- Academic Editor
Chromosomal microarray analysis (CMA) is the recommended genetic test for fetuses with increased nuchal translucency (NT); however, its use in Latin America remains limited. The objective of this study was to determine the prevalence of genetic testing in fetuses with increased NT in Panama and across Latin America.
We conducted a retrospective cohort study of 1512 women who underwent first-trimester screening in Panama, along with a systematic review and meta-analysis of studies reporting genetic testing in Latin America. A comprehensive literature search was conducted across MEDLINE (via PubMed), Epistemonikos, LILACS, BRISA, SciELO, and Google Scholar, covering studies from inception to June 2023 was updated to December 2023. The extracted data included population, setting, timing, and genetic testing methods. The Joanna Briggs Tool was used to assess the risk of bias. Pooled prevalence estimates were calculated using random-effects models.
Among 1236 fetuses in the Panamanian cohort, 77 (6.23%) had NT ≥95th percentile. The systematic review included 11 studies encompassing 842 fetuses diagnosed with increased NT. The overall proportion of fetuses undergoing invasive testing was 0.31 (95% confidence interval [CI]: 0.28–0.33). Anomalies were found in 63% of cases with increased NT. CMA was not reported in any of the studies.
Most patients in Latin America do not undergo invasive testing, and conventional karyotyping remains the most frequently performed method. To date, no studies have reported the use of CMA in this context. Therefore, the findings of this study highlight significant gaps in access to genetic testing, emphasizing the need for strategic initiatives to improve test availability and build capacity for implementing microarray analysis in the region.
The study has been registered on https://www.crd.york.ac.uk/prospero/ (registration number: CRD42023398899; registration link: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023398899).
Nuchal translucency (NT), a first-trimester sonographic marker characterized by
a subcutaneous accumulation of fluid behind the fetal neck [1, 2, 3, 4], has been
studied for over three decades. Increased fetal NT is associated with chromosomal
abnormalities, congenital heart defects, and structural anomalies. Defined by the
95th and the 99th percentiles, NT
Despite standardized NT measurements, international guidelines for managing
increased NT vary. For instance, the Society of Obstetricians and Gynaecologists
of Canada (SOGC) recommends genetic counseling, chromosomal microarray analysis
(CMA), and a detailed second-trimester ultrasound for NT
In Latin America, only one national prenatal screening program has been established [10, 11, 12]. However, significant barriers to accessing this program include a lack of genetic counselors, limited reimbursement policies, and inadequate medical genetics training. Moreover, the absence of national guidelines for prenatal genetic diagnosis has led to limited data on the availability and use of genetic and genomic testing for high-risk subgroups, such as fetuses with increased NT.
This study aimed to determine the prevalence of fetal structural anomalies in pregnancies with increased NT and the proportion of women who underwent invasive prenatal diagnosis due to increased NT in Latin America and other countries.
This retrospective study selected women who underwent sonographic examination at
the Hospital Punta Pacifica, Panama (November 2005–September 2018).
Informed consent was obtained from all participants. Singleton and multiple
pregnancies were included in the sample, with the maternal and fetal data
collected using questionnaires and ultrasound assessments. NT measurements,
recorded as the highest of three values, were considered to have increased if
they were above the 95th percentile using established reference ranges [13].
Patients with a Down syndrome risk
Quantitative variables were tested for normality using the Shapiro-Wilk test.
Moreover, normally distributed variables were compared using the t-test
(mean
This systematic review followed the PRISMA guidelines [14] (Supplementary Table 1) and was registered on https://www.crd.york.ac.uk/prospero/ (registration number: CRD42023398899; registration link: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023398899). Searches were conducted using databases such as MEDLINE (via PubMed), Epistemonikos, LILACS, BRISA, SciELO, and Google Scholar, without date restrictions, focusing on Spanish and Portuguese studies related to increased NT, genetic anomalies, and Latin America. The initial search (June 2023) was updated to December 2023.
Retrospective and prospective cohort studies on pregnant patients in Latin
America were included in the review, whereas case series (
Statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria), while a meta-analysis of proportions was conducted using the “meta” and “metafor” packages. A random-effects model was applied using the restricted maximum likelihood (REML) estimator to account for between-study variability.
Heterogeneity was assessed using the
A total of 1512 obstetric ultrasound examinations (of 1652 fetuses) were
performed. After excluding patients who were lost to follow-up, pregnant at the
time of data extraction, or had incomplete information, 1236 fetuses were
included in the final analysis. The mean maternal age of the participants was
31.9
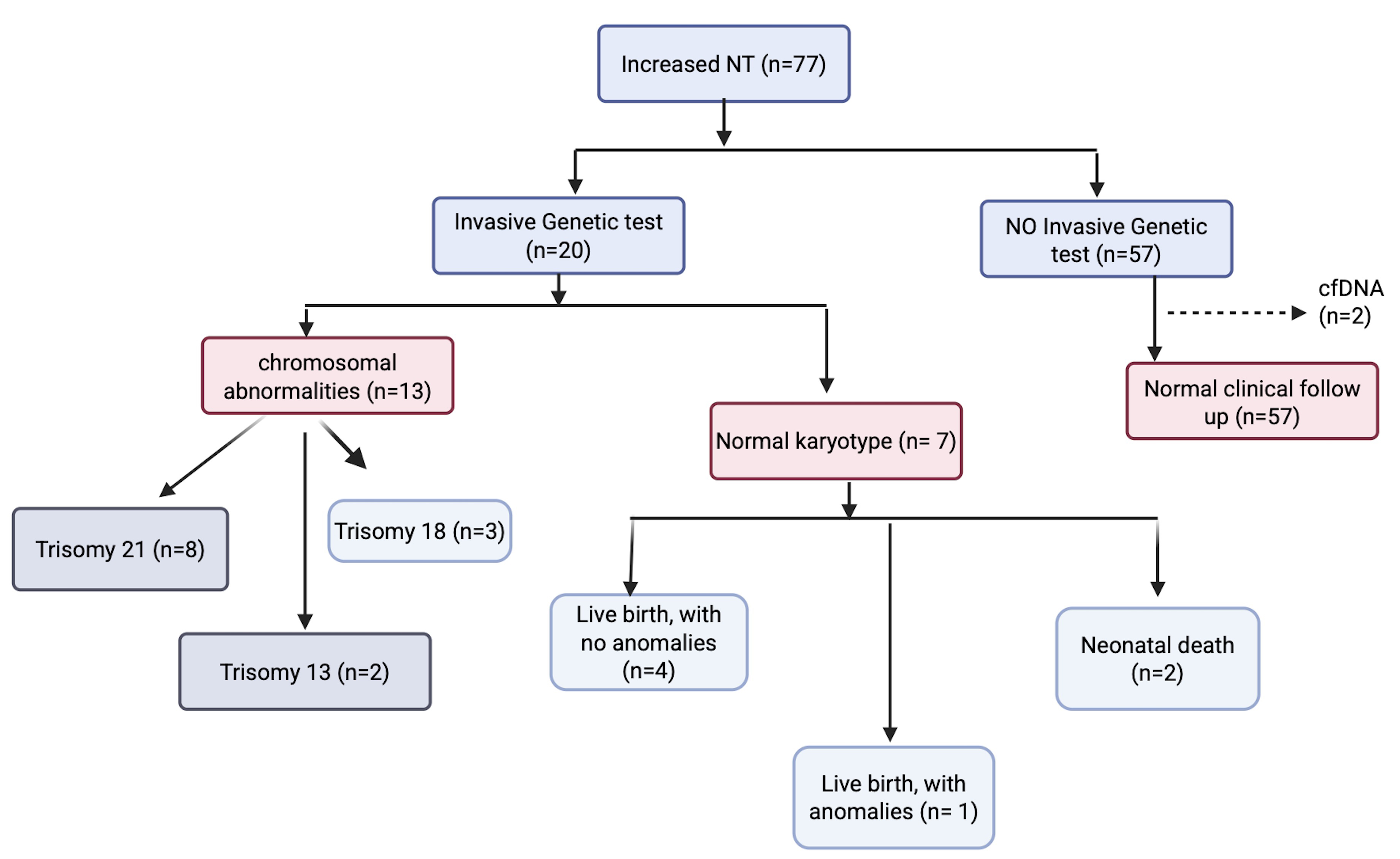 Fig. 1.
Fig. 1.
Flow chart of a Panamanian cohort of increased nuchal translucency (NT). cfDNA, cell free DNA.
As regards pregnancy outcomes, 998 fetuses (96.1%) resulted in live births, while 40 fetuses (3.9%) had a composite adverse perinatal outcome. Additional findings included abnormal nasal bone in 11 fetuses (0.9%), abnormal ductus venosus flow in 10 fetuses (0.8%), and abnormal tricuspid flow in nine fetuses (0.7%). The characteristics of the study population are presented in Supplementary Table 2.
In 77 fetuses (6.23%), 58 had live birth without anomalies (75.3%): 25 had adverse outcomes (32.5%). 4 termination of pregnancy (TOP), one stillbirth and two neonatal death lacked karyotype results. Structural abnormalities were noted in adverse outcomes, which are detailed in Table 1.
| Case number | NT (mm) | Sonographic markers | Risk 1/ | Structural abnormalities | Karyotype | Perinatal outcome |
| 314 | 3.2 | Absent nasal bone, tricuspid regurgitation | 140 | Hypoplastic left heart syndrome | No | Intrauterine death |
| 132 | 4.4 | Absent nasal bone | 3 | Cystic hygroma, upper extremity distal transverse disruptive syndrome | No | Missed abortion |
| 300 | 3.0 | Nasal bone present, abnormal ductus venosus, tricuspid regurgitation | 29 | No anomalies | No | Live birth |
| 411 | 4.5 | Normal nasal bone | 2 | Complete atrioventricular canal, cystic hygroma | Trisomy 18 | Missed abortion |
| 437 | 4.8 | Normal nasal bone, normal ductus venosus | 44 | Cystic hygroma | Normal | TOP |
| 1178 | 2.7 | Oligohydramnios | 518 | Isolated left clubfoot | No | Live birth, surgical repair |
| 133 | 4.6 | Normal nasal bone, normal ductus venosus, tricuspid valve normal | 10 | Holoprosencephaly, fetal megacystis, polydactyly | Trisomy 13 | Missed abortion |
| 542 | 5.6 | Normal nasal bone, normal ductus venosus, tricuspid valve regurgitation | 8 | Cystic hygroma, hypoplastic left heart syndrome | Normal karyotype | TOP |
| 600 | 8.9 | Absent nasal bone, normal ductus venosus, normal tricuspid valve flow | 5 | Cystic hygroma | Trisomy 18 | Spontaneous abortion |
| 699 | 2.7 | Normal nasal bone, abnormal ductus venosus, tricuspid regurgitation | 3 | Exomphalos, complete atrioventricular canal | Trisomy 21 | Spontaneous abortion |
| 732 | 6.6 | Absent nasal bone, tricuspid regurgitation, abnormal venous ductus | 4 | Cystic hygroma | Trisomy 21 | Missed abortion |
| 771 | 8.2 | Absent nasal bone, tricuspid regurgitation, abnormal four-chamber heart, absent bladder | 10 | Exomphalos | Trisomy 18 | Missed abortion |
| 789 | 6.5 | Absent nasal bone, tricuspid regurgitation, abnormal four chambers of the heart | 2 | Bilateral ulnar deviation | Trisomy 21 | Missed abortion |
| 626 | 7.3 | Absent nasal bone, tricuspid regurgitation, abnormal four chambers | 159 | Holoprosencephaly, exomphalos | Trisomy 13 | Missed abortion |
| 794 | 8.6 | Absent nasal bone, abnormal tricuspid Doppler | 2 | Cystic hygroma, hydrops fetalis, ascites, bilateral pleural effusion, mitral atresia, interventricular septal defect | No | Missed abortion |
| 630 | 8.7 | Cystic hygroma, absent nasal bone | 34 | Atrioventricular defect | 46, XY | Cesarean section, neonatal death |
| 1069 | 3.5 | Absent nasal bone, abnormal ductus venosus | 4 | Bilateral ulnar deviation, abnormal four chambers | Trisomy 21 | Missed abortion |
| 1204 | 3.3 | Absent nasal bone | 54 | None | Trisomy 21 | TOP |
| 1108 | 1.8 | Absent nasal bone, abnormal ductus venosus | 18 | Twin pregnancy discordant for anomaly, clenched hands, abnormal four-chamber view | No karyotype | Intrauterine death of one fetus |
| 805 | 14.3 | Absent nasal bone | 3 | Cystic hygroma, ectopia cordis | No karyotype | Missed abortion |
| 855 | 2.9 | Normal markers | 3680 | Absent left lower extremity | No karyotype | Missed abortion |
| 958 | 7.6 | Absent nasal bone | 3 | Cystic hygroma | No karyotype | Missed abortion |
| 1135 | 3.3 | Tricuspid regurgitation | 3 | Abnormal four-chamber view | No karyotype | Live birth |
| 1167 | 3.3 | Abnormal nasal bone | 3 | Double right ventricle outflow tract, abnormal pulmonary vein connection, imperforate anus with perianal fistula, left renal agenesis | 46, XY, negative for 22q11 deletion | Neonatal death, neonatal exam: micropenis, scrotalized lips, left and right microtia |
| 1393 | 9.2 | 3 | Atrioventricular septal defect | No karyotype | Spontaneous abortion | |
| 627 | 6.2 | 3 | Tricuspid atresia, levocardia | Normal | TOP | |
| 1325 | 1.9 | Normal markers | 9659 | None | Live birth, normal follow-up | |
| 698 | 9.8 | Absent nasal bone, intestinal hyperechogenicity | 6 | Renal agenesis | Trisomy 21 | Missed abortion |
| 1378 | 10 | 3 | Cystic hygroma | Trisomy 21 | Missed abortion |
MC, miscarriages; TOP, termination of pregnancy. “/” indicates a ratio or risk value (e.g., 1/140 or ½).
Invasive diagnostic procedures such as chorionic villous sampling (CVS) or amniocentesis were performed in 20 cases (23.5%), while two cases (2.3%) underwent cell-free DNA (cfDNA) testing, both leading to healthy live births. Among the TOP cases, one had trisomy 21, one stillbirth was linked to severe oligohydramnios, and two neonatal deaths involved complex congenital heart defects, including a complete atrioventricular canal and double outlet right ventricle with anomalous pulmonary venous connection.
Adverse outcomes occurred in 24 fetuses with normal NT (
The present systematic literature search identified 306 citations related to increased NT, genetic testing, and chromosomal anomalies in Latin America. After removing duplicates, 39 full-text studies were assessed, of which 11 met the inclusion criteria Fig. 2 (Ref. [14]). Collectively, these 11 studies included 842 fetuses with increased NT. Among them, nine studies applied the fetal medicine foundation (FMF) guidelines and NT measurements to evaluate the risk of Down syndrome and other aneuploidies [11, 17, 18, 19, 20, 21, 22, 23, 24]. One study used maternal age as the sole screening criterion [25], while another employed the “Fetal Test” software for risk assessment [22].
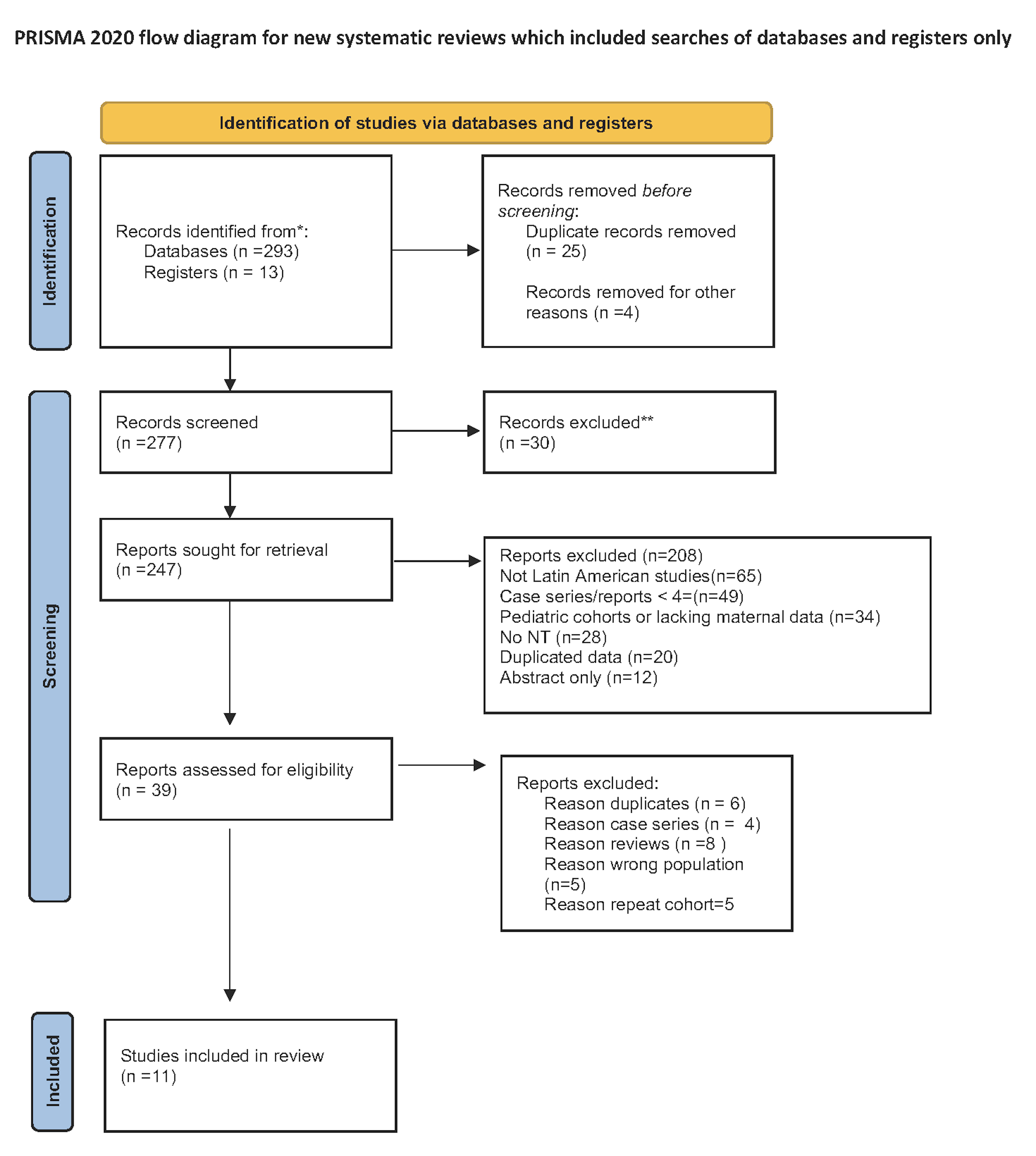 Fig. 2.
Fig. 2.
PRISMA 2020 flow diagram for new systematic reviews that included searches of databases and registers only. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **lf automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. For more information, visit: http://www.prisma-statement.org/.
The main characteristics of the studies included in the systematic review are presented in Table 2 (Ref. [11, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27]).
| Study | Year | Study period | Design | Setting | Screening criteria | GA at inclusion (weeks) | N | NT cutoff | Karyotype method | Chromosomal defects n, % (types) |
| Brizot, et al. [17] | 2001 | 1999–2001 | Prospective cohort | University Hospital Sao Paulo, Brazil | FMF Software, NT and Maternal age, Risk |
10–14 | 173 | Amniocentesis/CVS | 22, 12.7% (7 T21, 9 other) | |
| Saldanha, et al. [18] | 2009 | 2008–2009 | Prospective cohort | Hospital das Clinicas, Sao Paulo, Brazil | NT |
11–13.6 | 246 | Amniocentesis/CVS | 35, 14.2% (NA) | |
| Llanusa Ruiz, et al. [11] | 2009 | 2006–2007 | Retrospective cohort | Hospital Dr. Ramón González Coro, Cuba | NT |
10–13.6 | 43 | Amniocentesis/cordocentesis | 9, 20.9% (NA) | |
| Alcedo Ramírez, et al. [26] | 2009 | 2005–2007 | Retrospective | Genetic private lab, Bogotá, Colombia | Invasive testing (maternal age, sonographic markers, biomarkers) | - | 26 (6.9%) | Enlarged NT | Amniocentesis | 7, 26.9% (5 T21 and 2 structural alterations) |
| Mendoza-Caamal, et al. [20] | 2010 | - | Case series | Instituto Nacional de Perinatologia, Mexico | NT |
11–14 | 48 | CVS/Amniocentesis | 9, 18.9% (3 T21, 3 XO, 2 T 18, 1 47, XYY) | |
| González Herrera, et al. [21] | 2014 | 2006–2010 | Retrospective | National Prenatal Program, Cuba | NT |
11–13.6 | 71 (0.24%) | CVS/Amniocentesis | 40, 56.3%, (7 aneuploidies) | |
| Vieira, et al. [19] | 2013 | 2005–2011 | Prospective | Hospital Francisco Morato de Oliveira, Sao Paulo, Brazil | NT |
10–14 | 116 (3.8%) | CVS/Amniocentesis | 36, 31.0% (14 T21, 9 T18, 3 T13, 2 XO, 2 47XXY, 6 other) | |
| Huamán, et al. [22] | 2013 | 2007–2012 | Prospective | Instituto Latinoamericano de Salud Reproductiva, Perú | NT |
11–13.6 | 30 | CVS/Amniocentesis | 12, 40.0% (8 T21, 4 T18) | |
| Sepulveda, et al. [24] | 2009 | 2003–2007 | Retrospective | Ultrasound clinic, IVF Clinic (40% Twin pregnancies), Chile | NT and nasal bone | 11–14 | 16 (3.6%) | CVS | 5, 31.2% (3 T21, 1 T 18 and 1 X0) | |
| FMF software | ||||||||||
| Diez Chang and Bazán Lossio de Diez, [23] | 2019 | 2012–2019 | Retrospective | Clinica Santa Isabel, Lima, Perú | NT |
11–13 | NR | Amniocentesis | 17, 62.0% (7 T 21, 4 T 18, 5 XO and 1 T 22) | |
| Vázquez Martinez, et al. [25] | 2019 | 2006–2008 | Retrospective | National Prenatal Diagnosis Program, Hospital Gineco, Cuba | Increased NT | 10–13.6 | 73 | Amniocentesis/cordocentesis | 4, 5.5% (4 T21) |
GA, gestational age.
Four studies were conducted in Brazil and Mexico at academic university hospitals [17, 18, 19, 20]. Three studies from Cuba were conducted in provincial centers affiliated with the National Center of Medical Genetics at the Medical University of Havana [7, 21, 25]. The studies carried out in Peru, Chile, and Colombia were conducted in various settings, including in vitro fertilization clinics [22, 26], private genetic laboratories [23], and private clinics [24]. Invasive testing options included CVS and amniocentesis, although 36.6% of the patients did not undergo karyotype analysis.
The methodological quality assessment using the Joanna Briggs Institute (JBI) checklist revealed variability in study quality (Table 3, Ref. [11, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26]).
| Study | Sample frame appropriate? | Sampling method | Adequate sample size? | Subjects & setting described? | Valid condition identification? | Condition measured reliably? | Statistical analysis appropriate? | Response rate addressed? | Total score (0–8) | Risk of bias |
| Alcedo Ramírez, et al. [26] | No | No | No | No | Yes | Yes | Yes | No | 3 | High |
| Brizot, et al. [17] | Yes | Yes | Unclear | Yes | Unclear | Yes | No | No | 4 | High |
| Diez Chang and Bazán Lossio de Diez, [23] | Yes | No | No | No | Yes | Yes | No | No | 3 | High |
| Huamán, et al. [22] | Unclear | Yes | No | No | Yes | Yes | No | No | 3 | High |
| Llanusa Ruiz, et al. [11] | Yes | No | Yes | No | Yes | Unclear | No | No | 3 | High |
| Saldanha, et al. [18] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | Low |
| Sepulveda, et al. [24] | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | 7 | Low |
| González Herrera, et al. [21] | Yes | Yes | Unclear | No | Yes | Yes | No | No | 4 | High |
| Mendoza-Caamal, et al. [20] | No | No | No | No | Yes | Yes | No | No | 2 | High |
| Vázquez Martinez, et al. [25] | Yes | Yes | Yes | No | Yes | Yes | Yes | No | 6 | Moderate |
| Vieira, et al. [19] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | Low |
Three studies were classified as having a low risk of bias, one had a moderate risk, and seven exhibited a high risk of bias. Common limitations of high-risk studies include inadequate sample sizes, unclear sampling methods, and a lack of response rate reporting. Conversely, low-risk studies demonstrated robust study designs, appropriate statistical analyses, and comprehensive reporting of study settings and participants.
Fig. 3 illustrates the pooled proportion of fetuses with increased NT identified across the four studies. The overall proportion of increased NT cases was found to be 0.03 (95% CI: 0.03–0.04). Individual study estimates ranged from 0.02 (95% CI: 0.02–0.03) in Vázquez in 2008 to 0.04 (95% CI: 0.03–0.04) in Saldanha in 2009 [18, 23].
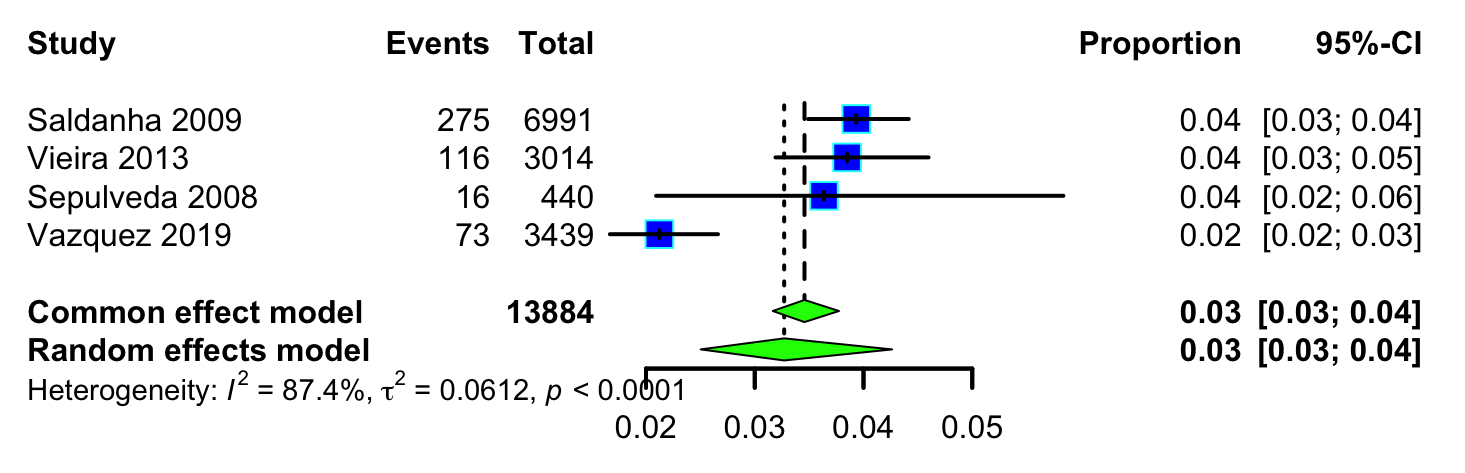 Fig. 3.
Fig. 3.
Pooled proportion of fetuses with increased NT.
There was evidence of substantial statistical heterogeneity (I2 = 87.40%)
and statistically significant Cochran’s Q test (p
Fig. 4 presents a forest plot of the proportion of fetuses undergoing invasive testing. The pooled proportion of invasive testing was 0.31 (95% CI: 0.28–0.33), with individual study estimates ranging from 0.29 to 0.39. Heterogeneity was moderate (I2 = 36.65%), with a non-significant Q-test (p = 0.11), indicating that the variability across studies was not statistically significant.
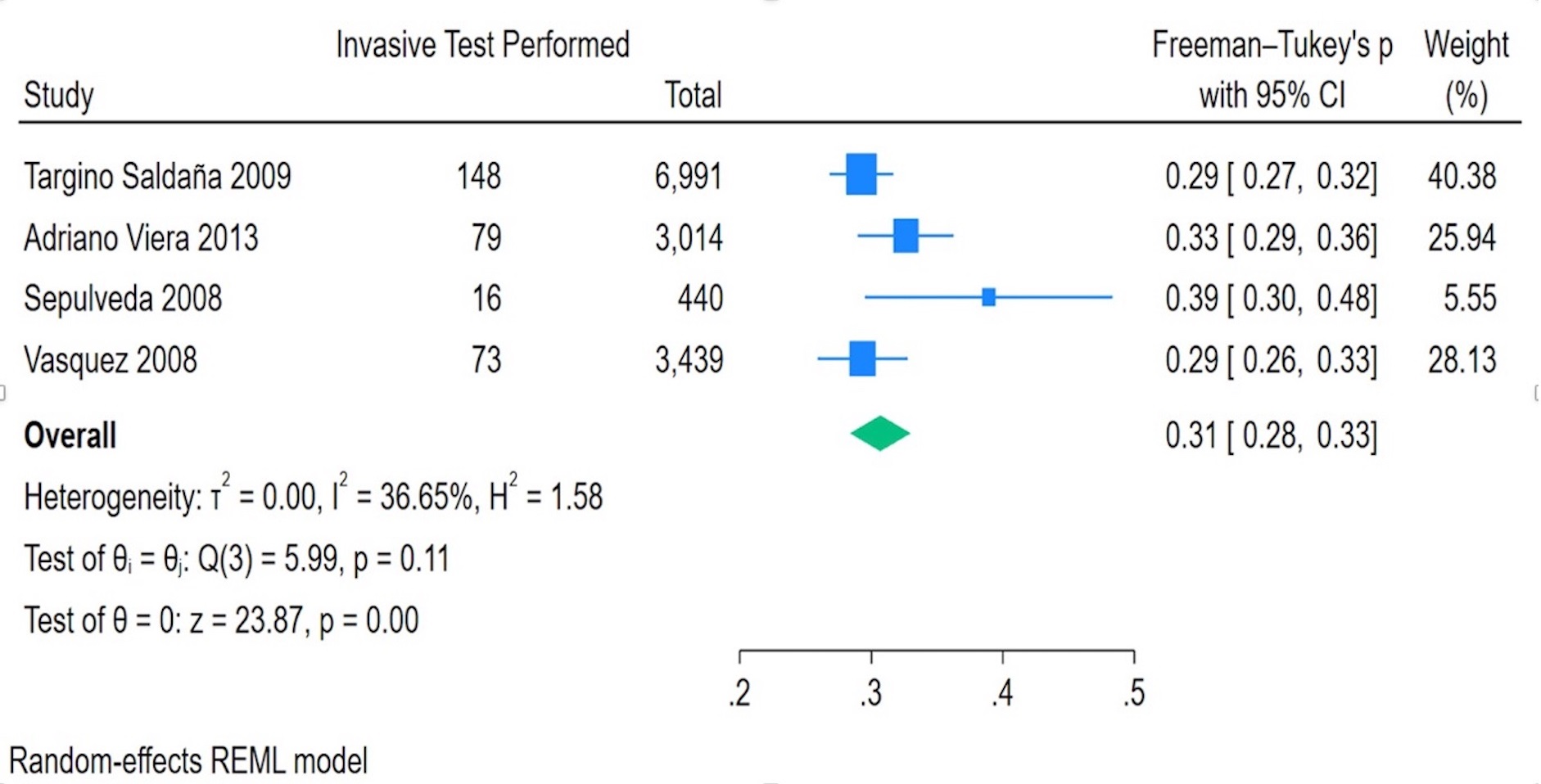 Fig. 4.
Fig. 4.
Invasive test performed in fetuses with increased NT.
A meta-analysis was conducted to determine the proportion of anomalies in cases with increased NT. The pooled estimate under the common-effect model indicated that 0.63 (95% CI: 0.59–0.68) of cases with increased NT were associated with anomalies. Similarly, the random-effects model yielded a proportion of 0.63 (95% CI: 0.52–0.73), albeit with a wider CI due to study variability (Fig. 5).
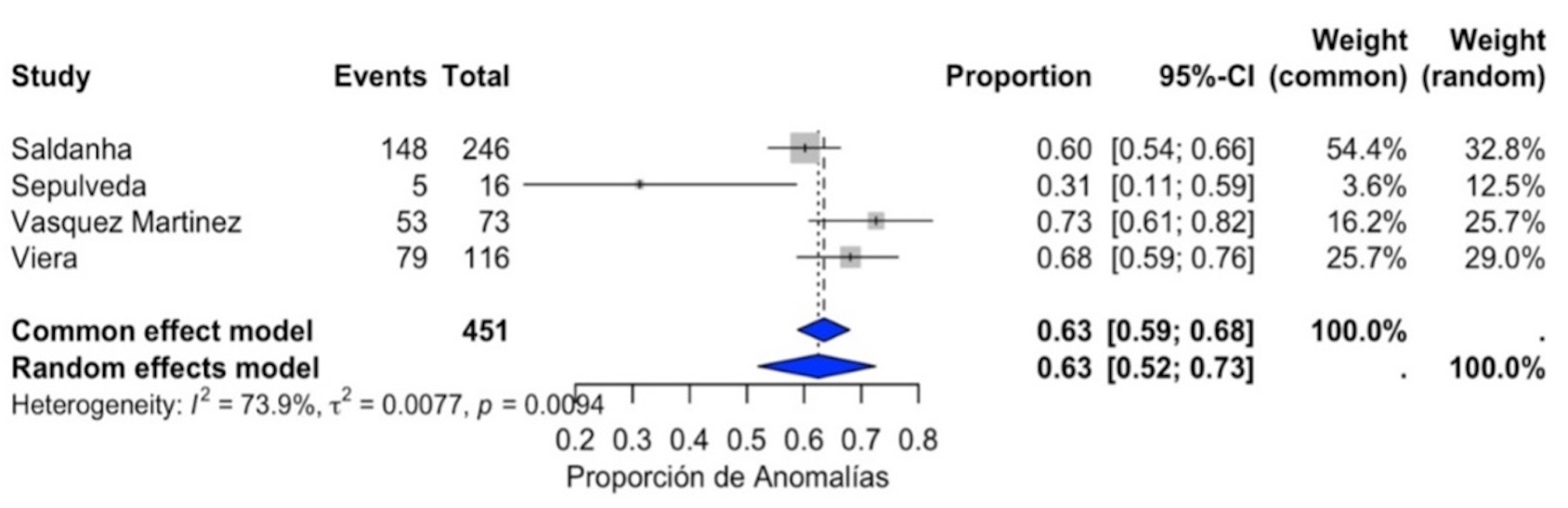 Fig. 5.
Fig. 5.
Prevalence of fetal anomalies in fetuses with increased NT.
The pooled proportion of cardiac anomalies was estimated to be 0.42 (95% CI: 0.1–0.73). The proportion of cardiac anomalies varied across studies, with Saldanha et al. (2009) [18] reporting 2.4%, Vieira et al. (2013) [19] reporting 7.0%, and the Panamanian cohort reporting 0.36%. The random effects model was used to account for heterogeneity, yielding a conservative estimate.
A total of 1512 pregnancies (1236 fetuses) from the Panamanian cohort and 842 fetuses from 11 Latin American studies were analyzed.
The prevalence of NT
| Variable | Panama | Latin America (SR) | North America (Canada, USA) | Europe (Mastromoro et al. 2023 [28]) | Asia (Hong Kong, Turkey) | Most Frequent Fetal Abnormalities |
| Total Cases Analyzed | 1236 fetuses | Varies (16–246 per study) | 226 cases (NT |
Meta-analysis: 59 studies, thousands of cases | Hong Kong: 300 cases (NT |
Congenital heart defects, cystic hygroma, abnormal nasal bone |
| NT |
77 fetuses (6.23%) | Ranges: 3.6–62% per study | 226 cases (all NT |
NT |
Higher NT linked to genetic conditions | Cystic hygroma, structural heart defects, skeletal dysplasias |
| Chromosomal Abnormalities | 11/19 MCs had aneuploidy (57.9%) | Varies: 10.5–62% per study | Canada: 51.3% aneuploidy | 34.35% aneuploidy for NT |
Turkey: 44.6% normal karyotype in NT |
Trisomy 21, Trisomy 18, Trisomy 13, Turner syndrome |
| Cystic Hygroma Prevalence | 9/19 MCs (47.4%) | Frequently observed, not always quantified | Canada: 47.8% had cystic hygroma | 1:285 pregnancies, strongly linked to aneuploidy and CHD | Turkey: Highly correlated with miscarriage and anomalies | Cystic hygroma associated with chromosomal and heart defects |
| Live Births with No Anomalies | 58/77 (75.3%) in NT |
Varies from 40–70% | Canada: 36.7% live births | 30–50% depending on NT severity | Turkey: Only 10.7% of NT |
Higher NT associated with congenital anomalies, but some cases remain normal |
| Pregnancy Loss (MC, Stillbirths, TOPs) | 25/77 (32.5%) in NT |
Varies (17.5–56%) | Canada: 46.9% terminated pregnancies, 6.6% intrauterine deaths | 30–50% pregnancy loss in NT |
Turkey: Higher loss rates in NT |
MC often associated with cystic hygroma and chromosomal abnormalities |
| Invasive Testing Rate (CVS/Amnio) | 20/77 (26.0%) | Varies (6.9–56%) | Canada: 100% underwent invasive testing | 50–70% invasive testing for NT |
Turkey: All patients with NT |
Most cases with NT |
| TOP | 4/77 cases (5.2%) | Ranges: 10–40% | Canada: 46.9% termination rate | 30–50% TOP rate in NT |
Turkey: 66.1% of NT |
TOP more common in cases with major structural anomalies and aneuploidy |
SR, systematic review.
The aneuploidy detection rate was 33% among MC with
karyotyping in Panama and varied from 10.5% to 62% across Latin America. In
contrast, North American and European studies reported aneuploidy rates of
34.35%–51.3% in fetuses with NT
The invasive testing uptake was significantly lower in Latin America (31%) than in North America and Europe (close to 100%). Notably, CMA has not been reported in any Latin American study, despite it being the recommended genetic test in developed countries. Pregnancy termination rates were lowest in Latin America (7.1%–7.8%), in contrast to 46.9% in Canada and 66.1% in Turkey, likely reflecting legal and cultural differences in prenatal decision-making.
This study highlights the low uptake of invasive genetic testing for fetuses with increased NT in the context of Latin America, despite its importance in detecting chromosomal and structural abnormalities. In the Panamanian cohort, only 23.5% underwent invasive testing, while a systematic review of 842 fetuses across 11 Latin American studies found a rate of 0.31 (95% CI: 0.28–0.33). These figures contrast sharply with those of North America and Europe, where nearly all high-risk pregnancies receive invasive testing [1, 2, 3].
Congenital heart defects (10.4%) and cystic hygroma with hydrops fetalis (13%) were found to be the most frequent fetal abnormalities in our cohort, aligning with global data showing structural anomalies in 30–50% of fetuses with increased NT [4, 5, 6]. However, unlike North America and Europe, where Turner syndrome is the most commonly reported chromosomal abnormality in increased NT cases (26.5%), no cases were reported in our review. This discrepancy likely reflects the low karyotyping and molecular testing rates in Latin America, thereby leading to potential underdiagnosis [7, 8, 9].
The absence of CMA data in this review highlights the significant gap between Latin America and developed countries. While the ACOG and SOGC recommend CMA as the first-line diagnostic tool for fetuses with increased NT due to its ability to detect submicroscopic chromosomal imbalances [7], none of the included Latin American studies reported CMA use, which suggests limited accessibility.
Besides, invasive testing rates also vary widely. While Latin America reports a
31% invasive testing rate, nearly 100% of patients with NT
Our findings emphasize the urgent need for expanded access to genetic testing and counseling in Latin America [34, 35, 36, 37, 38]. Although cfDNA testing is a viable noninvasive alternative, it fails to detect 2–10% of chromosomal aberrations, making it inadequate as a standalone test for high-risk cases [39, 40, 41]. Additionally, the absence of molecular testing in Latin America limits the identification of RASopathies and other monogenic disorders, which are increasingly recognized as contributors to increased NT in fetuses with normal karyotypes [42, 43, 44, 45]. Exome sequencing is increasingly recommended for cases when both CMA and karyotype are normal because identifies pathogenic variants in up to 10% of these cases [46]. Exome sequencing remains largely inaccessible in Latin America.
This study has several strengths. For instance, we conducted a systematic review of Latin American studies, incorporating data from multiple national and regional databases to ensure a comprehensive representation of prenatal diagnostic trends in the region. Additionally, our Panamanian cohort provides direct insights into local screening practices and their alignment with global recommendations.
However, the limitations of this study must also be acknowledged. First, the lack of large, multicenter, or population-based studies in Latin America restricts the generalizability of our findings. Second, no CMA or molecular testing data were available for either the Panamanian cohort or the systematic review, limiting the ability to detect submicroscopic chromosomal imbalances and monogenic disorders. Finally, differences in healthcare infrastructure, socioeconomic factors, and legal frameworks across Latin American countries may contribute to variability in prenatal diagnostic practices, requiring further investigation.
This study highlights the disparities in prenatal genetic testing for increased NT in Latin America, where only 31% of high-risk pregnancies undergo invasive testing, and no reported cases include CMA analysis. These findings contrast sharply with those of developed regions, where universal access to invasive and molecular genetic testing is standard practice. Therefore, efforts to improve genetic counseling, expand CMA availability, and standardize prenatal screening guidelines are urgently required to ensure that patients receive equitable access to prenatal diagnostics. Hence, addressing barriers such as the lack of genetic specialists, limited reimbursement, and legal restrictions on pregnancy termination will be essential to align Latin American prenatal care practices with international standards. Future research should focus on evaluating patient and provider perspectives regarding genetic testing uptake, assessing healthcare disparities, and conducting cost-effectiveness studies to support the implementation of CMA and expanded genetic testing programs in Latin America.
SOGC, Canadian Society of Obstetrics and Gynecology; CMA, chromosomal microarray analysis; NT, nuchal translucency; cfDNA, cell-free DNA; ACOG, American College of Obstetricians and Gynecologists; MC, miscarriages; TOP, termination of pregnancy; FMF, fetal medicine foundation; REML, restricted maximum likelihood; CVS, chorionic villous sampling; CHD, congenital heart defect; BMI, body mass index.
The datasets used and analyzed during the current study are available upon reasonable request.
TTH, AHV and AG conceived the study. TTH and AHN developed and conducted the search strategy. YCR, AHN and JMR assisted with clinical data aquisitation and verification.TTH, ICB, and AG jointly screened the titles and abstracts, performed the full-text screening, and conducted data extraction and bias evaluation. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study followed the ethical guidelines and the country-specific regulatory guidelines (RESEGIS 2397). This study was exempt from the Institutional Review Board of Hospital Punta Pacifica (CBI-21-104). This study was conducted in accordance with the principles of the Declaration of Helsinki. It was part of the standard of care in the first-trimester screening program. Written informed consent was obtained from all patients. The collected data were anonymously transferred to a central database, and coding and data analyses were performed by non-clinicians.
We thank all peer reviewers for their opinions and suggestions.
This work was funded with the support of the Secretaría Nacional de Ciencia, Tecnología e Innovación, and Sistema Nacional de Investigación Grant No.136-2022.
The authors declare no conflict of interest.
During the preparation of this work, the authors use ChatGPT-4o to improve readability and language. After using this tool, we reviewed and edited content as needed and took full responsibility for the final publication.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG39182.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
