Academic Editor: Govindan Dayanithi
The major hallmark of Parkinson’s disease (PD) is the
degeneration of dopaminergic neurons in the substantia nigra (SN), which is
responsible for the core motor symptoms of PD. Currently, there is no cure for
PD, and its prevalence is increasing, prompting the search for novel
neuroprotective treatments. Neuroinflammation is a core pathological process in
PD, evident by increased inflammatory biomarkers in the SN and cerebrospinal
fluid. Interestingly, epidemiological studies have reported a reduced risk of PD
in users of non-steroidal anti-inflammatory drugs compared to non-users,
suggesting the neuroprotective potential of anti-inflammatory drugs. Therefore,
this study aimed to: (1) test the efficacy of novel oral formulations of
edaravone (EDR) and curcumin (CUR) (which possess anti-inflammatory and
anti-oxidative properties) to alleviate motor and non-motor symptoms, and
associated pathology in the intrastriatal lipopolysaccharide (LPS) model of PD;
(2) investigate the expression of proteins linked to familial PD and markers of
autophagy in the intrastriatal LPS model treated with EDR and CUR.
Fifty-two C57BL/6 mice were divided into 4 groups, namely; (1)
control + vehicle; (2) LPS + vehicle; (3) LPS + EDR (made in vehicle) and (4) LPS
+ CUR (made in vehicle). 10
Parkinson’s disease (PD) is characterised by the cardinal motor features such as
bradykinesia, rigidity, resting tremors, and postural/gait disorders. These motor
features are largely associated with the degeneration of dopaminergic neurons in
the substantia nigra pars compacta (SNpc), which results in the
depletion of striatal dopamine, a neurotransmitter essential for the control of
movement [1]. PD is classically a motor disorder; however, it has a non-motor
aspect characterised by olfactory dysfunction, gastrointestinal dysfunction,
anxiety, depression, autonomic disturbance, and cognitive dysfunction that is now
well recognised, and evident to precede the onset of motor symptoms [2, 3]. Due
to the lack of treatments, it is the non-motor symptoms of PD that have a
significant effect on the patients’ quality of life [2]. Additionally, PD is
characterised by the presence of Lewy bodies/neurites in the surviving
dopaminergic neurons of the SN, and these are cytoplasmic inclusions consisting
of abnormal aggregates of
Currently, there are no disease-modifying therapies for PD, and the available
treatments such as levodopa, dopamine agonists, monoamine oxidase B inhibitors,
catechol-O-methyltransferase inhibitors, and deep brain stimulation, only provide
relief from motor symptoms [7]. Unfortunately, the beneficial effects of these
treatments decrease with time due to the progressive nature of PD, prompting the
search for novel treatment approaches [2, 7]. The current PD treatments focus on
preserving the levels of endogenous dopamine and do not target the core
pathological processes implicated in PD. Therefore, targeting the prominent
pathological mechanisms of PD, in combination with the available dopamine therapy
could have disease-modifying effects in patients. Neuroinflammation, oxidative
stress, and defective protein clearance are crucial events in the aetiology of
PD, and for this reason, we aimed to further explore these pathways in this
study. Neuroinflammation is a well-recognised phenomenon in PD patients, evident
by the presence of activated microglia cells in the SN of post-mortem PD brains,
and pro-inflammatory cytokines in the serum and cerebrospinal fluid [8, 9, 10]. These
findings are supported by epidemiological studies indicating a decreased risk of
PD in users of non-steroidal anti-inflammatory drugs compared to non-users,
emphasizing the importance of controlling inflammation in PD [11]. Additionally,
a multitude of genetic loci have been associated with the monogenic form of PD
which has Mendelian inheritance, and these include genes encoding for
In our recent work, we characterised the intrastriatal LPS model of PD which is
beneficial for understanding the neuroinflammatory aspect of the disease [21],
and for investigating the efficacy of antioxidants and anti-inflammatory
compounds. For this study, we selected to further explore edaravone and curcumin
which have been shown to have neuroprotective effects in-vivo due to
their antioxidant and anti-inflammatory properties [22, 23, 24]. These compounds can
be easily repurposed for clinical treatment of PD if they have disease-modifying
effects. Edaravone has already been approved by the Food and Drug Administration
for the treatment of amyotrophic lateral sclerosis in the USA, and by authorities
in Asian countries for the treatment of ischemic stroke. However, the drug is
administered twice a day intravenously due to its poor oral bioavailability which
could be inconvenient and distressful for patients [25]. In contrast, curcumin is
an active compound of turmeric, commonly used in Indian species with great
neuroprotective potential. Nonetheless, its progress to clinical use has been
impeded by its poor oral bioavailability associated with its physiochemical
properties [26]. Previously, Parikh and colleagues from our laboratory developed
novel oral formulations of edaravone (EDR) and curcumin (CUR) using soluplus, a
polymer as a drug carrier [27, 28]. These novel formulations had improved
physical properties and oral bioavailability, and were shown to have protective
effects in animal models of Alzheimer’s disease (AD) [29, 30]. Therefore for this
study, we aimed to investigate the therapeutic effects of EDR and CUR on motor
and non-motor symptoms of PD, and the associated pathological changes such as
reduced tyrosine hydroxylase (TH), increased
Curcumin was purchased from Chem-Supply Pty Ltd. (South Australia, Australia), soluplus was a gift from BASF Australia Ltd, and EDR was obtained from Suzhou Auzone Biotech, China. The novel oral formulation of CUR was prepared by combining soluplus and neat curcumin in a ratio of 1:10 and then dissolved in absolute ethanol [27]. The ethanol was evaporated using a rotary evaporator, and the novel formulation of CUR was left to dry in the desiccator. After drying, the CUR formulation was pulverised with mortar and pestle and dissolved in acidified drinking water.
All the animal experiments were approved by the Animal Ethics Committee of the
University of South Australia. Fifty-two C57BL/6 mice were purchased from the
Animal Resources Centre (Western Australia, Australia) when they were 9-weeks
old, and housed at the Core Animal Facility at the University of South Australia
for the duration of the experiment. The mice were housed in a pathogen-free
environment with a 12 h alternating light/dark cycle and had an unlimited supply
of food and water. LPS (E. coli 0111.4) was purchased from Sigma-Aldrich (USA)
and diluted to 5 mg/mL in sterile water and stored at –80 °C. C57BL/6
mice were trained for the buried food-seeking test and rotarod test before the
stereotaxic injections to ensure that they were accustomed to these tests.
Subsequently, the mice were randomly divided into 4 groups, namely: control +
vehicle (soluplus) (n = 12), LPS + vehicle (n = 15), LPS + EDR (made in vehicle)
(n = 13), and LPS + CUR (made in vehicle) (n = 12). A total of 10
 Fig. 1.
Fig. 1.The timeline of the study. C57BL/6 mice were injected
intrastriatally with 10
All the surgical procedures were conducted according to recent studies [21].
Briefly, the mice were deeply anaesthetised via inhalation of isoflurane and
mounted onto the stereotaxic frame (motorized stereotaxic apparatus, Stoelting,
USA). Once the mice were stable under anaesthesia, the skin on the cranium was
prepared with 2% chlorhexidine/70% ethanol, and an incision was made on the
scalp from the lambda and extended anteriorly to between the eyes. The bregma
point was identified by applying 3% hydrogen peroxide to the exposed cranium and
the two injection sites of the right striatum were identified using the following
coordinates from the bregma point (point A: +1.2 mm anterior-posterior, +1.5 mm
medial-lateral, –3.5 mm deep, and point B: –0.34 mm anterior-posterior, +2.5 mm
medial-lateral, and –3.2 mm deep) [31]. Subsequently, a micro-drill was used to
drill a hole at each of the injection sites, and a 10
Behavioural testing was conducted at 4- and 8-weeks after intrastriatal injection of LPS to determine if the animals display olfactory deficits (assessed by buried-food seeking test), anxiety-like behaviour (assessed by open field test), and motor deficits (assessed by rotarod test). These behavioural tests are described in more detail below.
The buried food-seeking test was used to assess olfactory function, and it was performed according to previous studies [21]. Briefly, mice fasted overnight for 14–18 h, were placed into a clean individual home cage. They were given up to 10 min to locate a standard chow pellet hidden underneath the bedding of the home cage. The time it took for the mice to find the hidden food was recorded as the latency time.
The open-field test was used to assess anxiety-like behaviour, and it was performed according to recent studies [21]. Firstly, the mice were placed in an open field arena that was divided into a peripheral and a central zone. Subsequently, they were allowed to explore the open field arena for 5 min, and their movement was tracked with ANY-maze, a video tracking software (Stoelting, USA).
The rotarod test assesses balance, endurance, and motor coordination, and it was
used to assess motor deficits in LPS injected mice [21, 32]. Briefly, the mice
were placed on a rotarod apparatus (Mouse RotaRod NG, Ugo Basile) set to
accelerate from 5–30 rpm within 5 min. The latency time was recorded for each
mouse, and this refers to the time it took for the mouse to fall off the rotating
rod or hang on the rotating rod and swung 360
After the last behavioural test at 8-weeks, the mice were humanely killed via intraperitoneal injection of sodium pentobarbitone (60 mg/kg). The brain tissues were collected fresh, sliced into different areas of interest such as the olfactory bulb and the striatum using brain matrix, and stored at –80 °C.
Fresh brain tissues (e.g., olfactory bulb and striatum) were homogenised in RIPA
buffer (50 mM tris, 150 mM sodium chloride, 1 mM ethylenediaminetetraacetic acid,
0.5% Triton X-100, 0.5% sodium deoxycholate, pH 7.4) plus cocktail protease
inhibitor using Precellys 24 Homogeniser (Bertin Technologies,
Montigny-le-Bretonneux, France). The protein concentration of the homogenates was
measured with a Micro-BCA
Each of the homogenates was diluted in a sample buffer in preparation for gel
electrophoresis. Western blotting was carried out as outlined in our previous
study [21]. Briefly, the proteins were electrophoretically separated on a sodium
dodecyl sulfate polyacrylamide gel for 90 min. The proteins on the gel were
transferred onto a nitrocellulose membrane for 90 min at 0.6 or 0.8 amps, and the
membrane was air-dried for 1 h to allow better adhesion of the proteins. The
nitrocellulose membrane was then blocked for 1 h with 5% milk or 5% BSA to
reduce non-specific binding and incubated overnight with specific primary
antibodies (Table 1). Subsequently, the nitrocellulose membrane was incubated for
1 h at room temperature with corresponding secondary antibodies for near-infrared
western blot detection (Table 1). The proteins on the nitrocellulose membrane
were visualized with Odyssey CLx imaging system (LI-COR Biosciences, USA), and
quantified with Image Studio Lite 5.2 (LI-COR
Biosciences, Los Angeles, LA, USA) using
| Antibodies | Company | Dilution | |
| Primary antibodies | |||
| Mouse anti-heat shock cognate protein of 70 kDa (HSC-70) | Santa Cruz Biotechnology | 5% BSA/TBST (1:1000) | |
| Mouse anti-lysosomal-associated membrane protein 1 (LAMP-1) | Santa Cruz Biotechnology | 5% BSA/TBST (1:500) | |
| Mouse anti- microtubule-associated protein 1A/1B-light chain 3 b (LC3) | Santa Cruz Biotechnology | 5% BSA/TBST (1:250) | |
| Mouse anti-parkin | Santa Cruz Biotechnology | 5% BSA/TBST (1:500) | |
| Mouse anti-PINK1 | Santa Cruz Biotechnology | 5% BSA/TBST (1:1000) | |
| Mouse anti-tyrosine hydroxylase (TH) | Sigma | 5% BSA/TBST (1:7000) | |
| Mouse anti- |
Abcam | TBST (1:15000) | |
| Rabbit anti-glial fibrillary acidic protein (GFAP) | Dako | 5% milk/TBST (1:2000) | |
| Rabbit anti-LRRK2 | Abcam | 5% BSA/TBST (1:500) | |
| Rabbit anti-Rab-10 | Abcam | 5% BSA/TBST (1:1000) | |
| Rabbit anti-Rab-10 (phospho-T73) | Abcam | 5% BSA/TBST (1:250) | |
| Rabbit anti- |
Santa Cruz Biotechnology | 5% BSA/TBST (1:300) | |
| Secondary antibodies | |||
| Donkey anti-rabbit IgG | Li-Cor Biosciences | TBST (1:20,000) | |
| Goat anti-mouse IgG | Li-Cor Biosciences | TBST (1:20,000) | |
Using GraphPad Prism 8 software (San Diego, CA, USA), we analysed the
behavioural data with 2-way ANOVA test, followed by Tukey’s multiple comparisons
test. In addition, the western blot data were analysed with the Kruskal-Wallis
test followed by Dunn’s multiple comparisons test. All the data are presented as
mean
Intrastriatal administration of LPS induced motor impairment at 4-weeks (p = 0.0360) and 8-weeks (p = 0.0023) in LPS injected mice versus controls, indicated by a reduction in latency time in the rotarod test (Fig. 2A). The impairment in motor function was not rescued by EDR or CUR. Secondly, the olfactory function was not altered by intrastriatal administration of LPS according to the buried food-seeking test (Fig. 2B).
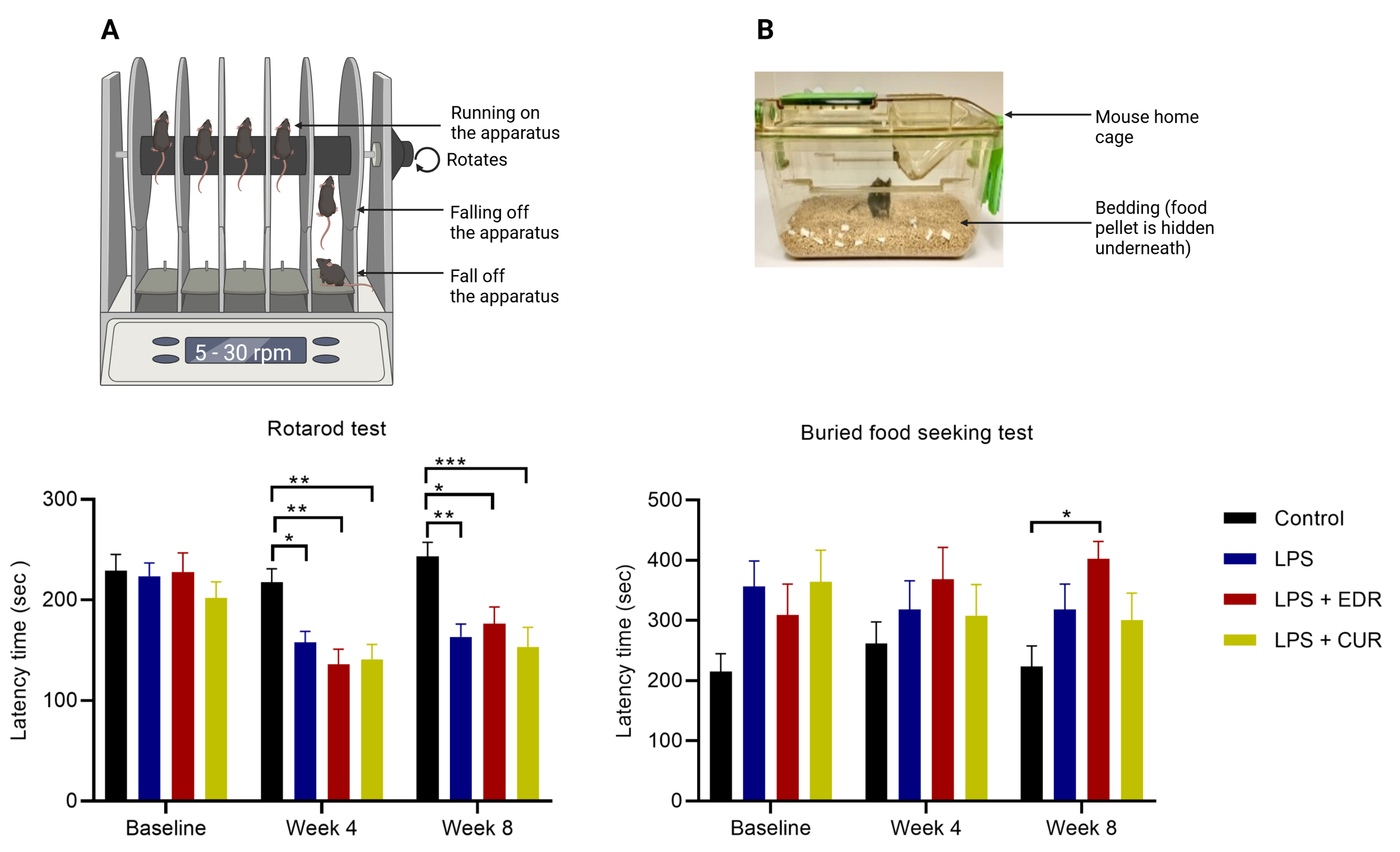 Fig. 2.
Fig. 2.The effects of EDR and CUR on motor and olfactory function
following intrastriatal administration of LPS. (A) The effects of EDR and CUR on
motor function assessed with the rotarod test (latency time in sec). (B) effects
on olfactory function assessed with buried food-seeking test (latency time in
sec). The results are presented as mean
The open-field test was used to examine the effects of LPS on voluntary movement
and anxiety-like behaviour. Intrastriatal LPS in C57BL/6 mice did not induce any
significant changes in the voluntary movement, illustrated by the total distance
travelled in the open field test (Fig. 3B). Also, there was a trend towards
reduction in the number of central zone entries in the open field test at 4-weeks
(p = 0.0660) and a significant reduction at 8-weeks (p = 0.0229) (Fig. 3C), accompanied by reduced central zone time at 8-week (p = 0.0005) (Fig. 3D) in LPS group compared to the control mice. Unlike EDR, the
number of central zone entries was not significantly different in controls versus
the CUR group at 4-weeks (p
 Fig. 3.
Fig. 3.The effects of EDR and CUR on voluntary movement and
anxiety-like behaviour following intrastriatal administration of LPS. (A)
Schematic of the open field arena. (B) Total distance travelled in the open field
(in metres denoted as m). (C) Number of central zone entries. (D) Central zone
time (sec). The results are presented as mean
The major neuropathological hallmarks of PD are the degeneration of the
nigrostriatal pathway, constituted by dopaminergic neurons of the SN and their
neuronal projections to the striatum, and the presence of Lewy bodies made up
predominately of
 Fig. 4.
Fig. 4.The effects of EDR and CUR on the protein expression of TH,
LAMP-1, HSC-70, and LC3 are involved in autophagy; therefore, we scrutinised the
effects of EDR and CUR on these proteins in LPS treated mice. Intrastriatal
administration of LPS did not alter the expression of LAMP-1 protein (p = 0.8499) (Fig. 5B) and LC3 (p
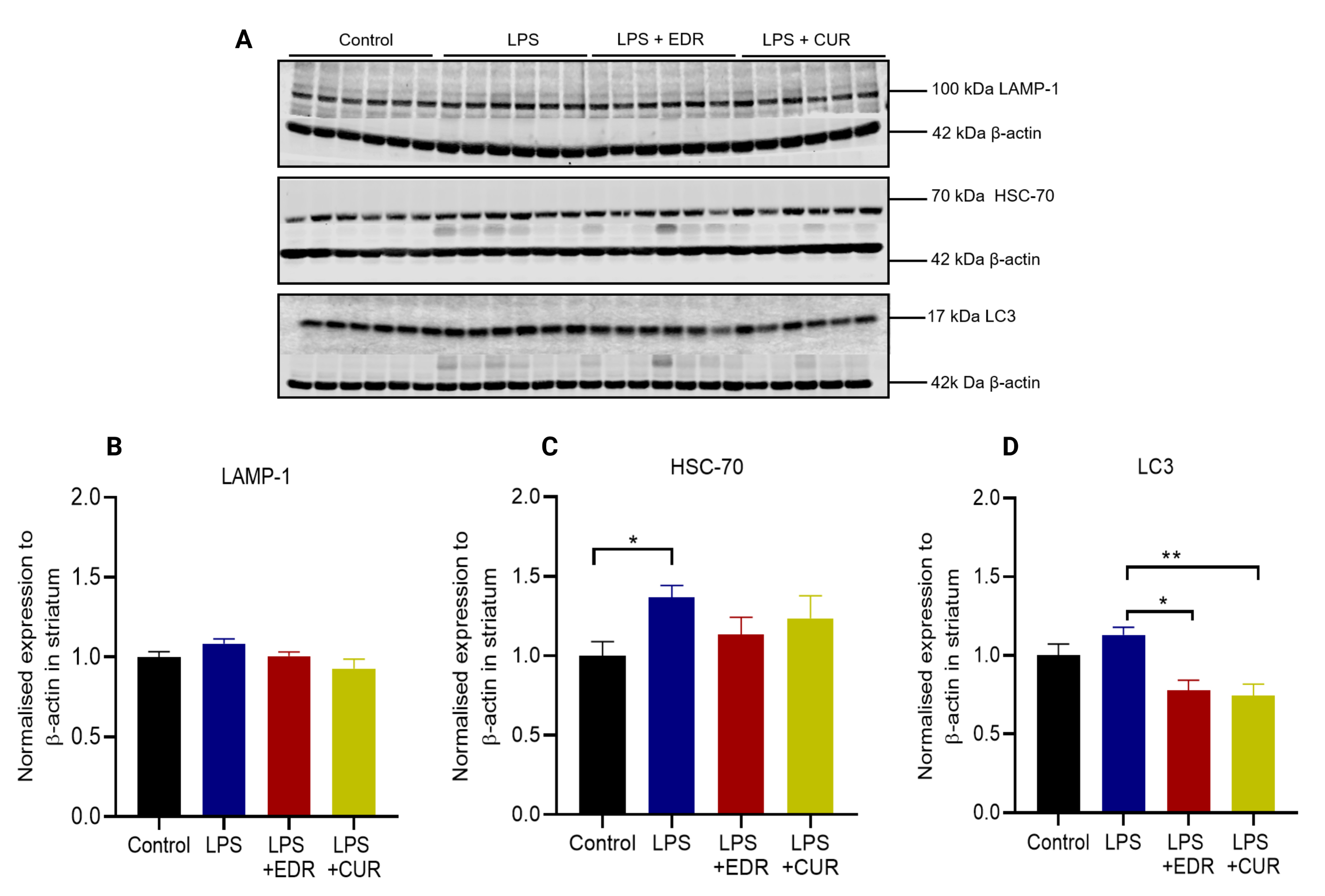 Fig. 5.
Fig. 5.The effects of EDR and CUR on the protein expression of LAMP-1,
HSC-70, and LC3 in the striatum following intrastriatal administration of LPS.
(A) Representative immunoblots for the protein expression of LAMP-1, HSC-70, and
LC3. (B) Densitometric analysis of LAMP-1. (C) Densitometric analysis of HSC-70.
(D) Densitometric analysis of LC3. The results are presented as mean
Mutations in the genes for PINK-1 and parkin are among the common causes of genetic PD, and the encoded proteins are essential for defence against oxidative stress, and clearance of defective mitochondria via autophagy. Intrastriatal administration of LPS induced a trend towards increased protein expression of PINK1 (p = 0.0736) (Fig. 6B) and parkin (p = 0.0858) (Fig. 6C). Interestingly, there was a significant reduction in the expression of parkin protein in the CUR group compared to the LPS group (p = 0. 0225).
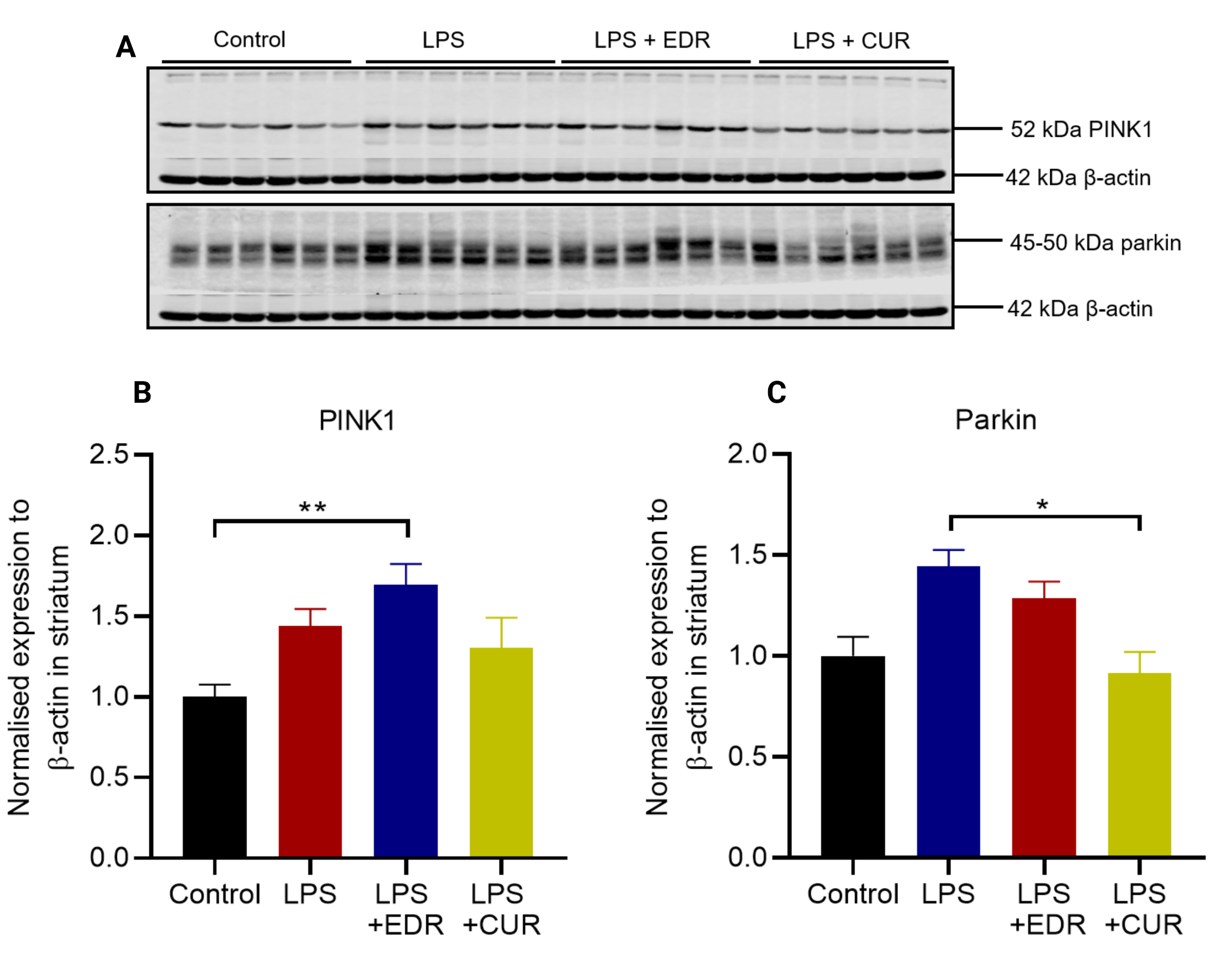 Fig. 6.
Fig. 6.The effects of EDR and CUR on the protein expression of PINK1
and parkin in the striatum following intrastriatal administration of LPS. (A)
Representative immunoblots for the protein expression of PINK1 and parkin. (B)
Densitometric analysis of PINK1. (C) Densitometric analysis of parkin. The
results are presented as mean
Mutations in LRRK2, a serine/threonine kinase are the most common cause of autosomal dominant PD [12]. LRRK2 is involved in autophagy via the modulation of Rab GTPases [23]. As a result, we examined the protein expression of LRRK2, Rab-10, and phospho-Rab-10 in the striatum. Intrastriatal LPS did not alter the expression of LRRK2 protein (p = 0.5650) (Fig. 7B) compared to controls. Additionally, there was a significant increase in Rab-10 protein (p = 0.0493) (Fig. 7C) with no alteration in its phosphorylation (p = 0.2026) (Fig. 7D) compared to the LPS group. In contrast, the phosphorylation of Rab-10 was significantly reduced in EDR (p = 0.0197) and CUR group (p = 0.0010) compared to controls. Although, there was a reduced phosphorylation of Rab-10 in EDR and CUR groups, this did not differ compared to the LPS group.
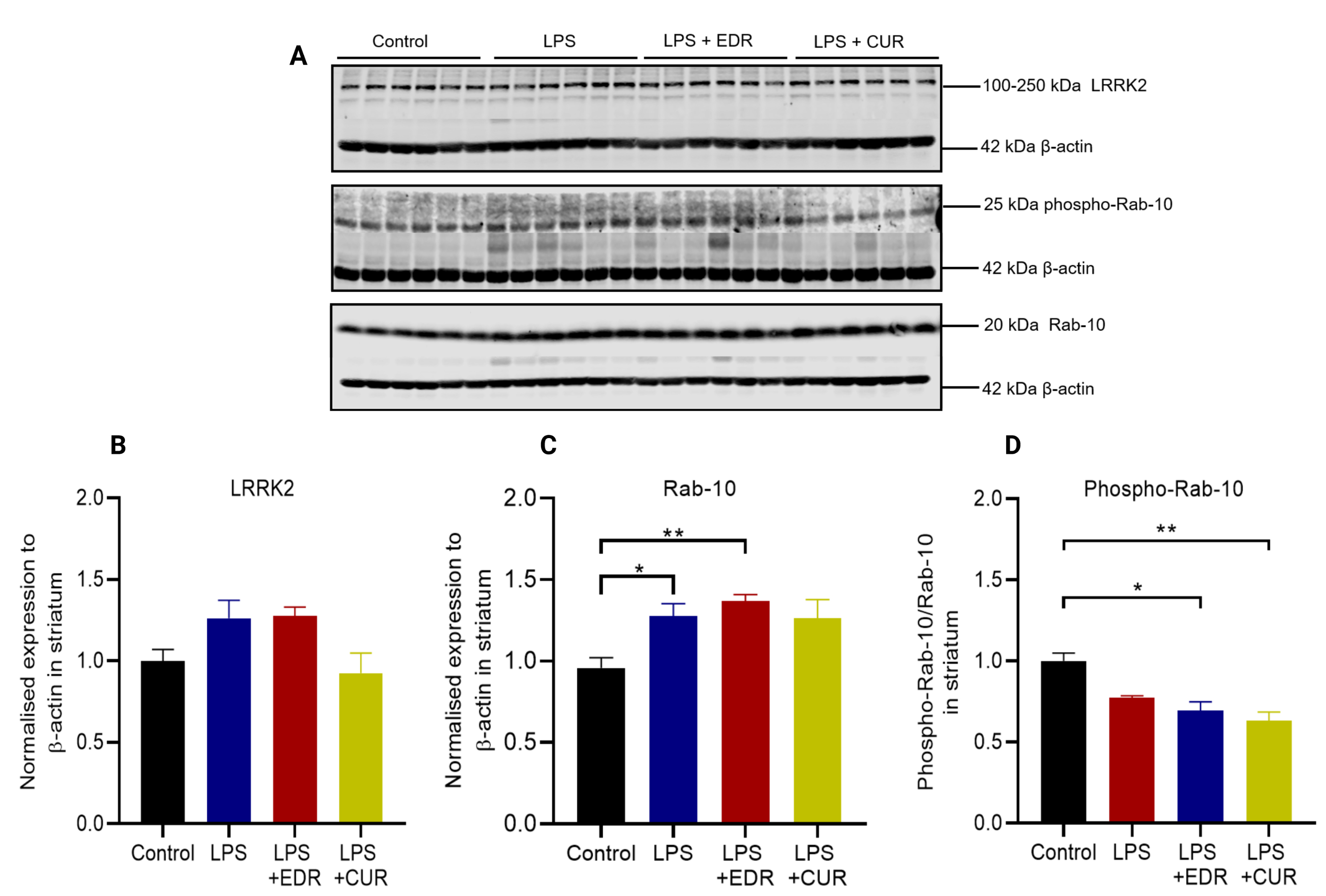 Fig. 7.
Fig. 7.The effects of EDR and CUR on the protein expression of LRRK2,
Rab-10, and phospho-Rab-10 in the striatum following intrastriatal administration
of LPS. (A) Representative immunoblots for the protein expression of LRRK2,
Rab-10, and phospho-Rab-10. (B) Densitometric analysis of LRRK2. (C)
Densitometric analysis of Rab-10. (D) Densitometric analysis of phospho-Rab-10.
The results are presented as mean
Intrastriatal LPS did not alter the expression of TH protein in the olfactory bulb compared to controls (p = 0.6668) (Fig. 8B). However, it induced astroglial activation indicated by increase expression of GFAP protein (p = 0.0155) (Fig. 8C). The administration of EDR or CUR did not alter the expression of GFAP protein in the olfactory bulb.
 Fig. 8.
Fig. 8.The effects of EDR and CUR on the protein expression of TH and
GFAP in the olfactory bulb following intrastriatal administration of LPS. (A)
Representative immunoblots for the protein expression of TH and GFAP. (B)
Densitometric analysis of TH. (C) Densitometric analysis of GFAP. The results are
presented as mean
Intrastriatal LPS increased the expression of HSC-70 protein in the olfactory bulb compared to the control group (p = 0.0014) (Fig. 9B). Interestingly, its expression was returned to the control level by oral CUR (p = 0.0422) but not EDR. Additionally, intrastriatal LPS did not alter the expression of LC3 protein in the olfactory bulb (p = 0.3301) (Fig. 9C), with no effects associated with EDR and CUR.
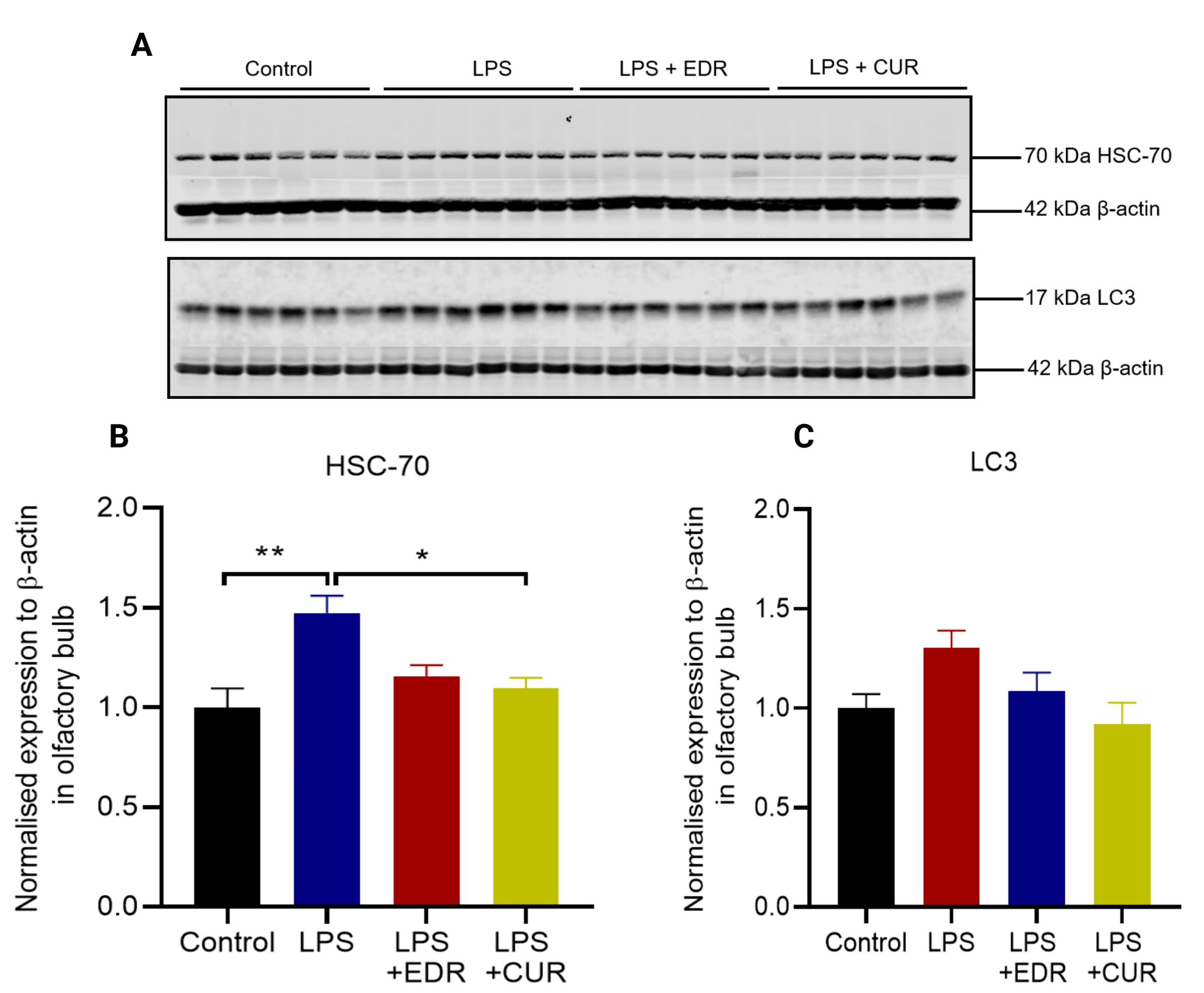 Fig. 9.
Fig. 9.The effects of EDR and CUR on the protein expression of HSC-70
and LC3 protein in the olfactory bulb following intrastriatal administration of
LPS. (A) Representative immunoblots for the protein expression of HSC-70 and
LC3. (B) Densitometric analysis of HSC-70. (C) Densitometric analysis of LC3. The
results are presented as mean
Intrastriatal LPS did not alter the expression of PINK1 protein (p = 0.3367) (Fig. 10B) and parkin protein (p = 0.3972) (Fig. 10C) compared to the controls. In contrast, CUR significantly reduced the expression of PINK1 protein compared to the LPS group (p = 0.0269).
 Fig. 10.
Fig. 10.The effects of EDR and CUR on the protein expression of PINK1
and parkin in the olfactory bulb following intrastriatal administration of LPS.
(A) Representative immunoblots for the protein expression of PINK1 and parkin.
(B) Densitometric analysis PINK1. (C) Densitometric analysis of parkin. The
results are presented as mean
LPS itself (LPS versus control) did not alter the expression of LRRK2 (p = 0.4591) (Fig. 11B) and phosphorylation of Rab-10 (p = 0.7527) (Fig. 11D), but there was a trend towards increased expression of Rab-10 protein (p = 0.0657) (Fig. 11C) in the olfactory bulb. On the other hand, CUR significantly decreased the protein expression of LRRK2 (p = 0. 0007) and returned the phosphorylation of Rab-10 (p = 0.0021) to the control levels when compared to the LPS group. Additionally, both EDR (p = 0.0253) and CUR (p = 0.0003) significantly reduced the expression of Rab-10 protein compared to LPS group.
 Fig. 11.
Fig. 11.The effects of EDR and CUR on the protein expression of LRRK2,
Rab-10, and phospho-Rab-10 in the olfactory bulb following intrastriatal
administration of LPS. (A) Representative immunoblots for the protein expression
of LRRK2, Rab-10, and phospho-Rab 10. (B) Densitometric analysis of LRRK2. (C)
Densitometric analysis of Rab-10. (D) Densitometric analysis of phopsho-Rab-10.
The results are presented as mean
This study focused on investigating the effects of EDR and CUR in LPS treated
mice. The main findings include: (1) Intrastriatal administration of LPS
impaired motor function, induced anxiety-like behavior and caused a reduction in
TH protein and increases in
Inflammation is a complex process mediated by multiple cell types and
markers. Intrastriatal administration of LPS increased the protein
expression of GFAP, an astrocytic marker in the striatum at 8-weeks after LPS
injection. It is important to note that reactive astrocytes have both pro- and
anti- inflammatory profiles which can exert both neuroprotective and neurotoxic
effects [10]. Increased GFAP in this study is consistent with our previous study
[21] whereby we used western blotting, and immunohistochemistry to illustrate
that LPS induced activation of both astrocytes and microglia in the striatum,
suggesting the involvement of inflammatory processes. The inflammation could have
increased the protein expression of
Abnormal secretion of
The clearance of
We showed in this study, and in our previous publication [21] that intrastriatal LPS induces inflammation and oxidative stress in the striatum, which may potentially cause mitochondrial dysfunction, a critical aspect in the pathogenesis of PD. Mutations in PINK1, parkin and LRRK2 are implicated in mitochondrial dysfunction in the nigrostriatal pathway; therefore, we examined their expression in non-genetic models of PD. The autophagic clearance of damaged mitochondria (referred to as mitophagy) is essential for mitochondrial quality control and reduction in reactive oxygen species (ROS) production. Mitophagy can be initiated in a PINK1/parkin-dependent manner, and deletion in these proteins in mice results in impaired mitochondrial biogenesis and respiration, increased sensitivity to oxidative stress, and reduced synaptic excitability [44, 45, 46]. We found that intrastriatal administration of LPS induced a trend towards increased expression of PINK1 and parkin in the striatum. Oral EDR and CUR did not have any noticeable effects on the expression of these proteins. PINK1 and parkin are not well studied in non-genetic models of PD, and additional studies are needed to further understand their role in sporadic PD. According to our findings intrastriatal injection of LPS did not have significant effects on mitochondrial proteins such as PINK1 or parkin in the striatum which may suggest intact mitochondrial integrity. However, it is important to note that we did not directly examine mitochondrial morphology or its function, and additional investigation is required to truly understand the effects of our LPS treatment regime on mitochondrial morphology/mitochondrial function as illustrated in LPS rat models of PD [47, 48, 49].
Lastly, LRRK2 protein acts through the Rab GTPases to modulate vesicular trafficking and autophagy [33]. PD mutations such as G2019S in the LRRK2 gene, increase LRRK2 kinase activity, which impairs PINK1/parkin-dependent mitophagy by increasing the phosphorylation of Rab-10 at threonine 73 [50, 51]. Rab-10 protein works downstream in PINK1/parkin-dependent mitophagy to recruit mitochondria to the autophagosome, which is fused with lysosome for degradation. This process is disrupted when Rab-10 protein is aberrantly phosphorylated by LRRK2 [50, 52, 53]. The inhibition or knockout of LRRK2 proteins which reduces its kinase activity, increases mitophagy, suggesting a therapeutic potential of targeting the LRRK2 pathway in PD [51, 54]. Based on the current study, we found that intrastriatal injection of LPS did not alter LRRK2 protein in the striatum. However, it increased Rab-10 protein with a reduction in its phosphorylation at threonine 73 suggesting that LPS may have also activated protein serine/threonine phosphatases. The increased Rab-10 protein in response to intrastriatal LPS could be a protective mechanism to enhance PINK1/parkin-dependent mitophagy. This doctrine is consistent with a study by Wauters and colleagues which demonstrated that increased expression of Rab-10 protein in LRRK2 mutant cells enhances mitophagy in a PINK1/parkin-dependent manner [53]. Of note, our finding for Rab-10 differs compared to a study by Rocha and colleagues [51] which showed that rotenone increased the phosphorylation of Rab-10 in the dopaminergic neurons, which may suggest variability in the pathways targeted in neurotoxin models. Therefore, a reduction of TH protein in the striatum, which may correlate with dopaminergic axonal degeneration, is likely associated with glial-induced inflammation which was not sufficiently suppressed by EDR and CUR. One could examine the levels of inflammatory cytokines, chemokines, and free radicals in the striatum, as well as the upstream pathways responsible for such response to ascertain the pathological mechanisms in the striatum. It was a limitation of our study that we did not examine the aforementioned parameters.
The presence of Lewy bodies (inclusions mostly composed of
Next, we examined the effects of intrastriatal LPS on the expression of
autophagic markers in the olfactory bulb that may be affected in non-genetic PD,
and to our knowledge they have not been examined in PD models. Firstly,
intrastriatal LPS did not alter the expression of LC3, which might suggest intact
autophagy. In contrast, there was increased expression of HSC-70 protein in the
olfactory bulb which indicates the activation of CMA. HSC-70 mediates the
clearance of
Anxiety has not been widely examined in LPS and other models of PD. Nonetheless, it has been shown in animal models of PD via the open field test and the elevated plus-maze test that lesions to the nigrostriatal pathway induce anxiety-like behaviour [56, 57]. Congruent with these findings, we showed that intrastriatal injection of LPS reduced central zone entries and time at 4 and/or 8-weeks which are indicative of anxiety-like behaviour. This result, however, is not consistent with our previous study when we did not find anxiety-like behaviours after LPS injections [21]. This discrepancy may potentially be explained by the fact that in the current study the control and LPS groups were receiving soluplus (120 mg/kg) orally in drinking water to match the treatment conditions of EDR and CUR groups between weeks 3 and 8 of the study. Unlike EDR, the number of central zone entries was not significantly different in the CUR group compared to controls, suggesting that CUR may have some therapeutic effects on anxiety-like behaviour, although, this was not significant compared to the LPS group. Regions of the brain such as the amygdala, prefrontal cortex, and hippocampus are vital in the modulation of anxiety [58]. We were unable to examine the pathological effects of intrastriatal LPS on these regions, and this would be an insightful addition for future studies.
In summary, intrastriatal LPS induced pathological changes of PD such as reduced
expression of TH protein and increased
ID performed all the experiments and wrote the manuscript. LB and XFZ were involved in conceptualization, methodology, supervision, and editing of the manuscript. SG was involved in student supervision and contributed to the critical review of the manuscript. All authors read and approved the final manuscript.
All the animal experiments reported in this manuscript were approved by the Animal Ethics Committee of the University of South Australia under the animal ethics U12-21.
Isaac Deng is a recipient of University of South Australia Postgraduate Award, Australia.
This research received no external funding.
The authors declare no conflict of interest.
