Academic Editor: Jen-Tsung Chen
The search for new antimicrobials is essential to address
the worldwide issue of antibiotic resistance. The present work aimed at assessing
the antimicrobial activity of Aesculus hippocastanum L. (horse chestnut)
bark against bacteria involved in urinary tract infections (UTIs).
Bioactive compounds were extracted from A.
hippocastanum bark using water and ethanol as solvents. The extracts were tested
against 10 clinical uropathogenic strains including five Gram-positive and five
Gram-negative bacteria. Staphylococcus aureus ATCC 6538 and
Escherichia coli ATCC 25922 were used as reference bacteria. The susceptibility
to antibiotics was assessed using the Kirby Bauer disc diffusion method and the
antibacterial activity of the extracts was evaluated using the well diffusion
method. The Minimum inhibitory concentration (MIC) and the minimum bactericidal
concentrations (MBC) were asseded by the microdilution method.
A. hippocastanum bark possessed a dry matter content of 65.73%. The
aqueous extract (AE) and ethanolic extract (EE) showed a volume yield of 77.77%
and 74.07% (v/v), and a mass yields of 13.4% and 24.3% (w/w) respectively. All
the bacteria were susceptible to amoxiclav, imipenem and ceftriaxone but the
clinical strains were resistant to at least one antibiotic. Kocuria
rizophilia 1542 and Corynebacterium spp 1638 were the most resistant
bacteria both with multidrug resistance index of 0.45. Except AE on
Proteus Mirabilis 1543 and Enterococcus faecalis 5960 (0 mm),
both AE and EE were active against all the microorganisms tested with inhibition
diameters (mm) which ranged from 5.5–10.0 for AE and 8.0–14.5 for EE. The MICs
of EEs varied from 1–4 mg/mL while those of AEs varied from 4–16 mg/mL. The
ethanolic extracts (EE) were overall more active than the aqueous
ones. The A. hippocastanum bark extracts had
overall weak antibacterial activity (MIC
Urinary tract infections (UTIs) are very common infections and occur at least
once in a lifetime. These infections are serious public health issues and are
responsible for nearly 150 million disease cases every year worldwide [1]. UTIs
are defined as any infection, commonly of bacterial origin, which occurs in any
part of the urinary system [1]. When UTIs are localized in urethra, they are
called urethritis, cystitis (when localized in the bladder), pyelonephritis
(infection of the kidneys), and vaginitis (infection of the vagina) [2, 3]. UTIs
are more likely to occur in women, because, compared to men, their urethra is
shorter and there is relative proximity between the urethra and the anus [4]. In
addition, the prevalence of UTIs among sexually active young women has been
reported to vary from 0.5 to 0.7 person per year, while this incidence rate among
young men was only 0.01 [5]. Otherwise, the so-called uropathogenic bacteria is
responsible for 80–90% of UTIs but other germs such as Staphylococcus
saprophyticus, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella
pneumoniae, Proteus mirabilis, Acinetobacter baumannii, Streptococcus, and
Enterococcus faecalis are sometimes also involved [6]. The UTIs are
easily managed with antibiotics [7] such as trimethoprim-sulfamethoxazole
(TMP-SMX), nitrofurantoin, or fosfomycin for 3–5 days [8] and sometimes by
cephalosporins (fluoroquinolones; cefixime) and
However, unfortunately, resistance to antibiotics, which spares no area, has made UTIs more difficult to treat. Several recent studies have reported a considerable increase in antibiotic resistance in uropathogens and an unprecedented number of multidrug-resistant germs worldwide [10, 11]. To respond to this dangerously worrying situation, research teams from all over the world are permanently evaluating the use of potential alternatives to antibiotics such as nanoparticles [12], probiotics [13, 14], phage therapy [15], antimicrobial peptides [16] and medicinal plants [17, 18]. Among these alternatives, medicinal plants seem to offer the most credible evidence [19], considering that plants have been used for millennia in the treatment and prevention of various diseases, including bacterial infections and some of these herbal remedies have proven to be effective in preventing and treating UTIs [19]. In this context, certain plants such as Aesculus hippocastanum which are very widespread (and therefore available) but scarcely utilized deserve to be investigated for their antimicrobial properties.
A. hippocastanum, commonly known as horse chestnut and buckeyes, is a rapidly growing ornamental tree belonging to the family Hippocastanaceae [20]. Trees of this plant are grown up to 30 m in height and 1 m thick with short stem having a rounded crown. A. hippocastanum is highly adapted in polluted environment and is commonly used for the treatment and prevention of many diseases [20]. Its composition rich in esculin (over 17%), fraxin (over 7%) and proanthocyanidins (over 6% of epicatechin and over 5% of procyanidin A2) (Fig. 1, Ref. [21]) gives it anti-inflammatory, vasoprotective, antidiabetic, anti-inflammatory, gastroprotective, neuroprotective, anticancer, hepatoprotective and antibacterial properties [20, 21].
Thus, the aim of this work was to assess the antibacterial properties of ethanolic and aqueous A. hippocastanum bark extract against some bacteria involved in urinary tract infections.
The plant material used in this study was the bark of Aesculus
hippocastanum. The plant was collected in June 2021 near the Medical Institute
of the Peoples’ Friendship University of Russia (MG23 + 9H Obroutchevski, Moscow,
Russia). After harvest, the plant was taken directly to the laboratory where it
was dried at 37
The microorganisms used for the screening of antimicrobial activity consisted of five Gram positive bacteria and 5 Gram negative. The five Gram + were Kocuria rhizophila 1542, Enterococcus avium 1669, Staphylococcus simulans 5882, Corynebacterium spp 1638 and Enterococcus faecalis 5960 while the five Gram—were Proteus mirabilis 1543, Morganella morganii 543, Citrobacter freundi 426, Acynetobacter baumannii 5841 and Achromobacter xylosoxidans 4892. Staphylococcus aureus ATCC 6538 and Escherichia coli ATCC 25922 were used as standard Gram + and Gram - respectively. All strains were provided by the Department of Microbiology and Virology of the Peoples’ Friendship University of Russia.
Dimethyl sulfoxide (DMSO) was purchased from BDH Laboratories, VWR International Ltd., USA. We also used BHIB (Brain Heart Infusion Broth) (HiMedia™ Laboratories Pvt. Ltd., India), Muller Hinton Agar (MHA HiMedia™ Laboratories Pvt. Ltd., India), Sabouraud Dextrose Broth (SDB, HiMedia™ Laboratories Pvt. Ltd., India) and all other reagents and chemicals used were of analytical grade.
Ethanolic solution (80%, v/v) and distilled water were used as solvents because
they have been reported to be efficient solvents for the extraction of bioactive
compounds in the medicinal plants used in this study. As we described in our
previous investigation [18] thirty grams (30 g) of vegetal material was weighed
and added to 270 mL of the solvent in separate conical flasks. The flasks were
covered tightly and were shaken at 200 rpm for 24 h and 25
For each plant extract, the crude extract was dissolved in the required volume
of DMSO (5%, v/v) to achieve a concentration of 128 mg/mL. The extracts were
sterilized by microfiltration (0.22
Bacteria were cultured for 24 h at 37
The modified Kirby–Bauer’s disk method described in our previous study [22] was
used to study the antibiotic sensitivity of the bacterial strains, and the
following eight antibiotics disks were used: amoxicillin, 30
| Antibiotics/limits of inhibition diameters (mm) | ||||||||||||
| CIP | CAZ | AMC | CTR | TR | TE | NIT | AMP | IMP | CAC | FO | ||
| Interpretation | R | d |
d |
d |
d |
d |
d |
d |
d |
d |
d |
d |
| I | 16–20 | 15–17 | 14–17 | 14–20 | 14–15 | 15–18 | 14–17 | 14–16 | 14–15 | 15–17 | 13–15 | |
| S | d |
d |
d |
d |
d |
d |
d |
d |
d |
d |
d | |
| AMC, Amoxycillin; AMP, Ampicillin; CZ, Cefazolin; CAC, Cefazolin/clavulanic acid; CAZ, Ceftazidime; CTR, Ceftriaxone; CIP, Ciprofloxacin; FO, Fosfomycin; IMP, Imipenem; NIT, Nitrofurantoin; TE, Tetracyclin; TR, Trimethoprim. | ||||||||||||
The well diffusion method previously described [18] was used to assess the
antimicrobial activity of the extracts. Briefly, 15 mL of sterile Muller Hinton
Agar (for bacteria) was poured into petri dishes and 100
MIC is the lowest concentration of antibacterial agent that completely inhibits
the visible bacterial growth. The MIC of the extracts was determined using the
microbroth dilution method as previously described without any modification [24].
Briefly, 100
MBCs were determined by subculturing the wells without visible growth (with
concentrations
Tolerance level of tested bacterial strains against aqueous and ethanolic extract was determined using the following formula:
Tolerance = MBC/MIC
The characteristic of the antibacterial activity of extracts was determined by
the tolerance level indicating the bactericidal or bacteriostatic action against
the tested strains. When the ratio of MBC/MIC is
The dry matter, water content, ethanolic (EE) and distilled water extract (AE) yields of Aesculus hippocastanum bark are presented in Table 2. We found that A. hippocastanum bark possessed a dry matter content of 65.73%. Dry matter is an indicator of the amount of constituents (excluding water) in the sample tested. The drying allowed the removal of water from the plant in order to standardize the extraction and to make this work reproducible if authors from other regions project to work with the same plant. Furthermore, we obtained an extraction volume yield of 74.07% with distilled water as the solvent and 77.77% with ethanol. The difference in extraction volume yields can be explained by losses during the extraction process. Indeed, as reported by [18] we noticed that the filtration of ethanolic extracts was faster (less than 5 minutes for 300 mL) compared to the aqueous extract which took much longer (more than 50 minutes on average for 300 mL) and required in average 6 filter changes. Despite the filtration, the EAs still looked cloudy while the EEs were completely clear. This residue cloudiness could therefore explain the higher mass yield in AE (24.3%) compared to EE (13.4%). Several authors [17, 18, 26, 27] reported observations similar to our findings while others [28, 29] found opposite results. Therefore, as Arsene et al. [18] pointed out in their previous work, the extraction performance depends on several factors, including the method used, extraction time, the solvents, and the equipment used. In addition, Mouafo et al. [17] reported that the high yields of phytoconstituent does not necessarily imply better antimicrobial activity and this was further assessed in the present work by evaluating the antimicrobial activity of each of the extracts using the well diffusion method and the determination of minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC).
| Aqueous extract | Ethanolic extract | |
| Volume yield (%) | 74.07 | 77.77 |
| Mass yield (%) | 24.3 | 13.4 |
| Dry matter of the fresh bark of A. hippocastanum = 65.73% (Water content = 34.27%). | ||
The susceptibility of uropathogenic tests to eleven (11) antibiotics was
evaluated by the Kirby Bauer disc method, the Multidrug resistance Index (MDR)
was calculated, and the results were reported in Table 3. As shown in Table 3, no
bacteria were resistant to amoxiclav, imipenem and ceftriaxone while 7/12 were
resistant to ampicillin, 6/12 to trimethoprim, 5/12 to tetracycline and
ceftazidime/clavulanic acid, 4/12 to ceftazidime, 3/12 to fosfomycin, 2/12 to
nitrofurantoin and 1/12 to ciprofloxacin. Interestingly, both standard bacteria
(E. coli ATCC 25922 and S. aureus ATCC 6538) were sensitive to
all antibiotics while the clinical strains were resistant to at least one
antibiotic. Among clinical strains, MDRs ranged from 0.09 to 0.45. K.
rizophilia 1542 and Corynebacterium spp 1638 were the most resistant
bacteria with MDRs of 0.45 each. The results observed with these clinical strains
are similar to those reported in other studies [10, 11]. While the strongest
resistance was observed on ampicillin (which is an antibiotic from the
| NIT | TE | CTR | AMC | FO | CAZ | IPM | CAC | CIP | AMP | TR | MDR | ||
| Gram + | K. rizophilia 1542 | 10 |
13 |
22 |
22 |
28 |
10 |
23 |
6 |
30 |
13 |
21 |
0.45 |
| E. avium 1669 | 21 |
6 |
30 |
25 |
31 |
23 |
27 |
24 |
15 |
6 |
6 |
0.36 | |
| S. simulans 5882 | 23 |
20 |
19 |
27 |
38 |
11 |
34 |
11 |
26 |
6 |
20 |
0.27 | |
| Corynebacterium spp 1638 | 20 |
32 |
32 |
36 |
14 |
6 |
27 |
6 |
26 |
9 |
10 |
0.45 | |
| E. faecalis 5960 | 21 |
11 |
22 |
27 |
31 |
17 |
24 |
6 |
32 |
6 |
6 |
0.36 | |
| S. aureus ATCC 6538 | 22 |
32 |
30 |
35 |
38 |
25 |
28 |
25 |
32 |
20 |
31 |
0.00 | |
| Gram - | P. mirabilis 1543 | 19 |
6 |
30 |
25 |
31 |
23 |
27 |
24 |
30 |
16 |
6 |
0.18 |
| M. morganii 1543 | 15 |
6 |
33 |
17 |
13 |
23 |
22 |
23 |
22 |
10 |
6 |
0.36 | |
| C. freundi 426 | 21 |
30 |
27 |
35 |
40 |
12 |
37 |
10 |
30 |
6 |
22 |
0.27 | |
| A. baumannii 5841 | 26 |
16 |
27 |
35 |
32 |
19 |
34 |
17 |
30 |
21 |
14 |
0.09 | |
| A. xylosoxidans 4892 | 6 |
11 |
23 |
36 |
6 |
16 |
32 |
16 |
20 |
20 |
6 |
0.36 | |
| E. coli ATCC 25922 | 21 |
30 |
24 |
20 |
40 |
18 |
32 |
16 |
24 |
26 |
24 |
0.00 | |
| AMC, Amoxycillin; AMP, Ampi5cillin; CZ, Cefazolin; CAC, Cefazolin/clavulanic acid; CAZ, Ceftazidime; CTR, Ceftriaxone; CIP, Ciprofloxacin; FO, Fosfomycin; IMP, Imipenem; NIT, Nitrofurantoin; TE, Tetracycline; TR, Trimethoprim; R, Resistant; I, Intermediate; S, Sensitive. | |||||||||||||
Fig. 2 presents the inhibition diameters of ethanolic (EE) and aqueous (AE) extract of Aesculus hippocastanum bark on the tested microorganisms. Except AE on Proteus Mirabilis 1543 and Enterococcus faecalis 5960 (0 mm), both AE and EE were active on all microorganisms tested with inhibition diameters (mm) which ranged from 5.5–10.0 for AE and 8.0–14.5 for EE.
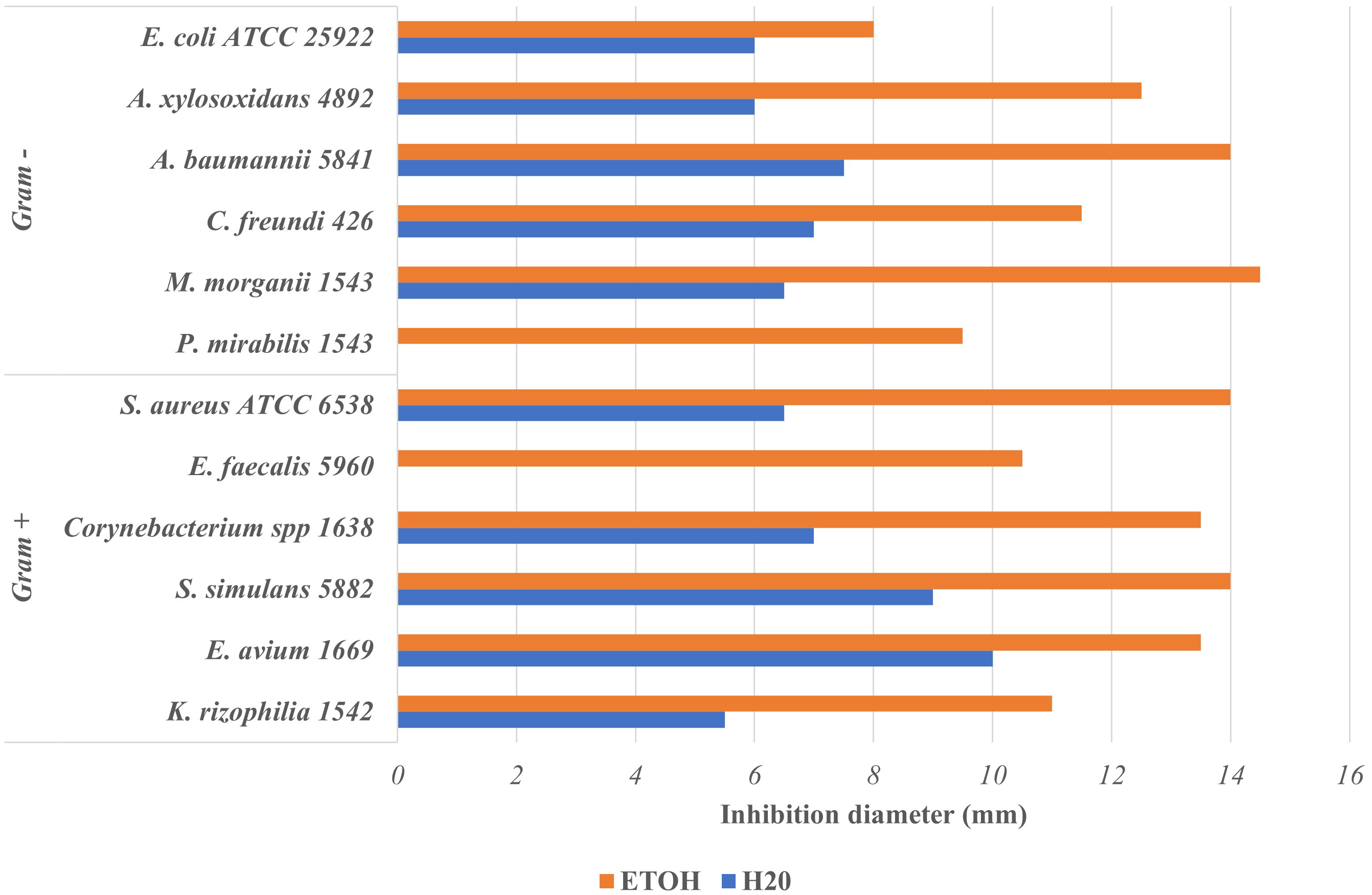 Fig. 2.
Fig. 2.Inhibition diameter of ethanolic and aqueous Aesculus hippocastanum bark extract on tested uropathogenic bacteria.
The ethanolic extracts (EE) were overall more active than the aqueous ones. Consequently, this means that ethanol extracts more compounds with antimicrobial properties compared to water although we found above that the extraction yields with water was higher than that with ethanol. Several authors have reported that compounds with antimicrobial activity such as flavonoids, polyphenols, tannins and alkaloids are generally insoluble in water but soluble in ethanol [32, 33]. Other authors such as Arsene et al. [18], Mouafo et al. [17] and Evbuomwan et al. [34] also pointed out that ethanol extracts more antimicrobial compounds from plant materials as opposed to water. In addition, we found that extracts of A. hippocastanum bark were both active against Gram + bacteria as against Gram - bacteria. To our knowledge, the antibacterial properties of this plant have not yet been investigated but our findings suggest that A. hippocastanum bark has constituents exhibiting a broad-spectrum antimicrobial.
Most of the research conducted on A. hippocastanum focused on its properties to regulate circulatory system imbalances and relieve attacks of hemorrhoids [19]. In a study conducted by Owczarek et al. [19] on the composition of A. hippocastanum bark, it has been reported that this plant presents mainly two groups of compounds: coumarins and proanthocyanidins. Among the coumarins there was mainly esculin (over 17%) and fraxin (over 7%) while among the proanthocyanidins, the authors found epicatechin (over 6%) and procyanidin A2 (over 5%) (Fig. 1) [19]. Owczarek et al. [19] finally concluded that, in total, about 40% of the A. hippocastanum bark extract could be attributed to simple phenolics compounds detectable by LC-PDA. Unfortunately, due to limited ressources we were unable to assess the composition of our extracts in order to compare with the data in the literature. However, we can hypothesize that the antimicrobial properties of A. hippocastanum bark can be attributed to all its components or specifically to esculin [35], to fraxin [36] or proanthocyanidins [37] because these compounds (from other plants) have been reported to have antimicrobial properties. Notwithstanding our findings, further investigations are required to confirm or refute our hypothesis.
After evaluating the antibacterial properties of aqueous (AE) and ethanolic (EE)
extracts of Aesculus hippocastanum bark against the uropathogens tested
using the well diffusion, we investigated the minimal inhibitory concentrations
(MIC) and minimal bactericidal concentrations (MBC) of the two extracts. MIC and
MBC are two very important elements in the search for new antimicrobials and
respectively provide the minimum concentrations required to inhibit or kill the
microorganism tested. Table 4 presents the MIC, the MBC, and the MBC/MIC ratio of
our 2 extracts on the uropathogens investigated. Similarly to the
inhibition
diameters, ethanolic extracts (EE) showed the best antimicrobial activity with
the lowest MIC and MBC. The MICs of EEs varied from 1–4 mg/mL while those of EAs
varied from 4–6 mg/mL. Almost all the MBCs of AEs were indeterminate (
| Aqueous extract | Ethanolic extract | ||||||
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | ||
| (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | ||||
| Gram + | K. rizophilia 1542 | 16 | - | 4 | - | ||
| E. avium 1669 | 4 | - | 2 | 64 | 32 | ||
| S. simulans 5882 | 16 | - | 2 | 16 | 8 | ||
| Corynebacterium spp 1638 | 64 | 64 | 1 | 4 | 32 | 8 | |
| E. faecalis 5960 | 4 | 64 | 16 | 2 | 64 | 32 | |
| S. aureus ATCC 6538 | 16 | 64 | 4 | 2 | 16 | 8 | |
| Gram - | P. mirabilis 1543 | 8 | - | 2 | 64 | 32 | |
| M. morganii 1543 | 2 | - | 1 | 64 | 64 | ||
| C. freundi 426 | 16 | 64 | 4 | 2 | 32 | 16 | |
| A. baumannii 5841 | 4 | - | 2 | 64 | 32 | ||
| A. xylosoxidans 4892 | 4 | - | 2 | 64 | 32 | ||
| E. coli ATCC 25922 | 16 | - | 2 | 16 | 8 | ||
With water as a solvent, the highest antimicrobial activity was observed against
E. faecalis 5960, S. aureus ATCC 6538 and C. freundi
426. Although the MIC and MBC of AE were high against these bacteria, AE was
found to be bactericidal against S. aureus ATCC 6538 and C.
freundi 426 since the MBC/MIC ratio was 4 for both bacteria. Indeed, Mondal et
al. [25] reported that when the ratio MBC/ MIC is
The search for new antimicrobials is essential for overcoming antibiotic
resistance in the management of bacterial diseases including urinary tract
infections (UTIs). In this study we evaluated the antibacterial properties of
aqueous and ethanolic extracts of Aesculus hippocastanum bark against
ten (10) clinical uropathogenic bacteria and two (2) standard bacteria. The
results showed that, except against few bacteria, the extracts had overall weak
antibacterial activity (MIC
UTI, Urinary tract infection; MIC, Minimum Inhibitory Concentration; MBC, Minimum Bactericidal Concentration; EE, Ethanolic extract; AE, Aqueous Extract; MDR, Multidrug Resistance.
MMJA & PIV designed the research study. KYK & MMJA performed the research. MMJA analyzed the data. KYK, MMJA, VGE, MVA, MMA, AAA and PIV wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
This study has been supported by the RUDN University strategic Academic Leadership Program.
This research received no external funding.
The authors declare no conflict of interest.

