-
- Academic Editor
-
-
-
Chromosomal microarray analysis (CMA) is the recommended genetic test for fetuses with increased nuchal translucency (NT); however, its use in Latin America remains limited. The objective of this study was to determine the prevalence of genetic testing in fetuses with increased NT in Panama and across Latin America.
We conducted a retrospective cohort study of 1512 women who underwent first-trimester screening in Panama, along with a systematic review and meta-analysis of studies reporting genetic testing in Latin America. A comprehensive literature search was conducted across MEDLINE (via PubMed), Epistemonikos, LILACS, BRISA, SciELO, and Google Scholar, covering studies from inception to June 2023 was updated to December 2023. The extracted data included population, setting, timing, and genetic testing methods. The Joanna Briggs Tool was used to assess the risk of bias. Pooled prevalence estimates were calculated using random-effects models.
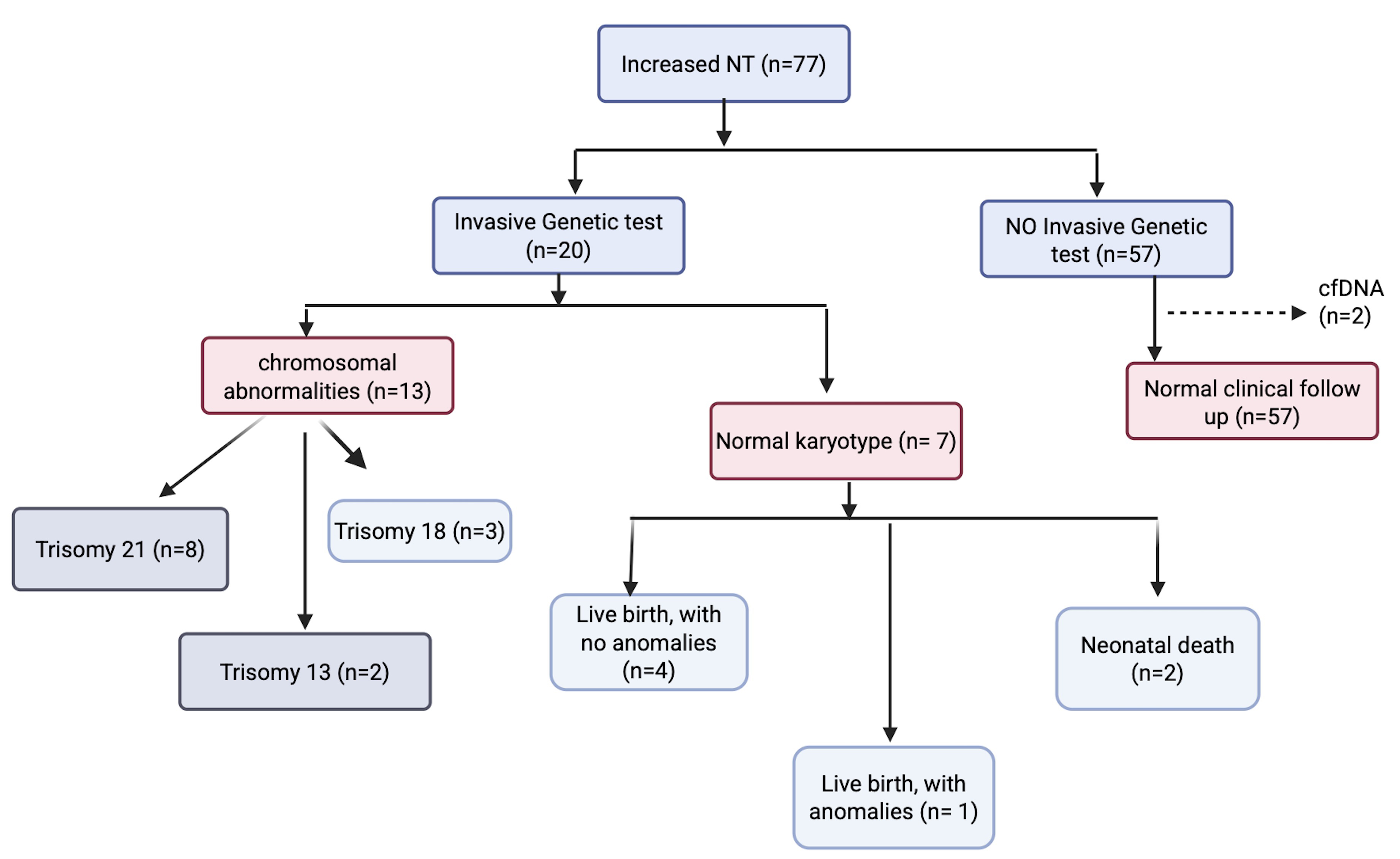
Among 1236 fetuses in the Panamanian cohort, 77 (6.23%) had NT ≥95th percentile. The systematic review included 11 studies encompassing 842 fetuses diagnosed with increased NT. The overall proportion of fetuses undergoing invasive testing was 0.31 (95% confidence interval [CI]: 0.28–0.33). Anomalies were found in 63% of cases with increased NT. CMA was not reported in any of the studies.
Most patients in Latin America do not undergo invasive testing, and conventional karyotyping remains the most frequently performed method. To date, no studies have reported the use of CMA in this context. Therefore, the findings of this study highlight significant gaps in access to genetic testing, emphasizing the need for strategic initiatives to improve test availability and build capacity for implementing microarray analysis in the region.
The study has been registered on https://www.crd.york.ac.uk/prospero/ (registration number: CRD42023398899; registration link: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023398899).