Academic Editor: Giuseppe Annunziata
With several medicinal and aromatic species, the Asteraceae family is one of the
largest angiosperm families. The genus Warionia is represented in this
family by only one species, Warionia saharae. In Moroccan traditional
medicine, this species is widely used to treat gastrointestinal problems.
Essential oil of this plant (EoWs) was studied for possible myorelaxant and
antispasmodic activities to rationalize some of the traditional uses. In this
investigation, hydrodistillation was used to obtain the essential oil from the
aerial part of the dry plant extract (EoWs), which was then analyzed using gas
chromatography coupled to mass spectrometry (GC/MS). The major compounds
identified in the EoWs are nerolidyl acetate (21.44%),
Warionia saharae Ben-them ex Benth. & Coss. is an endemic plant of North Africa regions. This plant is native to Morocco, and it is the sole species in the genus Warionia (Asteraceae) [1]. The plant may reach a height of one to three meters. The shrub has a strong trunk covered in a gray peel, a structure of highly wavy terminal leaf bouquets, and a profusion of yellow flowers. The latex that pours out of peel injuries glues to hands in a very tenacious way; picking the leafed stems of this bush clears a very strong and spicy odor [2]. Afessas, abessas, or tazart nîfiss are some of the native Berber names for this plant [3]. W. saharae leaves are used in traditional medicine to treat inflammatory disorders, gastrointestinal problems, and epileptic seizures [4]. Until recently, W. saharae had not sparked the curiosity of scientists, and just a few articles on this species had been published. The hydromethanolic extract of W. saharae exhibits significant glucosidase inhibitory activities, according to Rechek et al. [4]. The essential oil and crude extracts of this plant were found to have antibacterial, cytotoxic, antioxidant, and anti-inflammatory properties against the cancer cell line “KB cells” [4, 5].
Essential oils and their constituents have been utilized to treat a wide range of human ailments since ancient times. Essential oils are used in aromatherapy, medicine, fragrances, and cosmetics. Their use is limited to their varied biological functions [6]. Heghes et al. [7] 2019 identified 39 plant species containing essential oils with antispasmodic activities. They found that the main mechanisms of antispasmodic action were achieved via inhibiting voltage-dependent calcium channels, modifying potassium channels, and intracellularly controlling AMPc. Several investigations have identified various biological activities of essential oils [8], but the antispasmodic effect, while mentioned in traditional medicine literature, has received far less experimental attention.
The objectives of this study were to investigate at the chemical composition of essential oils extracted from aerial parts of W. saharae in the southern region of Morocco and to study their antispasmodic activities.
W. Saharae aerial parts were collected from southeastern region of
Morocco (Errachidia: 31
One hundred grams of air-dried W. saharae aerial part were cut into
small pieces and placed in a round-bottom flask with 0.1 L distilled water, and
the essential oil were extracted by hydro-distillation after 2 hours using a
Dean-Stark apparatus. The obtained oil was kept in a dark sealed container at 4
The volatile components of W. saharae essential oils were characterized
using a gas chromatography coupled with mass spectrometry GC/MS (Shimadzu model
QP2010, Kyoto, Japan). Helium (3 mL/min) was employed as the carrier gas in a BPX
25 capillary column (30 m
All chemicals used were of analytical grade. Calcium chloride (CaCl
Essential oil dissolved in DMSO was prepared by mixing 10
The Krebs-Hemseleit Buffer (KHB) utilized in this investigation had a pH of 7.4
and was held at 37
New Zealand rabbits (1.5–2 kg), Wistar rats (200–350 g) and Swiss albino mice (20–25 g) of both sexes were utilized. They were housed in standard laboratory animal housing conditions. Animals fast for 18 hours before each experiment and have unrestricted access to water. The experiments were carried out ethically in conformity with the principles outlined in the Declaration of Helsinki, and the study was authorized by the Faculty of Sciences institutional review board in Oujda, Morocco (01/20-LBBEH-04 and 09/01/2020).
Under the antispasmodic experiments, when the animal was anesthetized by light ethyl ether inhalation and euthanized, the abdominal cavity was opened and most of the internal organs were visualized in place, including the digestive mass. It is necessary to notice the state of all organs and precisely that of the intestine, of which sometimes the presence of intestinal alterations can distort the results, and before any experiments it is checked that the organ contracted well in a KHB medium rich in potassium.
The basic spontaneous contractions of the rabbit jejunum are larger and easier to evaluate than those of the rat jejunum. Therefore, it is a good model for studying the spontaneous myorelaxant activity. However, if you want to study the antispasmodic activity and their mechanism of action, rat jejunum contraction induced by CCh and KCl is more practical and cheaper. For each experience 6 animals were used.
We used the mice in the toxicity test because they are very sensitive compared to the other animals, so they are more adapted for the toxicity tests of Natural products.
Acute toxicity of EoWs was evaluated following the recommendations by OECD-Guidelines [9]. For this study, 30 mice were divided into five groups of six mice each. After fasting for 18 hours with unrestricted access to water, EoWs treatment was started by giving each mouse a single dose of:
-0.5, 1, 1.5 and 2 g EoWs/kg body weight solubilized in gelatin (5%)
-gelatin (5%) solubilized in distilled water for the control group
The animals were fed after treatment, followed by weight registration and 14-day observation of general indicators of toxicity symptoms, behavior, and mortality. At the end of the investigation, the mice were euthanized by cervical dislocation, and the weights of organs such as the right and left kidney, liver, and heart were collected while the intestines and stomach were inspected.
Antispasmodic effect of W. saharae L. essential oil (EoWs) was
evaluated utilizing isolated jejunum preparations from rabbit and rat: 2 cm long
animal small intestine segments were suspended in 10 mL tissue baths using KHB
solution with regular oxygenation (bubbling) and kept at 37
The vehicle DMSO was used to dissolve EoWs, which was then added to the organ
bath. At the maximum dose we used (50
The amplitude of the jejunum contraction is measured used an isotonic transducer (B. Braun Melsungen AG Type 362722 # 203, Germany) related to the intestine mounted in organ isolated bath (10 mL). The graph tracing related to the contractile response of the intestine was recorded using recording cylinder (B. Braun Melsungen AG 861 062 Type, No. 1696, Germany). To calibrate the system: a weight was used to calibrate the signal transducer (1 g equivalent to 100% of contraction for rat jejunum, and 2 g equivalent to 100% of contraction for rabbit jejunum).
In isolated spontaneously contracting rabbit jejunum, the action of the EoWs
(10, 30, and 50
The following experiments were performed:
-The antispasmodic action of EoWs (5, 10, 30
-In the absence and presence of the EoWs, concentration response curves for
carbachol (CCh) were produced. Following a 1-hour stabilization period in KHB,
the jejunum section was treated with cumulative doses of CCh (0.03–30
-To evaluate whether calcium channel was involved in the effect of EoWs in
jejunum, the tissue first stabilized in normal KHB solution, then replaced with a
calcium-free KHB (NaCl, 121.7; KCl, 4.7; CaCl
In the last experiment, the impact of EoWs (30
The results were expressed as mean
The essential oil EoWs was extracted directly from the dried aerial section of W. saharae from the Errachidia region (Morocco) and characterized using GC-MS. On a dry weight basis, the obtained essential oil yields were around 0.5% (w/w). Fig. 1 and Table 1 illustrate the chromatogram and chemical content and composition of this essential oil, respectively. These findings indicate that the plant is very rich in a wide range of chemical components.
 Fig. 1.
Fig. 1.Typical GC-MS chromatogram of the essential oil of W. saharae. The peaks obtained correspond to 16 compounds representing 99.9% of the aromatic compounds present in this plant.
| Pic number | Chemical compound | Retention Time (min) | Abundance (%) |
| 1 | 4(10)-Thujene | 5.73 | 1.90 |
| 2 | (+)-4-Carene | 6.43 | 1.19 |
| 3 | p-Cymene | 6.57 | 1.57 |
| 4 | Cineole | 6.70 | 5.34 |
| 5 | ɣ-Terpinene | 7.13 | 1.95 |
| 6 | Linalool | 7.78 | 16.48 |
| 7 | 1-Terpinen-4-ol | 9.10 | 10.93 |
| 8 | p-menth-1-en-8-ol | 9.30 | 5.37 |
| 9 | Geraniol | 10.19 | 1.97 |
| 10 | 11.64 | 2.01 | |
| 11 | E-7-Tetradecen-1-ol | 13.26 | 1.56 |
| 12 | 7-Heptadecene, 1-chloro- | 13.48 | 2.67 |
| 13 | 9-Cedranone | 13.90 | 2.42 |
| 14 | Nerolidyl acetate | 14.51 | 21.44 |
| 15 | Agarospirol | 15.52 | 3.72 |
| 16 | 15.81 | 19.47 | |
| Total | 16.00 | 99.99 |
The chromatographic profile of this essential oil allowed for the identification
of 99.99% of the aromatic compounds present in this plant, the most important
are, in increasing order, nerolidyl acetate (21.44%),
The acute toxicity study with EoWs revealed that there was no toxicity or mortality at the tested levels (1, 1.5, and 2 g EoWs/kg body weight). All of the animals behaved normally when it came to eating, moving, and drinking water. The weights of the mice during the experience period did not change between the control and EoWs-treated groups.
The results of this study (Fig. 2A) showed that EoWs at concentrations ranging
from 10
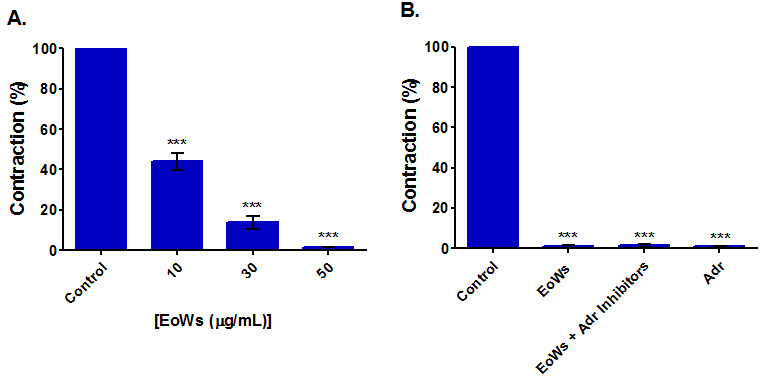 Fig. 2.
Fig. 2.Myorelaxant effect on spontaneous contractions of the rabbit jejunum of the W. saharae essential oil (EoWs). (A) Influence of EoWs applied at different concentrations. (B) Influence of 50 μg/mL EoWs applied in the presence and the absence of Adrenergic inhibitors (Adr Inhibitors) (Prazosin (0.5 μM) + yohimbine (0.5 μM) + propranolol (0.5 μM)). (Adr: Adrenaline). ***p
Rabbit basal jejunal contractions are fully suppressed in the presence of 50
g/mL EoWs, even in the presence of three adrenergic inhibitors (propranolol,
prazosin, and yohimbine, all used at 0.5
According to the results of this study (Fig. 3), EoWs at doses ranging from 5 to
30 g/mL suppressed rat jejunal tone elicited by 25 mM KCl with an IC
 Fig. 3.
Fig. 3.Effect of W. saharae essential oil (EoWs). (A) Influence of EoWs on rat jejunum precontracted with carbachol 1 μM. (B) Influence of EoWs on rat jejunum precontracted with KCl 25 mM (B). *: p
The concentration-response effects of CCh and CaCl
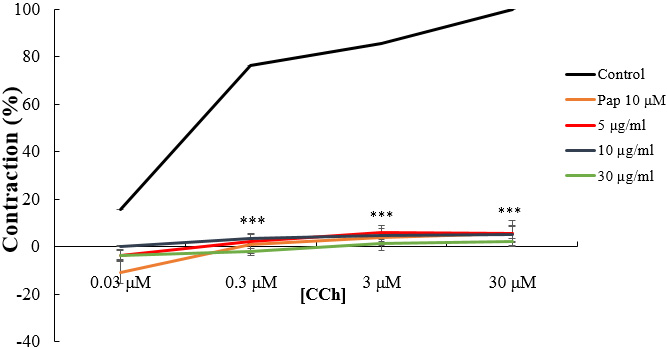 Fig. 4.
Fig. 4.Concentration-response curves of CCh in the absence and
presence of increasing concentrations of W. saharae essential oil (EoWs)
and Papaverine (Pap). ***p
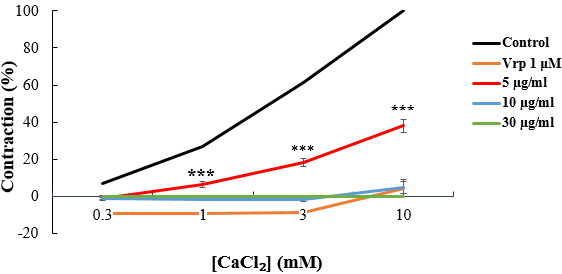 Fig. 5.
Fig. 5.CaCl
When the tissue was pre-contracted with CaCl
We tested the effectiveness of 30 g/mL EoWs with pharmacological inhibitors that
are often used to reduce KCl-induced contraction (atropine is used to inhibit
muscarinic receptors, l-Name is used to inhibit NOS, methylene blue is
used to inhibit GC pathway, and hexamethonium is used to inhibit nicotinic
receptors). The results show (Fig. 6) that after 20 minutes of pre-treatment with
pharmaceutical drugs, the influence of EoWs alone was more effective in lowering
KCl-induced contraction than the presence of 1
 Fig. 6.
Fig. 6.Effect of W. saharae essential oil (EoWs, 30
The same result was obtained when KCl was replaced with CCh (Fig. 7). In the
absence of the positive control, EoWs (30 g/mL) completely suppressed the
contraction caused by CCh; however, the contraction caused by carbachol (1
 Fig. 7.
Fig. 7.Effect of W. saharae essential oil (EoWs, 30
The essential oil was extracted and analyzed using GC-MS from the dried areal
parts of W. saharae. The essential oil yields were about 0.5% (w/w) on
a dry weight basis. Sellam et al. [10] found a 1.1% essential oil
extraction yield from W. saharae. For Mezhoud, the extraction yield was
around 0.85% (plant grown in Algeria) [11]. These yield variations might be
attributed to extraction techniques, ecotype, or aerial component employed (stem,
leaf or flower). The composition of the EoWs was presented as a ratio of numerous
molecules from the monoterpene, sesquiterpene, terpene esters, and monoterpenol
groups. The oil’s distinctive and aromatic odor may be explained by its high
concentration of identified oxygenated compounds (Table 1). Several
investigations have found nerolidyl acetate,
To assess a substance’s potential risk to humans, toxicity studies in animal models are commonly conducted. The study of effect EoWs at concentrations of 0.5, 1.0, 1.5, and 2.0 g/kg body weight showed no signs of toxicity or mortality over a 14-day observation period, nor did the mice’s weight change, and even after the animal was euthanized, the weight of vital organs such as the kidneys, liver, and heart, as well as the aspect of the stomach and intestine, did not change. After investigating these factors, we detected no evidence of toxicity. Based on these results, we may infer that our plant is relatively safe in the case of acute intake of up to 2 g/kg body weight of albino mice.
The myorelaxant activity of W. saharae essential oil has been examined
in the rabbit jejunum, which is characterized by contractions whose amplitude and
frequency are rhythmic (do not change over time), owing to the slow waves
generated by the Cajal interstitial cells [15]. Furthermore, the waves are
clearer and larger than the rat’s, making them easier to examine and comprehend.
As a result, we chose to concentrate our studies on the myorelaxant impact of
EoWs, specifically on the rabbit jejunum. With an IC
Several investigations have indicated that intestine smooth muscle contraction
occurs as a result of an increase in free calcium in the cytoplasm, which
subsequently activates the contractile elements [18]. The Ca
Previously, it was established that catecholamines and adrenergic drugs reduced
spontaneous contractions of the rabbit jejunum [23]. For this reason, we selected
three adrenergic inhibitors: prazosin, yohimbine, and propranolol, which are
recognized for their competitive action on
Carbachol, a structural analogue of acetylcholine, was employed to evaluate the
cholinergic receptor pathway, since it has the advantage of not being degraded in
the physiological medium by the action of acetylcholinesterases. In the presence
of EoWs, we examined two different cholinergic inhibitors: atropine, a muscarinic
antagonist, and hexametonium, a nicotinic inhibitor. EoWs appear to operate via
muscarinic receptors but not nicotinic receptors. Then this oil may therefore
induce the activation of M2 and M3 muscarinic receptors found in the intestine
[25]. Furthermore, muscarinic receptor activation can stimulate non-selective
calcium (NSC) channels in the plasma membrane, resulting in a membrane potential
and Ca
We obtained the same results, when we progressively added calcium in the presence of EoWs or verapamil. These results, thus, suggest that EoWs operated as an agonist through one of the two muscarinic receptor contraction pathways. Similar observations were done with plant extracts from Nepeta cataria L. [28], Satureja hortensis [29], and Satureja obavata [30].
Previous research has found that the antispasmodic activity of medicinal plants
is generally mediated by blocking calcium channels [31, 32]. Similarly, we aimed
to determine if our essential oil’s antispasmodic activity is driven by similar
pathways. The effect of EoWs on rat jejunal contractions generated by 25 mM KCl
was studied for this purpose, and the results showed that this essential oil
significantly reduced the maximum tone of the jejunum created by the latter in a
dose-dependent manner, with an IC
We also explored whether the activity of our plant essential oil is mediated by nitric oxide (NO) or guanylate cyclase to better understand the mechanism of action. l-NAME (NG-nitro-l-arginine methyl ester) [37, 38] and methylene blue [39] were used as selective inhibitors of these pathways, respectively. The administration of both inhibitors had a significant effect on EoWs-induced relaxation. As a result of these observations, our extract is likely to function directly through the NO and guanylate cyclase signaling pathways.
This antispasmodic activity can be linked to the chemical components found in
this essential oil that have spasmolytic properties, such as Cineole [40],
1-terpinen-4-ol [41], terpineols [42], and linalool [43]. It should also be
mentioned that
The antispasmodic activity of W. saharae essential oil (EoWs) on isolated rabbit and rat small intestine was here studied. The EoWs demonstrated a significant spasmolytic effect by acting on several pharmacological pathways such as NO, guanylate cyclase, VGC channels, and muscarinic receptors. These results support the traditional usage of this plant to treat intestinal disorders and will allow us to explore future prospects for the treatment of intestinal spasms using natural chemicals derived from this medicinal plant.
MA (Mohammed Aziz) conceived and designed research. OA, MM, HM conducted the experiments. OA, MA (Mohamed Addi), MA (Mohammed Aziz) wrote the manuscript. MA (Mohamed Addi), MM contributed to software analysis. CA, AK analyzed the data. CH, JTC critical analysis of the data, review and editing manuscript. All authors contributed to editorial changes in the manuscript.
The experiments were carried out ethically in conformity with the principles outlined in the Declaration of Helsinki, and the study was authorized by the Faculty of Sciences institutional review board in Oujda, Morocco (01/20-LBBEH-04 and 09/01/2020).
Mustapha Badraoui, Karim Ramdaoui, and Mohammed Joudar are acknowledged for technical support and animal breeding. The authors would like to thank the head of the chemistry department, Abdelmonaem Talhaoui of the Faculty of Sciences of Oujda (Mohamed Premier University).
This work was funded by the budget allocated to research at Mohamed the First University by the Ministry of National Education, Vocational Training, Higher Education and Scientific Research.
The authors declare no conflict of interest. JTC is serving as one of the Guest Editor of this journal. We declare that JTC had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to GA.
