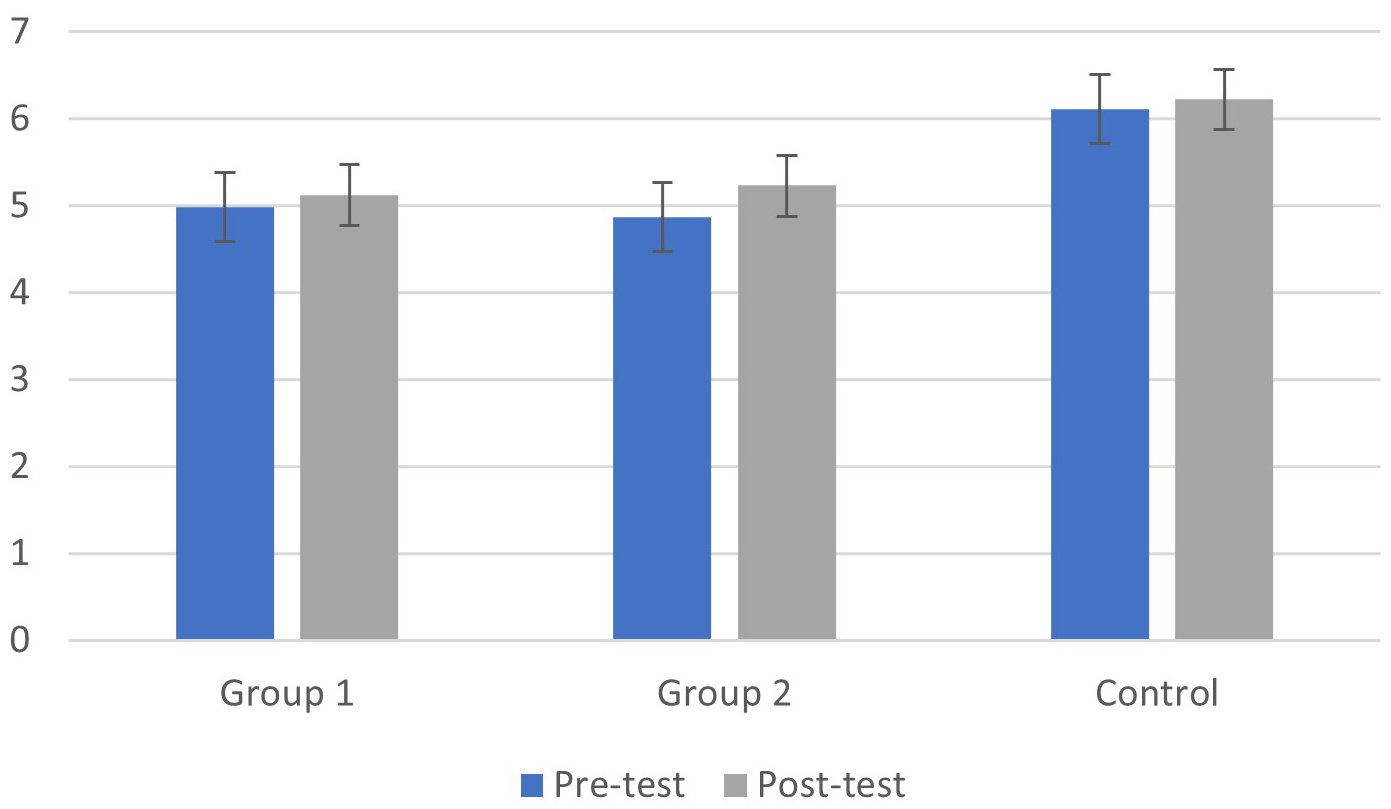 Fig. 2.
Fig. 2.Means (M) and Standard Deviations (SD) of P300 amplitude in the pre and post-test phases for the cognitive fog and control groups.
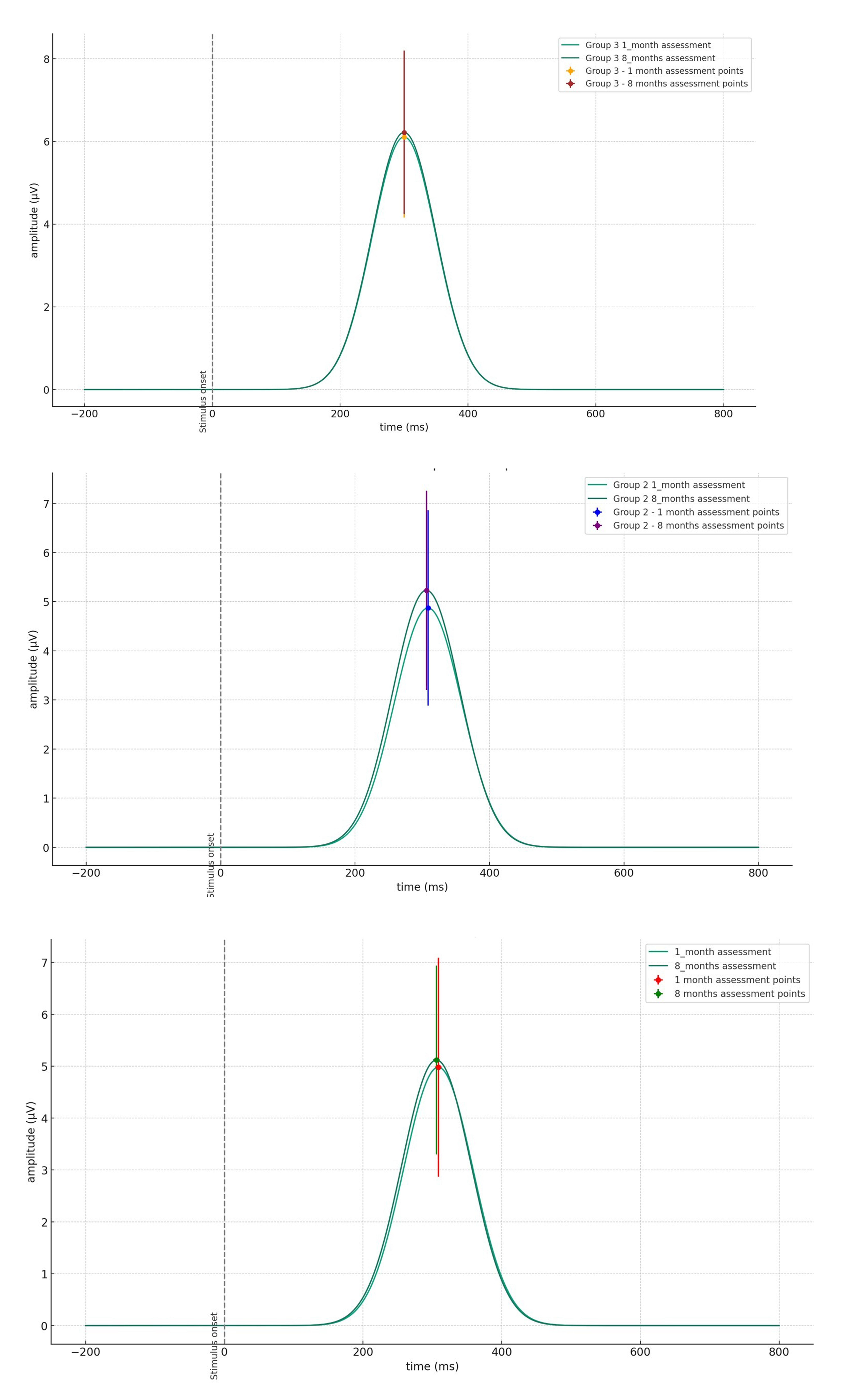 Fig. 3.
Fig. 3.ERP waveform (P300) in the pre and post-test phases for cognitive fog groups (with and without anosmia) and control group. ERP, Event-Related Potentials.
As shown in Fig. 4, the group with cognitive fog had significantly lower levels
of alpha band rhythms than healthy controls (main effect of groups: F(1, 39) =
15.02, p
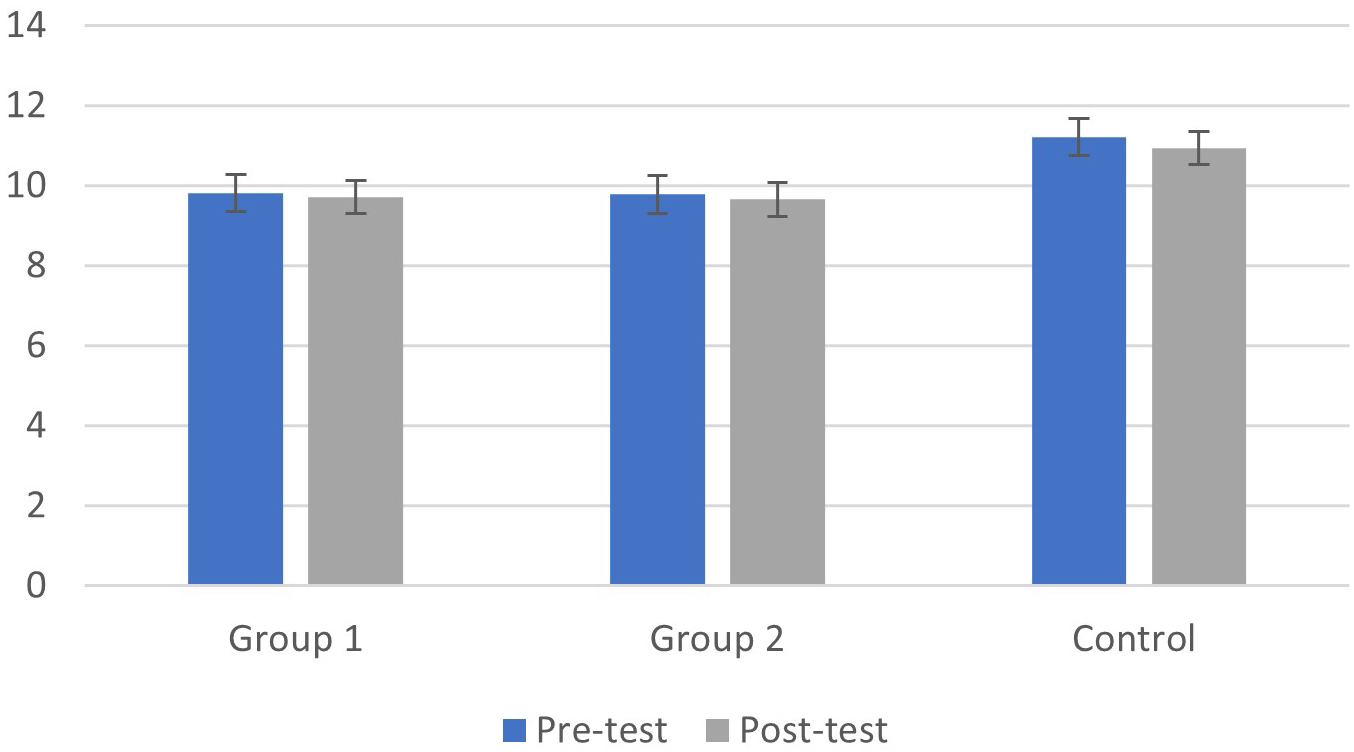 Fig. 4.
Fig. 4.Means (M) and Standard Deviations (SD) of alpha band rhythm in the pre and post-test phases for the cognitive fog and control groups.
As shown in Fig. 5, the group with cognitive fog had significantly lower levels
of beta band rhythms than healthy controls (main effect of groups: F(1, 39) =
11.15, p
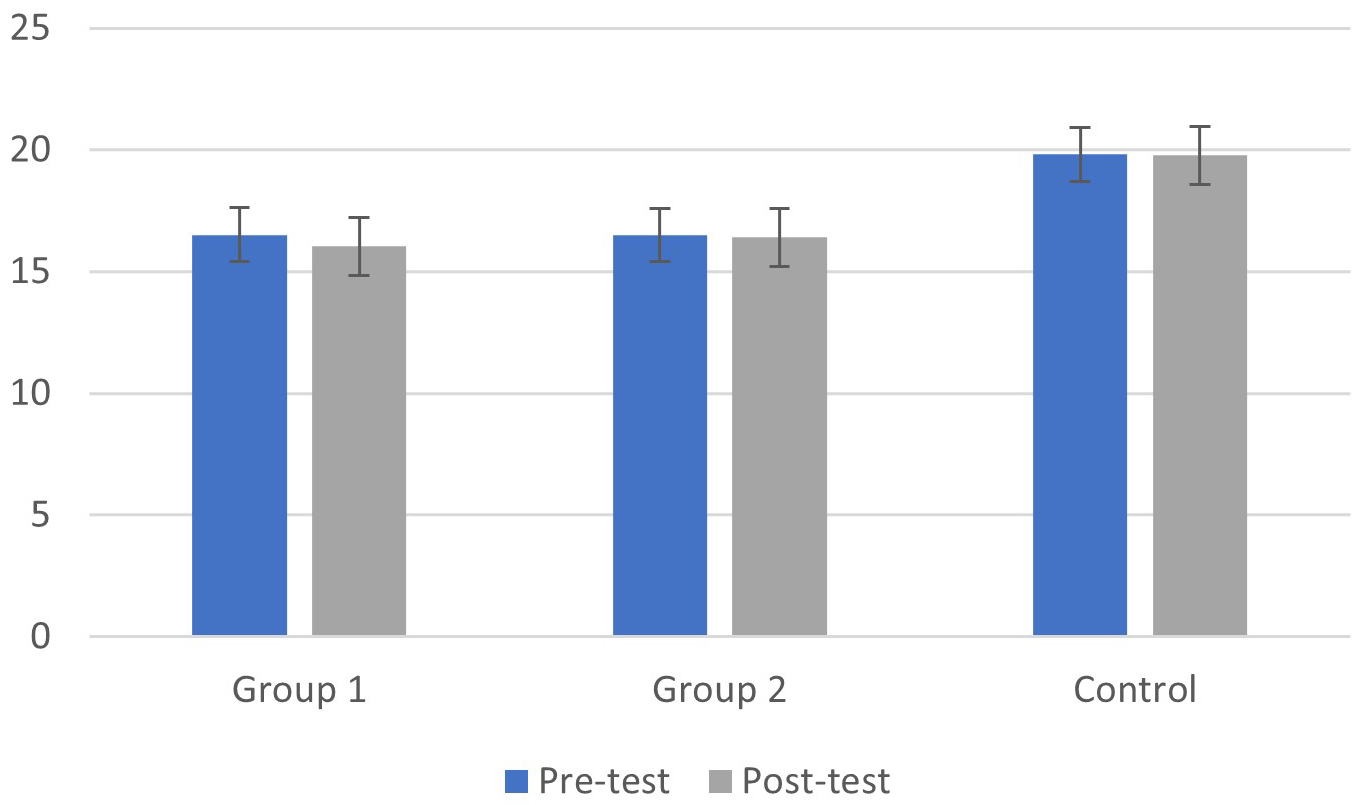 Fig. 5.
Fig. 5.Means (M) and Standard Deviations (SD) of beta band rhythm in the pre and post-test phases for the cognitive fog and control groups.
As shown in Fig. 6, the group with cognitive fog had significantly higher levels
of theta band rhythms than healthy controls (main effect of groups: F(1, 39) =
5.80, p
 Fig. 6.
Fig. 6.Means (M) and Standard Deviations (SD) of theta band rhythm in the pre and post-test phases for the cognitive fog and control groups.
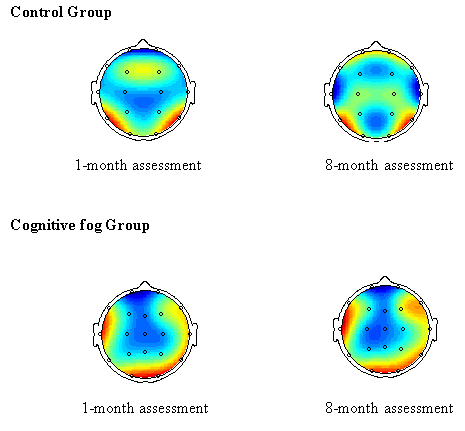 Fig. 7.
Fig. 7.Topographic brain map of electroencephalography (EEG).
The overweight participants (BMI between 25 and 29.9), representing 30% of the sample, exhibit more severe neurophysiological symptoms compared to the normal-weight participants (BMI between 18.5 and 24.9). In more detail, patient n.2: BMI = 27.3; P300 Latency = 318 ms; P300 Amplitude = 3.95 µV. Patient n.11: BMI = 26.9; P300 Latency = 317 ms; P300 Amplitude = 5.21 µV. Patient n.13: BMI = 28.1; P300 Latency = 319 ms; P300 Amplitude = 4.89 µV. Patient n.16: BMI = 27.8; P300 Latency = 319 ms; P300 Amplitude = 4.83 µV. Patient n.19: BMI = 25.5; P300 Latency = 316 ms; P300 Amplitude = 3.67 µV. Patient n.20: BMI = 28.6; P300 Latency = 319 ms; P300 Amplitude = 4.56 µV. While statistical analyses were not conducted on this specific subgroup due to sample size limitations, these results serve as a preliminary observation that could suggest an association between higher BMI and the severity of neurophysiological symptoms. Further research with a larger sample size would be necessary to validate and confirm this potential relationship.
In this study, the effects of a previous Covid-19 infection on brain cortical activity were evaluated in a sample of patients with cognitive fog symptoms in the medium and long term. Specifically, the main objective of this research was to investigate whether the cognitive symptom reported by patients as cognitive fog correlated with actual alterations in neurophysiological parameters of P300 and alpha, beta, and theta bands.
The results of this study are consistent with previous knowledge regarding the associations between P300 parameters and patient-reported symptoms. The role of P300 as a neurophysiological correlate of cognitive functions has been previously reported in both normal samples [50, 51] and clinical groups [52]. In the present study, this correlation was also confirmed in cases of cognitive fog, showing a longer latency and reduced amplitude of the P300 compared to the control group. This data suggests a slowdown in the brain’s response to significant stimuli, indicating possible impairment or deterioration of cognitive functions. The prolonged latency of P300 may reflect reduced efficiency in information processing or a diminished capacity of the central nervous system to promptly detect and respond to stimuli. This slowdown could negatively impact the ability to process information, maintain attention, and make decisions, thus contributing to cognitive fog symptoms.
Further changes were observed in the rhythms of alpha, beta, and theta brain waves in the cognitive fog group compared to the control group, suggesting an association with cognitive symptoms. Alpha waves are generally associated with a state of relaxation and a decrease in brain activity in the absence of external stimuli [53, 54]. Variations in alpha waves could indicate impairment in the ability to relax or concentrate, both of which are fundamental for cognitive functions. In our results, we observed a significant decrease in alpha waves in some patients with cognitive fog. This could suggest difficulty in achieving a relaxed mental state or a decrease in attention, both of which influence cognitive performance. Moreover, beta rhythms, known for their association with sensory information integration and motor activity execution [55], were reduced in the cognitive fog group compared to the control group. This reduction could indicate impairment in the brain’s ability to effectively integrate environmental information and translate it into appropriate motor actions. This result suggests that individuals with cognitive fog may have difficulty processing sensory information and coordinating motor activities efficiently, with possible impacts on various cognitive domains such as attention, memory, concentration, and decision-making skills [36, 37]. Regarding theta waves, we observed an increase in activity in the cognitive fog group. Theta waves are commonly associated with memory and decision-making processes [56, 57]. An increase in theta wave activity could indicate greater brain effort in attempting to compensate for the decrease in cognitive functions. This increase may reflect brain adaptation to recover compromised cognitive functions from previous Covid-19 infection. However, this additional effort could also lead to increased mental fatigue and a reduction in cognitive resources available for other activities. The second objective of the study was to investigate whether any alterations in P300 parameters and beta band rhythms persisted over time. From the results of this research, it emerged that the problems of slowness in P300 and the alterations in beta band rhythms in individuals affected by cognitive fog persist even after recovery from Covid-19. Although slight improvements were observed eight months after recovery, the neurophysiological parameter alterations related to cognitive fog remained present. This suggests that, despite the disappearance of acute symptoms of the disease, cognitive functioning connected to these neurophysiological indices may remain compromised [58, 59, 60].
Finally, the third objective was to examine whether there were significant differences in the alteration of brain cortical activity between subjects who presented only cognitive fog and those who presented cognitive fog associated with anosmia. Specifically, in line with the hypothesis of neuroinvasion through the olfactory tract [28, 29], we expected that the group of subjects with cognitive fog associated with anosmia would exhibit more intense cognitive symptoms. Contrary to this expectation, the results of this study showed that the values of P300 and beta band rhythms in subjects with only cognitive fog and those with cognitive fog associated with anosmia did not show significant differences. This does not exclude the possibility that cognitive fog may be due to virus neuroinvasion through other pathways such as the vagus nerve or trigeminal nerve [27].
The key findings of this research have revealed three important discoveries regarding cognitive fog symptoms due to Long-Covid: (a) a significant connection has been found between cognitive fog symptoms occurring after Long-Covid and neurophysiological alterations, particularly in the brain waves of P300 and beta frequency bands; (b) the identified neurophysiological alterations appear to persist in the long term, suggesting that the effects of Long-Covid on brain function may endure over time; (c) the presence or absence of anosmia does not seem to be significantly correlated with the observed neurophysiological alterations. This suggests that anosmia may not be a determining factor in the neurophysiological consequences of Long-Covid.
This study provides a significant contribution as it adds crucial knowledge about the relationship between cognitive fog and neurophysiological alterations in the context of Long-Covid. Understanding this connection could open new perspectives in the diagnosis and management of health-related cognitive impairments [61, 62, 63]. Moreover, recognizing the persistence of neurophysiological alterations in the long term emphasizes the importance of carefully assessing patients with Long-Covid to prevent and address potential cognitive deficits [64, 65, 66]. The main strengths of this research can be identified in its longitudinal nature, which allowed for the observation of symptoms and neurophysiological alterations over time, providing an in-depth perspective on the persistence of cognitive fog due to Long-Covid. Additionally, the inclusion of a comparison group with and without anosmia further allowed exploration of the correlation between this condition and cognitive fog, contributing to the understanding of the complex manifestations of Long-Covid. These combined aspects make this study a significant contribution to the scientific literature regarding the long-term effects of Covid-19 on the brain and provide valuable foundations for the development of targeted clinical interventions to support patients affected by Long-Covid.
However, there are also some limitations to consider. For instance, the study sample might be limited in terms of age, potentially leading to underrepresentation of specific demographic groups. This aspect should be considered in future studies to ensure greater representativeness of the results. Additionally, the relationship between cognitive fog and neurophysiological components requires further investigation and exploration. The absence of pre-infection P300 data does not exclude the possibility that EEG alterations may serve as a predisposing factor for cognitive fog, rather than representing a response to the infection itself. The data should be considered cautiously because the group of healthy subjects was drawn from a database within the hospital, but we are not aware of whether they contracted the virus or not. This leads us to conclude that all observed differences were likely to be attributed to the presence of COVID-19 along with cognitive fog. Essentially, it is not clear which attribution we can solely attribute to cognitive fog. The limitation is related to the absence of pre-infection data; for this reason, we cannot establish a causal link with absolute certainty due to the absence of pre-infection data. Future research should directly compare individuals with COVID-19-related brain fog to those without, collecting complete pre-infection data to better understand the virus’s role in neurocognitive symptoms.
It is important to note that in the current study, measures of the severity of symptoms related to cognitive fog were not available. In future investigations, it may be beneficial to consider incorporating quantitative measures of symptomatology to provide a more comprehensive and detailed assessment of the extent of the disorder. While connections between these phenomena have been observed, the exact mechanism of brain function slowing and its link with mood and other factors are not yet fully understood. Therefore, it is essential to encourage further multidisciplinary and longitudinal studies on patients affected by Long-Covid and other relevant conditions. It is important to acknowledge that the manifestation of cognitive fog may vary depending on different conditions and variables involved, which could influence its relationship with neurophysiological parameters depending on the underlying cause. Furthermore, confounding factors such as depression and chronic fatigue syndrome could independently influence brain function, emphasizing the need for more in-depth investigations into the complex interaction among these variables.
In conclusion, further research is needed to achieve a comprehensive understanding of this complex phenomenon. Future studies should include a larger sample involving a wide range of ages and other demographic characteristics to obtain more representative and generalizable results. Adopting a multidisciplinary approach would be desirable, considering psychosocial factors such as mood, stress, and quality of life.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Material preparation, data collection and analysis were performed by AG, RS & RAF. The first draft of the manuscript was written by RAF & RS. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Approval was obtained from the ethics committee of the Madonna della Consolazione Polyclinic Nursing Home (protocol code: 2022-269). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
