- Academic Editor
This is an open access article under the CC BY 4.0 license.
Investigations have highlighted the detrimental neurological outcomes associated with tramadol exposure, yet studies addressing histopathological changes in the cerebellum following prenatal exposure remain limited. Therefore, this study aimed to elucidate alterations in cerebellar architecture induced by tramadol administration during gestation, particularly during the critical period of neuronal differentiation, while evaluating the potential neuroprotective role of L-carnitine.
A cohort of eight male pups was euthanized at two postnatal time points: one and three weeks after birth. Each age group was divided into four experimental categories: Group I (control); Group II (L-carnitine), where pregnant rats received L-carnitine; Group III (tramadol), where offspring from tramadol-exposed mothers were assessed; Group IV (tramadol + L-carnitine), which included pregnant rats administered both tramadol and L-carnitine. Treatments began on gestational day 7 and continued until day 21. Pups were sacrificed on postnatal days 7 and 21 following treatment, and cerebellar samples were subjected to histological and immunohistochemical analyses to evaluate oxidative stress markers. Data were analyzed using GraphPad Prism v7.01 and expressed as the mean ± SEM; significance (p < 0.05) was assessed using a t-test or one-way ANOVA with the Tukey–Kramer post hoc test.
Prenatal tramadol exposure resulted in significant histological alterations in the developing cerebellar cortex of the postnatal offspring. Noteworthy findings included the persistence of the external granular layer, degeneration of Purkinje cells with pericellular halos and vascular congestion, all of which correlated with oxidative stress markers. In contrast, L-carnitine co-administration facilitated a restoration of normative cerebellar architecture.
These findings indicate that tramadol exposure during pregnancy elicits substantial degenerative changes in the cerebellar cortex, highlighting L-carnitine co-treatment as a promising strategy to mitigate these tramadol-mediated adverse effects.
Tramadol is a synthetic analgesic drug closely related to opioid compounds such as codeine and morphine. Its pharmacodynamic profile involves both opioid and non-opioid mechanisms: It binds to µ-opioid receptors. It inhibits the reuptake of neurotransmitters like serotonin (5-HT) and norepinephrine, contributing to its analgesic effects [1]. Structurally, tramadol is 2-(dimethylaminomethyl)-1-(3-methoxyphenyl) cyclohexanol, with its hydrochloride form demonstrating solubility in ethanol and water [2]. Tramadol, approved by the Food and Drug Administration (FDA) as a potent analgesic, is primarily used for moderate to severe pain. However, as a Schedule IV controlled substance, it requires careful restriction due to potential misuse and addiction risks [3]. Particularly during pregnancy warrants caution, as it has been correlated with adverse outcomes such as increased rates of abortion, stillbirth, low birth weight and congenital malformations [4].
The cerebellum, located in the hindbrain beneath the occipital lobe, is crucial for motor control, behavioral regulation, homeostasis and cognitive functions such as language and attention [5]. It features an outer layer of gray matter (Cerebellar cortex) and an inner layer of white matter (Cerebellar medulla), including three layers: Internal granular, Purkinje cell and external molecules [6]. Due to its prolonged maturation, the cerebellum is vulnerable to developmental influences, increasing the risk of disorders [5]. At birth, the cerebellum is still underdeveloped, undergoing myelination primarily postnatally, with cellular proliferation continuing into early postnatal stages [7]. In rodents, the cerebellum has inner white matter and outer grey matter, with a mature cortex forming by three weeks postpartum as the external granular layer regresses [8, 9]. The molecular layer, positioned between the Purkinje and external granular layers, becomes the most superficial and has a high density of synapses and various cells [5]. The granule cell layer is dense with granule cells, while the Purkinje cell layer features large cell bodies with prominent nuclei [10].
Tramadol has been documented to disrupt vital enzymatic functions within the rat cerebellum and interfere with the metabolism of several amino acids, thereby affecting cerebellar neuronal pathophysiology [1] and raising concerns about congenital malformations with increased opioid prescriptions during pregnancy [1, 11].
The L-carnitine has been documented as a support for fatty acid transport for energy and is essential for brain and lung maturation in utero. It modulates brain metabolism, enhances cell membrane stability and exhibits neuroprotective properties [12, 13].
This study aims to investigate the biochemical and histological changes in rat offspring’s cerebellum due to tramadol exposure during pregnancy and the potential neuroprotective effects of L-carnitine.
The research was carried out at the Animal Facilities of the Animal House, Faculty of Medicine, Minia University, Egypt. The study took place between December, 2024 and February, 2025.
The drug used in this study is Tramadol hydrochloride (HCL), obtainable from Alkan-Pharm in Egypt in the form of Tamol (225 mg tablets). It was administered via oral gavage at a dosage of 50 mg/kg each day [14]. Additionally, L-carnitine is sourced from Global, NAPI Company, Egypt and is available in 500 mg capsules under the name Carnitol.
After receiving approval from the Committee of Animal Research Ethics, two female Sprague-Dawley rats were housed with one male for mating, with fertilization confirmed through daily vaginal smear examinations. The presence of sperm indicates the start of gestation as day one. On day 7 of pregnancy, the rats were divided into four groups, each consisting of eight male offspring, with four animals allocated for analysis at one week and four at three weeks of age. The rats were housed individually in plastic cages under standard laboratory conditions, including a temperature range of 24 to 30 °C, a 12 hrs light/dark cycle and unrestricted access to a standard diet and water.
Group I (control group): Pregnant rats were fed standard laboratory food and provided with water.
Group II (L-carnitine group): Pregnant rats were administered L-carnitine, dissolved in normal saline, by gastric gavage once a day starting from day 7 of their pregnancy.
Group III (tramadol group): Pregnant rats received tramadol at a dosage of 50 mg/kg daily through a gastric tube from day 7 of their pregnancy [14].
Group IV (tramadol and L-carnitine group): Pregnant rats were treated with a combination of tramadol and L-carnitine dissolved in normal saline via gastric gavage once a day beginning on day 7 of pregnancy. Four male offspring from each group were euthanized under deep anesthesia at each time point, one week and three weeks after birth, for subsequent analyses using an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). After ensuring the absence of reflexes, euthanasia was completed by cervical dislocation at two different ages One week and three weeks after birth.
Before any surgical or invasive procedures, animals were anesthetized using an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) to ensure adequate anesthesia and analgesia. At the end of the experiment, the animals were euthanized using a lethal dose of the same anesthetic combination administered intraperitoneally, in accordance with institutional ethical guidelines. The cerebella were removed and divided into two halves; one half was prepared for light microscopy, immunohistochemistry and morphometric analysis. The other half was homogenized for biochemical testing.
Using a polytron homogenizer, frozen cerebellar tissues were processed in ice-cold 0.01 M phosphate buffer (pH 7.4). Following a 15-minute centrifugation at 5000 rpm, the supernatants were collected and used to assess various biochemical parameters. The levels of Malondialdehyde (MDA) and reduced Glutathione (GSH) were determined calorimetrically in the cerebellar tissue homogenates using specific colorimetric test kits (Catalogue No. for MDA, MD 25 29; for GSH, GSHGR 25 11) obtained from Bio-diagnostic (Giza, Egypt). The method for GSH measurement is based on the reaction of GSH’s sulfhydryl group with Ellman’s reagent (5,5-dithiobis). The measurement of MDA is based on its interaction with thiobarbituric acid.
After fixation, the samples were dehydrated in graded alcohol, cleared in xylene, embedded in paraffin wax, and cut into 5 µm slices. Hematoxylin and Eosin (H&E) staining was performed for histological analysis: The sections were dewaxed in xylene, stained with hematoxylin for 7 min, rinsed, stained with eosin for 3 min, dehydrated, cleared, and cover-slipped for light microscopy [15].
Immunohistochemical staining was conducted using anti-Glial Fibrillary Acidic Protein (anti-GFAP) and anti-Proliferating Cell Nuclear Antigen (anti-PCNA) antibodies. Paraffin-sections 5 µm were prepared following the manufacturer’s protocols. Endogenous peroxidase activity was inhibited by deparaffinizing in xylene and rehydrating through graded alcohols, followed by treatment with 0.1% hydrogen peroxide for 15 min. After washing with phosphate-buffered saline, sections were incubated in Ultra Vision Block for 5 min to reduce non-specific staining. Primary antibodies were incubated for 2 hrs with anti-GFAP and 60 min, followed by anti-PCNA at room temperature. Antibodies included polyclonal rabbit anti-GFAP (Catalogue No. FNab03426, Lot WT230512, Wuhan Fine Biotech Co., Ltd., Wuhan, Hubei, China, dilution 1:50) and monoclonal mouse anti-PCNA (Catalogue No. P8825, Sigma-Aldrich, Giza, Egypt, dilution 1:100). The antibody-antigen reaction was visualized using Diaminobenzidine chromogen (SIGMA FAST™, Lot 0000442429; SigmaAldrich, St. Louis, MO, USA; Catalog D4418; CAS 91952) and the Ultra Vision One Detection System (HRP polymer; Epredia Lab Vision, Catalog TL125HLJ; Lab Vision Corp., 47777 Warm Springs Blvd, Fremont, CA 94539, USA). Sections were counterstained with hematoxylin, dehydrated through alcohol, cleared in xylene, and mounted under coverslips with the permanent medium.
The mean area percentage of GFAP and PCNA immunopositivity was measured at
GraphPad Prism (version 7.01, GraphPad Software, San Diego, CA, USA) was used to
analyze quantitative data. The mean and Standard Error of the Mean (SEM) for each
group was calculated by presenting results as Mean
The treatment with tramadol reduced the activity of the antioxidant enzyme GSH while, the level of MDA increased. In contrast, when L-carnitine was given alongside, there was a notable rise in GSH levels and a significant decrease in MDA levels.
In the control Postnatal Day 7 (PND7) group, the rat cerebellar cortex displayed its typical structure, consisting of the external granular layer (EGL), molecular layer (MCL), Purkinje cell layer (PCL) and internal granular layer (IGL) (Fig. 1a). The EGL contained small, rounded cells in two or three rows with deeply stained nuclei and scant cytoplasm. The MCL had migrating cells arranged perpendicularly to the pial surface, while Purkinje cells were in a single row, characterized by rounded vesicular nuclei and pale cytoplasm. The IGL was filled with clumps of rounded cells with similar features (Fig. 1b).
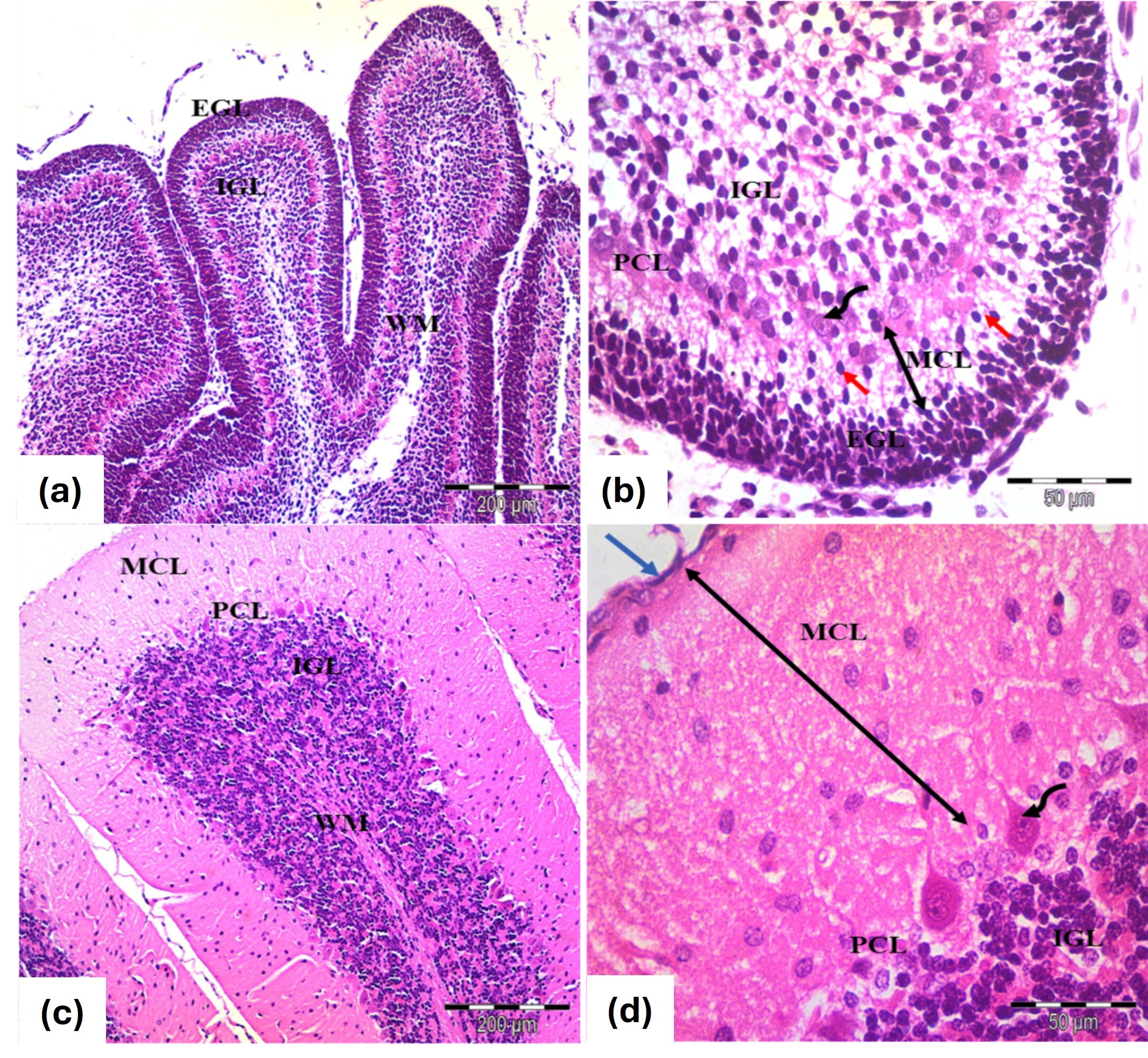 Fig. 1.
Fig. 1.
Photomicrographs of the cerebellar cortex from (a,b) 7 days old
and (c,d) 21 days old control groups. (a,b) Cerebellar cortex of 7 days old
rats. Exhibit an external granular layer EGL with closely packed cells and
rounded, deeply stained nuclei. The molecular layer MCL contains migrating cells
(red arrows), while the Purkinje cell layer PCL features rounded vesicular nuclei
and pale cytoplasm (curved arrows). The inner granular layer GCL is densely
populated with rounded cells. (c,d) Cerebellar cortex 21 days old rats. EGL:
Disappears, leaving remnants of pia mater (blue arrow), MCL: Thickens
(double-head black arrows), PCL: Flask-shaped or oval cells with large vesicular
nuclei and MCL shows decreased cellularity alongside an increase in granule
cells. WM: Indicates white matter fibers. Stain: H&E. Mag. (a,c)
In the control PND21 group, the cerebellar cortex showed the adult architecture with an outer MCL, middle PCL and inner granular cell layer (GCL), marking the disappearance of the EGL. Granule cells migrated through the MCL, while Purkinje cells were well differentiated with oval-shaped nuclei and the granule cell population appeared dense (Fig. 1c,d).
The histological structure closely mirrored that of the control group at the 7- and 21-days marks (Fig. 2a–d).
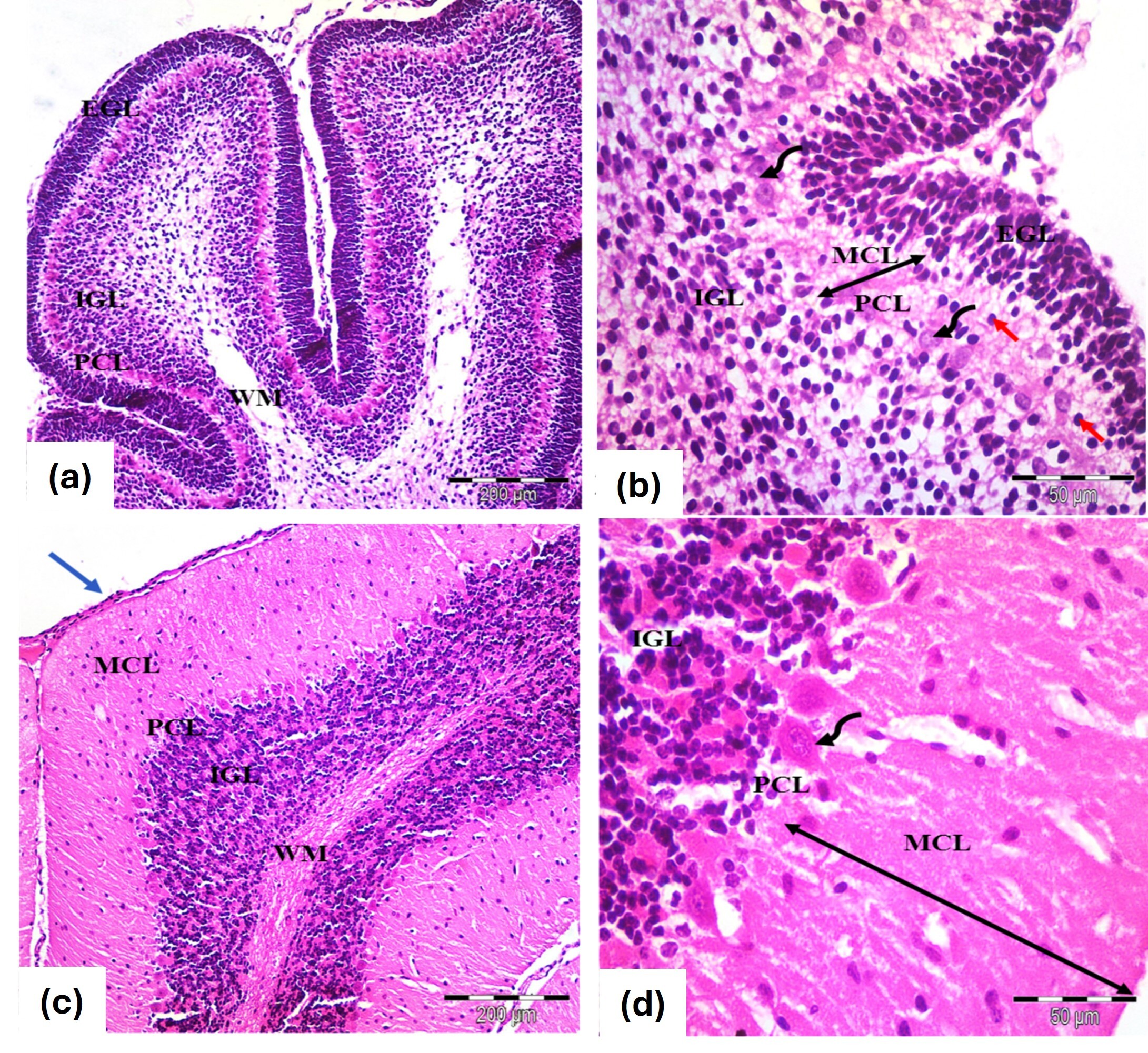 Fig. 2.
Fig. 2.
Photomicrographs of the cerebellar cortex (a,b) 7 days old and
(c,d) 21 days old in albino rats from the L-carnitine group. Similar
histological features to the controls. (a,b) Cerebellar cortex of 7 days old
rats. Exhibit an external granular layer EGL with closely packed cells and
rounded, deeply stained nuclei. The molecular layer MCL contains migrating cells
(red arrows), while the Purkinje cell layer PCL features rounded vesicular nuclei
and pale cytoplasm (curved arrows). The inner granular layer GCL is densely
populated with rounded cells (c,d) Cerebellar cortex 21 days old rats. EGL:
Disappears, leaving remnants of pia mater (blue arrow), MCL: Thickens
(double-head black arrows), PCL: Flask-shaped or oval cells with large vesicular
nuclei and MCL shows decreased cellularity alongside an increase in granule
cells. WM: Indicates white matter fibers. Stain: H&E. Mag. (a,c)
At PND7, the cerebellar cortex showed a reduced thickness of the EGL and an increased thickness of the molecular cell layer (MCL), with extravasated blood in the EGL (Fig. 3a). Purkinje cells were irregularly shaped, with distortion and shrinkage, displaying pyknotic nuclei (Fig. 3b). By PND21, remnants of the EGL persisted, along with increased MCL thickness. Purkinje cells displayed further distortion and degeneration, with visible extravasated blood (Fig. 3c,d).
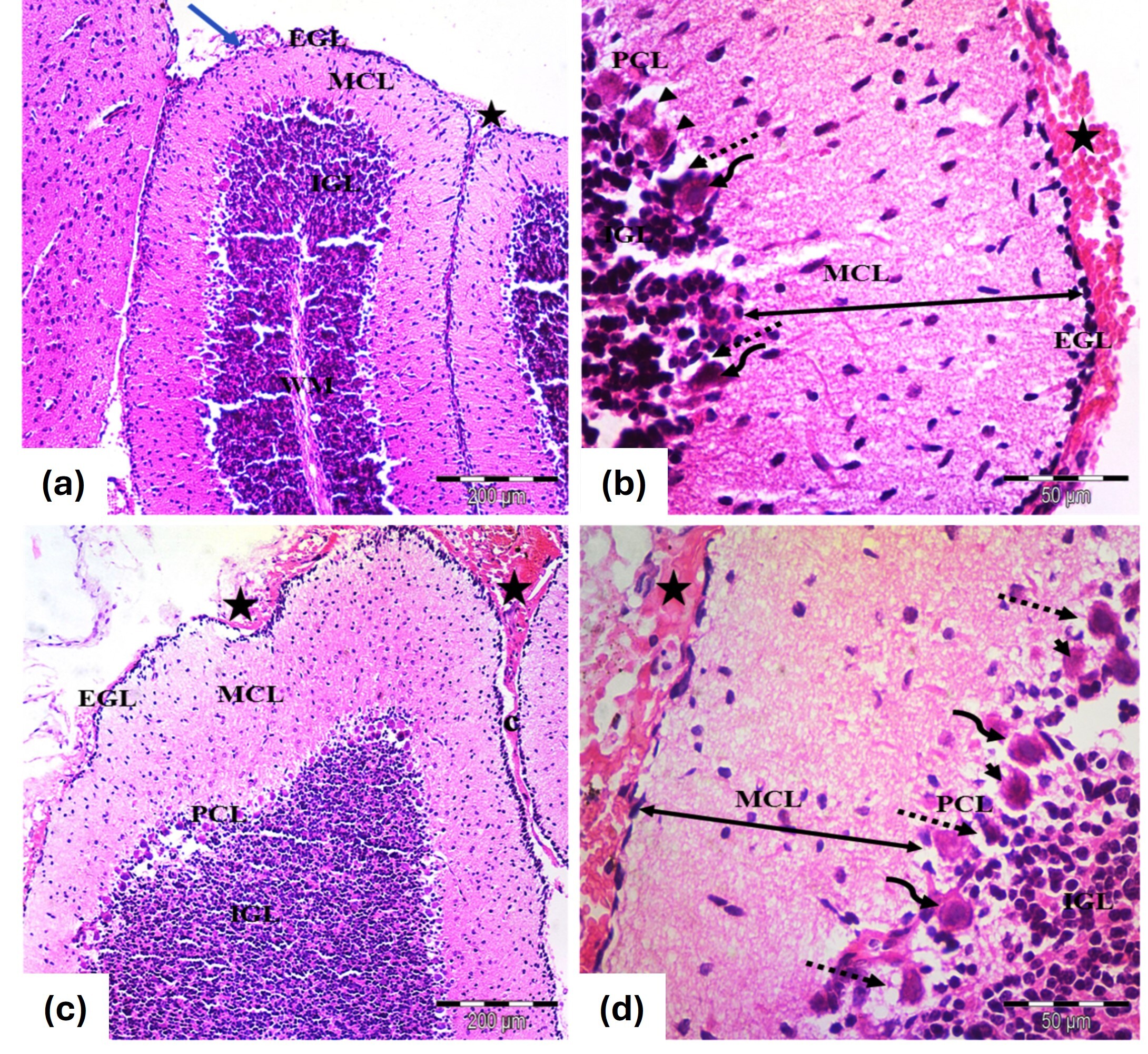 Fig. 3.
Fig. 3.
Photomicrographs of the cerebellar cortex from the tramadol
group of (a,b) 7 days old and (c,d) 21 days old rats. (a) Cerebellar cortex of 7
days old rats; Identified layers include EGL, MCL, PCL and IGL. The EGL is
thinner, while the MCL is thicker. Extravasated blood (star) is seen under the
pia mater (blue arrow). (H&E
At PND7, the cerebellar cortex showed nearly standard architecture across primary layers, with EGL and MCL thicknesses comparable to controls and morphologically normal Purkinje cells (Fig. 4a,b). By PND21, the typical cortical structure was restored, with no EGL under the pia mater and predominantly normal Purkinje cells, though mild vascular congestion was observed (Fig. 4c,d).
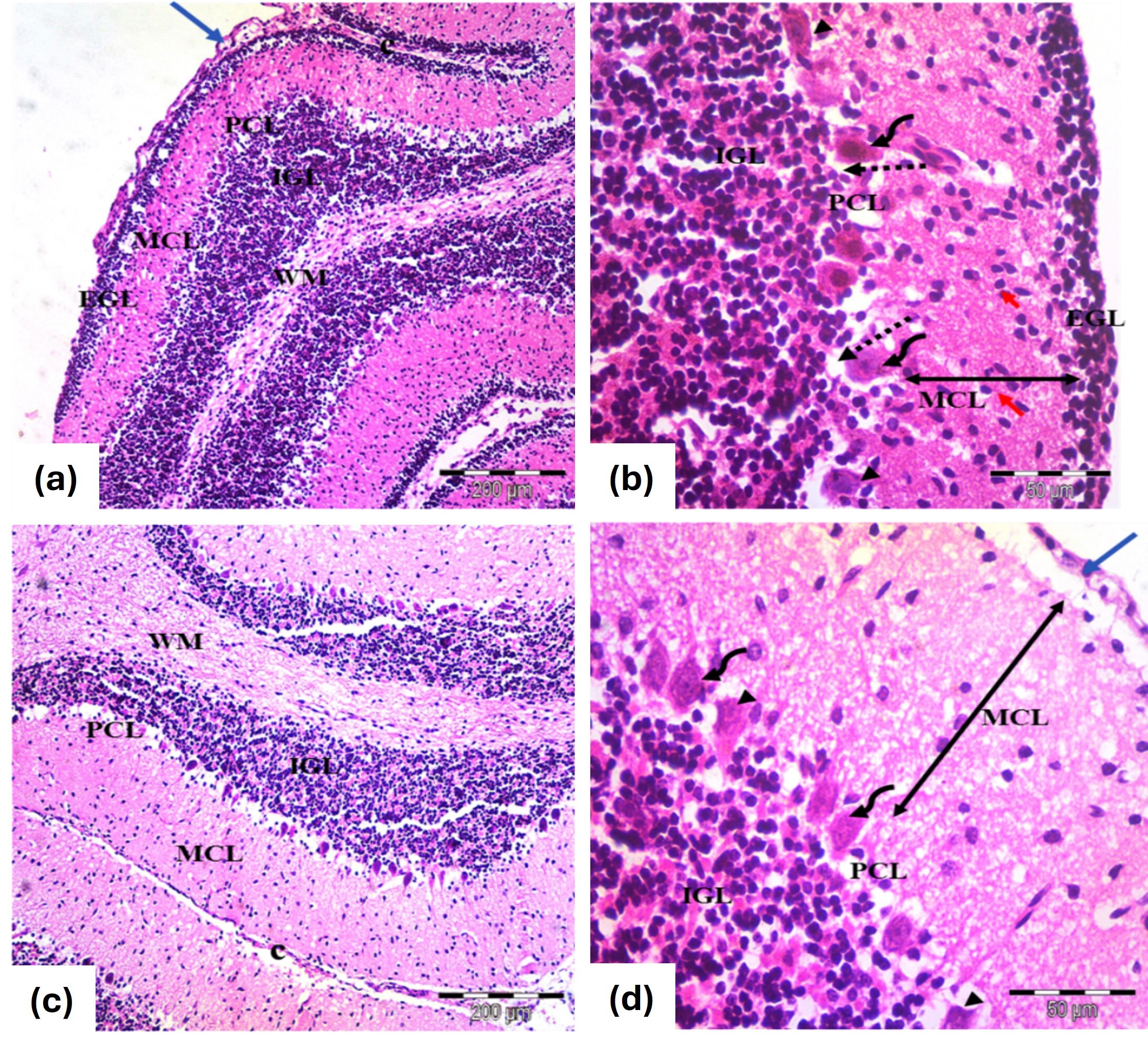 Fig. 4.
Fig. 4.
Photomicrographs of the cerebellar cortex from the
tramadol-carnitine group, showing (a,b) 7 days old and (c,d) 21 day old groups.
(a) Cerebellar cortex of 7 days old rats; EGL with rounded, darkly stained nuclei (blue arrow) , normal MCL and PCL with rounded nuclei and some disfigured cells (H&E
Concerning GFAP immunostaining, astrocytes were observed as star-shaped cells displaying delicate processes. The control group (Fig. 5a,b) and the carnitine group (Fig. 5c,d) demonstrated a typical distribution of GFAP immunoreactivity, showing a mild positive response in the astrocytes and their extensions throughout all layers of the cerebellar cortex. However, the tramadol group reveals strong GFAP immunoreactivity with many larger astrocytes with thick multiple ramified processes if compared to control and carnitine groups (Fig. 5e,f). Also, many hypertrophic and intensely stained astrocytes with large cell bodies in white matter appeared in this group (Fig. 5g,h). In contrast, the tramadol-carnitine group reveals small astrocytes with short, thin processes if compared to the tramadol group (Fig. 5i,j).
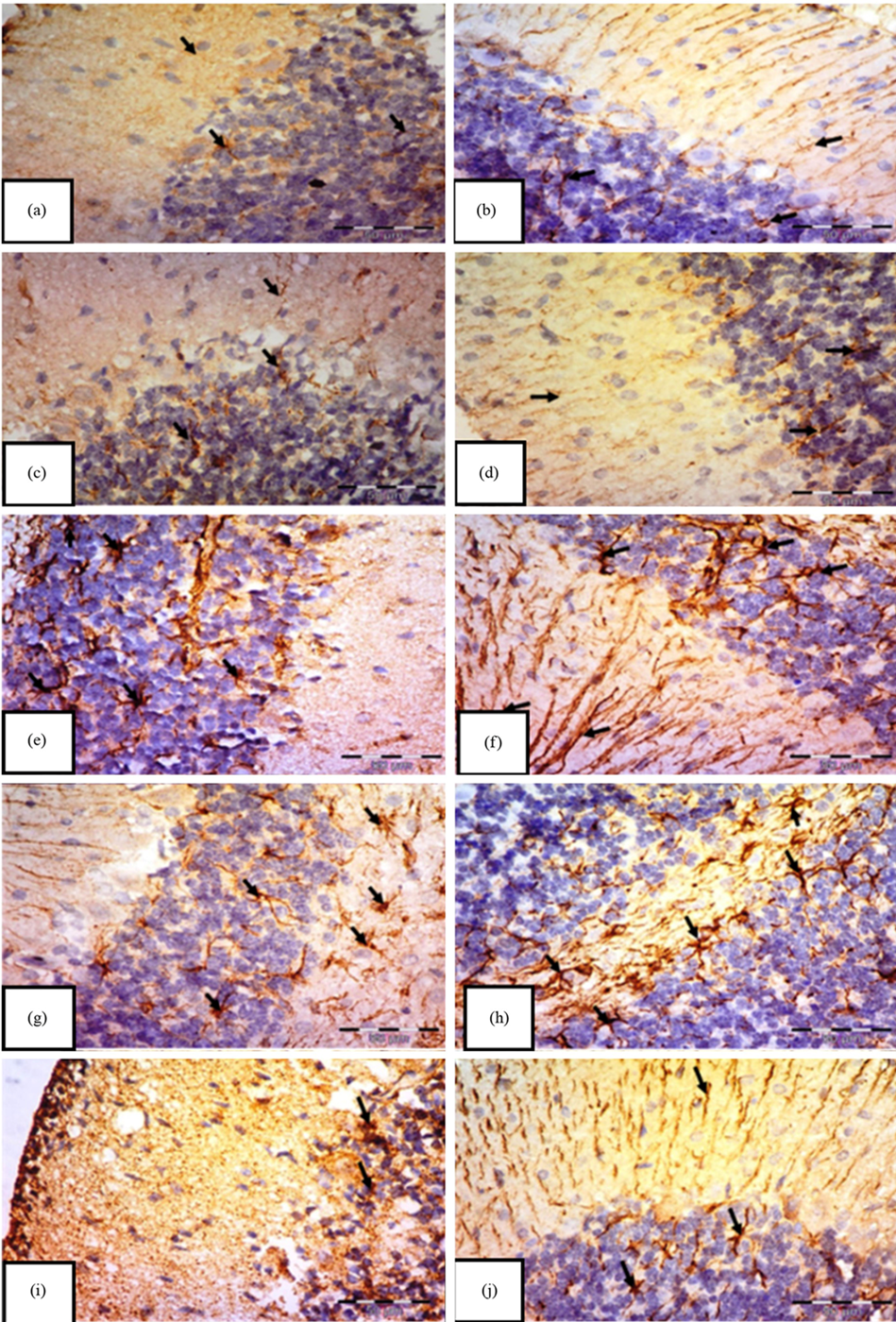 Fig. 5.
Fig. 5.
Immunohistochemical staining of Glial Fibrillary Acidic Protein (GFAP) in the cerebellar cortex of albino rats.
Groups: control (a,b), carnitine (c,d), tramadol (e,f), white matter (g,h), and tramadol–carnitine (i,j), each at 7 and 21 days. Controls and carnitine groups show few small astrocytes (arrows). Tramadol group shows numerous hypertrophic astrocytes with thick processes. White matter shows many enlarged astrocytes, while tramadol–carnitine shows a moderate number of small astrocytes. Mag.
Morphometric analysis of GFAP immunopositivity (Table 1) indicated that there
was no significant difference between the control group and the carnitine-treated
group (p = 0.459 for PND7 and p = 0.526 for PND21). In
contrast, the tramadol group exhibited a significant increase in GFAP
immunoreactivity when compared to both the control and the carnitine groups
(p
| Group | Day | Mean |
p-value vs. Control (#) | p-value vs. Tramadol ($) | p-value vs. Day 7 (&) |
| Control | 7 | 17.21 |
– | – | – |
| Control | 21 | 17.46 |
– | – | – |
| L-carnitine | 7 | 19.64 |
0.459 | – | – |
| L-carnitine | 21 | 20.48 |
0.526 | – | – |
| Tramadol | 7 | 34.58 |
– | ||
| Tramadol | 21 | 40.77 |
– | ||
| Tramadol + L-carnitine | 7 | 25.74 |
0.0009* | 0.0117* | 0.0007* |
| Tramadol + L-carnitine | 21 | 28.09 |
0.0018* | 0.0192* | 0.0004* |
#Control group, $L-carnitine group, &Tramadol group and
*p
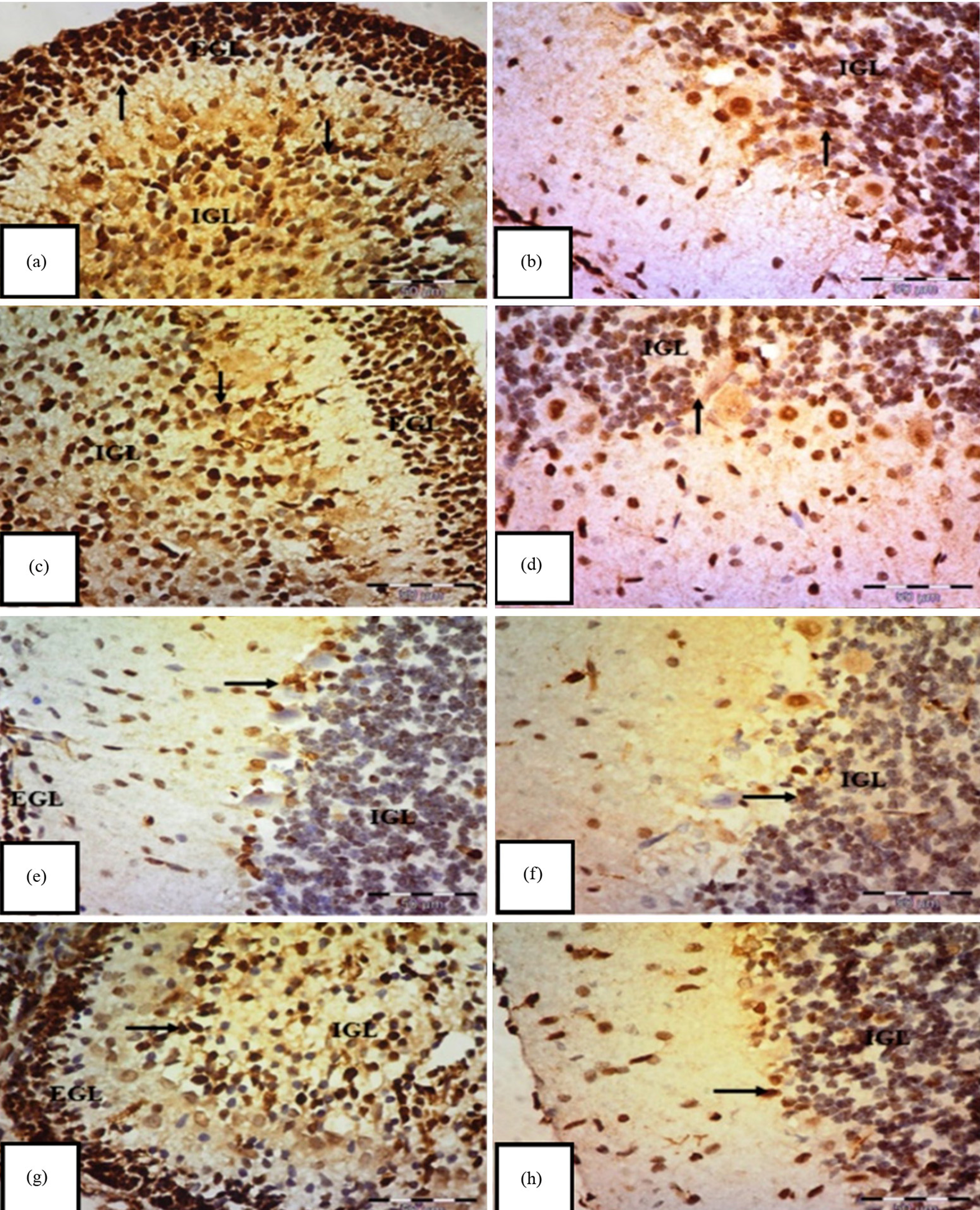 Fig. 6.
Fig. 6.
Immunohistochemical staining of Proliferating Cell Nuclear Antigen
(PCNA) in the transverse section of the cerebellar cortex of an albino rat.
(a,b) Control group shows a strong positive immune reaction to PCNA, with
brownish nuclear staining of external granular cells (EGL) in the internal
granular layer (IGL) (arrows). (c,d) Carnitine group also displays positive
nuclear staining of EGL in the IGL (arrows). (e,f) Tramadol group shows mostly
negative expressions in EGL and IGL cells, with only a few faint to moderate
expressions (arrows) and (g,h) tramadol-carnitine group exhibits positive nuclear
staining of EGL in the IGL (arrows). Mag.
Morphometric analysis to assess PCNA immunopositivity (Table 2) showed that
there was an insignificant difference between the control group and carnitine
treated group p
| Group | Day | Mean |
p vs. Control (#) | p vs. Tramadol ($) | p vs. Day 7 (&) |
| Control | 7 | 35.57 |
– | – | – |
| Control | 21 | 31.45 |
– | – | – |
| L-carnitine | 7 | 35.48 |
– | – | |
| L-carnitine | 21 | 33.48 |
0.674 | – | – |
| Tramadol | 7 | 15.41 |
– | ||
| Tramadol | 21 | 14.59 |
– | ||
| Tramadol + L-carnitine | 7 | 22.36 |
0.0086* | ||
| Tramadol + L-carnitine | 21 | 22.16 |
0.0010* | 0.0002* | 0.0051* |
#Control group, $Carnitine group, &Tramadol group and
*p
Tramadol consistently increased MDA levels, indicating higher oxidative stress, while decreasing GSH concentrations, a key antioxidant. In contrast, carnitine alone had no significant effect on these markers. However, when carnitine was co-administered with tramadol, it significantly reduced MDA levels (Fig. 7a,b) and partially restored GSH levels (Fig. 7c,d), suggesting a protective role for carnitine against tramadol-induced oxidative stress. In addition, tramadol increased GFAP levels (Fig. 8a,b), indicating significant astrogliosis and reduced PCNA (Fig. 8c,d), suggesting impaired cellular proliferation. Carnitine’s simultaneous administration mitigated these effects, reducing GFAP levels and partially restoring PCNA, indicating its protective role against tramadol’s adverse impact.
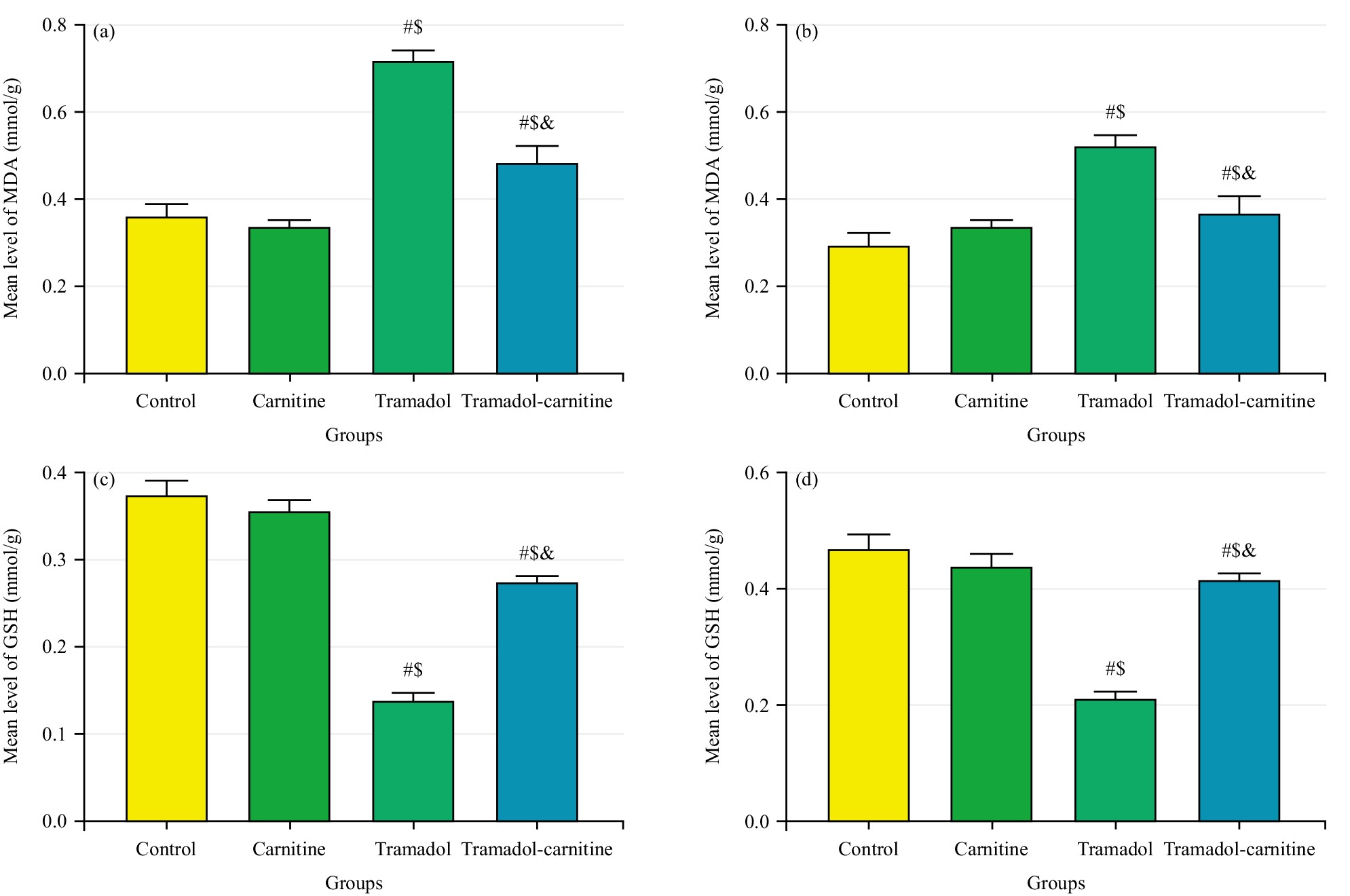 Fig. 7.
Fig. 7.
Comparison of MDA and GSH Levels in cerebellar tissues at day 7 and 21. (a) Mean MDA levels (mmol/g) at day 7. (b) Mean MDA levels (mmol/g) at day 21. (c) Mean GSH levels (mmol/g) at day 7. (d) Mean GSH levels (mmol/g) at day 21. #Control, $Carnitine, &Tramadol.
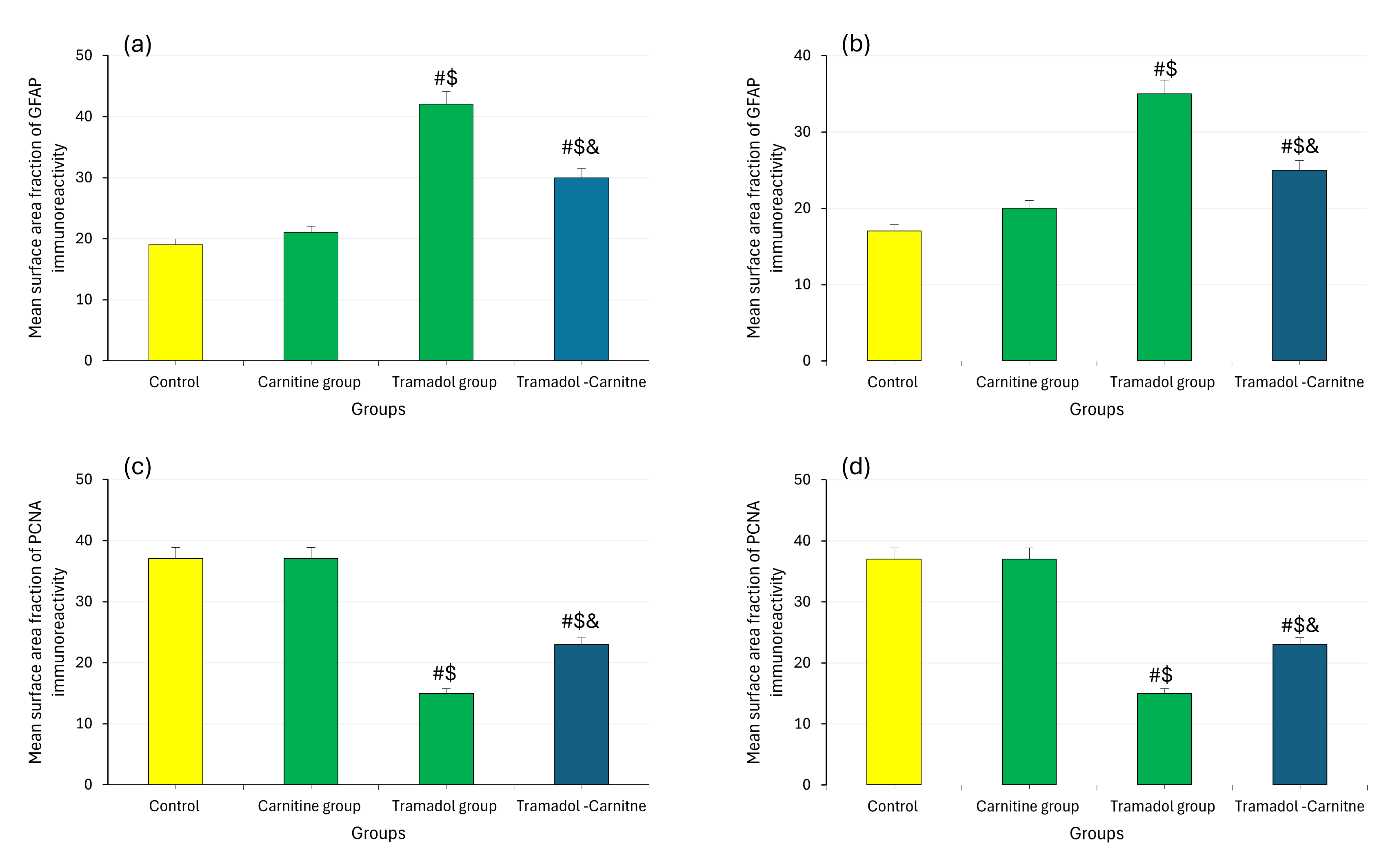 Fig. 8.
Fig. 8.
Analysis of MDA, GSH, GFAP and PCNA in cerebellar tissues at day 7 and 21. (a) GFAP immunoreactivity at day 7. (b) GFAP immunoreactivity at day 21. (c) PCNA immunoreactivity at day 7. (d) PCNA immunoreactivity at day 21. #Control, $Carnitine, &Tramadol.
The cerebellum is vital in balance, motor control and cognitive processes like behaviours and emotions [16]. Its prolonged development makes it particularly susceptible to environmental damage, with significant growth occurring during the third trimester in humans [17].
Rats were chosen for this study because their postnatal neurodevelopment parallels human gestation [16]. The rat cerebellum matures within the first three postnatal weeks, during which significant structural changes occur, making it an ideal model to examine tramadol-induced changes during this critical growth period [1]. Tramadol hydrochloride, an opioid used for moderate to severe pain, is believed to have low dependency potential but may adversely affect the nervous system [18]. Given the rise in opiate use globally [19]. This study clarifies tramadol’s effects on the cerebellar cortex of postnatal male albino rats after prenatal exposure.
Additionally, L-carnitine, which can cross the blood-brain barrier and influence brain metabolism [20], was investigated for its potential protective effects on the cerebellar cortex following prenatal tramadol treatment.
The study evaluated GSH and MDA concentrations to identify tramadol-mediated oxidative stress and examined L-carnitine’s potential antioxidant effects. The tramadol group showed a significant decrease in GSH levels in the cerebellum, indicating increased oxidative stress. In contrast, the tramadol-carnitine group exhibited a notable rise in GSH compared to the tramadol group. This supports findings from Mehranpour et al. [21] that long-term tramadol use lowers GSH.
The MDA, a byproduct of lipid peroxidation, significantly increased in the tramadol group, consistent with previous findings [22, 23].
Light microscopy revealed no differences between control and L-carnitine groups at both ages, with the cerebellar cortex displaying a typical layered structure. Prenatal exposure to tramadol altered the cerebellar architecture, particularly the Purkinje cell layer, leading to distorted and degenerated cells [10].
Immunostaining for GFAP in the tramadol group indicated astrogliosis, supported by morphometric data showing increased GFAP immunopositivity. The tramadol-carnitine group exhibited lower GFAP percentages, suggesting reduced astrogliosis due to L-carnitine’s protective effects as Ferreira and McKenna [24] reported. Additionally, PCNA immunostaining in the tramadol group showed substantial changes in both PND7 and PND21, highlighting the impact of tramadol on cell proliferation.
This study has limitations, including its focus on male pups, which may overlook sex-related differences in development. The short assessment periods of one and three weeks postpartum may miss long-term neurodevelopmental effects and behavioral evaluations are needed to assess the functional consequences of cerebellar alterations. Additionally, variability in tramadol dosing during gestation and potential observer bias in histological analysis further complicate the results. Addressing these issues is crucial for a better understanding of tramadol’s effects and L-carnitine’s potential neuroprotective role.
This investigation shows that tramadol can cause neurotoxic effects on cerebellar development during pregnancy, leading to altered structure and oxidative stress. The L-carnitine may mitigate these effects by protecting cerebellar integrity and promoting neurogenesis. These findings highlight L-carnitine’s potential to protect against opioid-induced brain damage. With increased tramadol use in pregnant women, more clinical trials are necessary to evaluate its long-term effects on fetal brain development and the benefits of L-carnitine supplementation.
This study highlights the significant effects of prenatal tramadol exposure on cerebellar development, indicating notable changes in offspring. The L-carnitine may have neuroprotective properties that could mitigate tramadol’s harmful effects. These findings underscore the importance of understanding maternal medication’s impact on fetal brain development and propose strategies to protect neurological health. Further research is needed to investigate the impact of tramadol on cerebellar development and the protective role of L-carnitine, with the aim of conducting long-term studies and clinical trials to refine treatment approaches.
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
The author confirms sole responsibility for the conceptualization, methodology, investigation, data analysis, writing, and final approval of the manuscript.
Ethical approval for the study was granted by the Institutional Animal Care Committee, Faculty of Medicine, Minia University, Egypt (Approval No: 1382/12/2024; Date: 09/12/2024). The research was conducted with a focus on minimizing animal use and ensuring their well-being. All experimental procedures were performed efficiently and with care to limit stress-induced variables.
Not applicable.
This research received no external funding.
The author declares no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
