-
- Academic Editor
-
-
-
Endometrial thickness is a key factor in determining the suitability for embryo transfer (ET) and influences the success of assisted reproductive technology (ART) outcomes. The aim of this study is to compare endometrial thickness in patients undergoing frozen-thawed embryo transfer (FET) who received platelet-rich plasma (PRP) injections alongside standard estrogen therapy with those receiving standard estrogen therapy alone.
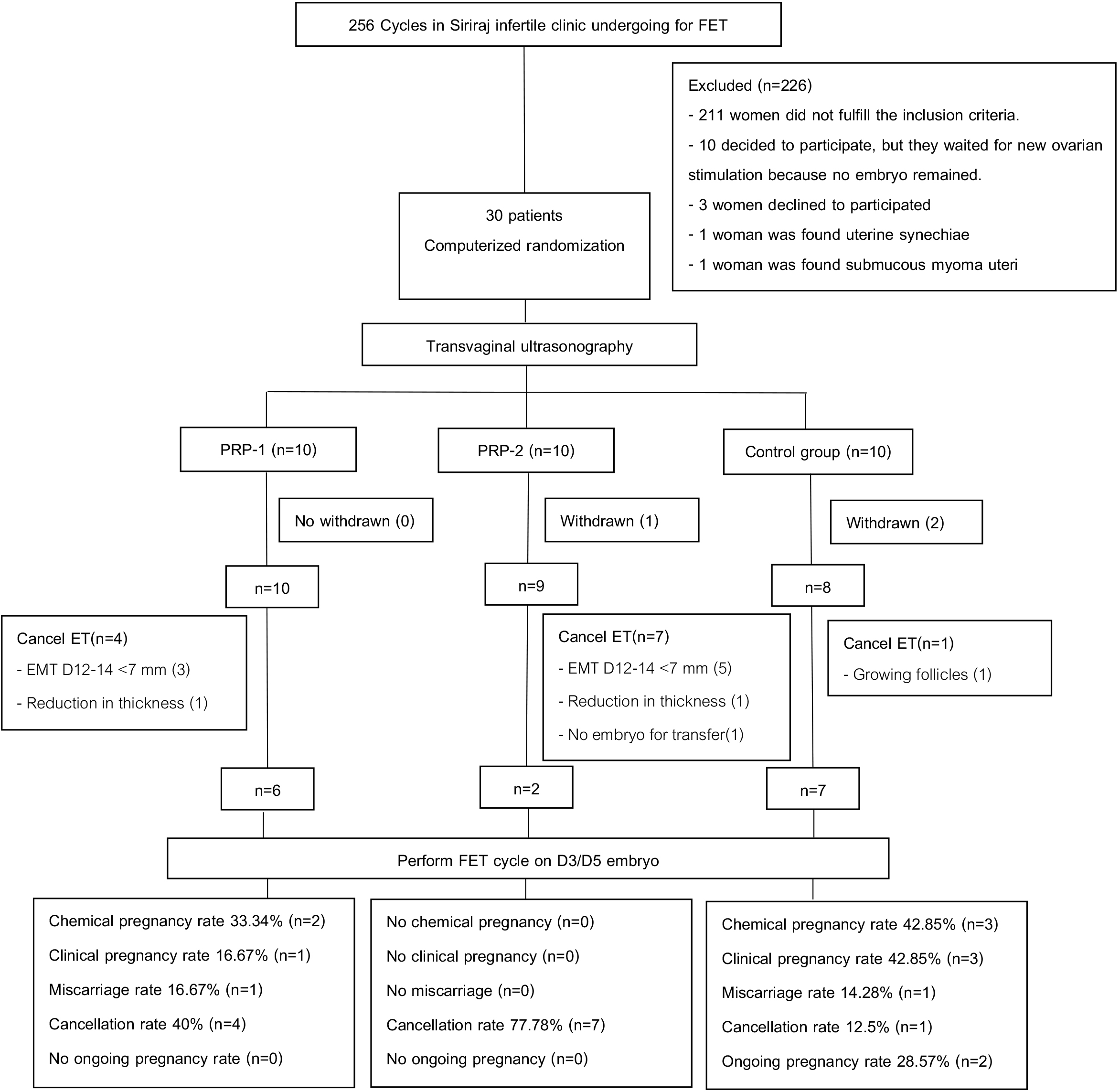
In this randomized controlled trial (RCT), a total of 30 infertile women from Siriraj Infertility Clinic undergoing FET were enrolled and randomly assigned to three groups. Group 1 (PRP-1 group) received a single intrauterine PRP instillation on day 8 (D8) of the cycle prior to ET; Group 2 (PRP-2 group) received two intrauterine PRP instillations on D8 and D10 before ET; and Group 3 (Control) received standard estrogen therapy alone. Endometrial thickness, chemical pregnancy rate, clinical pregnancy rate, abortion rate, and cycle cancellation rate were recorded.
The mean differences in endometrial thickness on D8 and D12 were compared. The PRP-1 group showed the greatest increase in endometrial thickness compared to the other groups; however, the difference was not statistically significance (1.52 ± 1.10 in PRP-1, 0.72 ± 0.72 in PRP-2, 1.43 ± 0.88 in the Control group; p = 0.153). Chemical pregnancy rates were comparable across groups, with 33.34% in PRP-1 group and 42.85% in the control group. Similarly, clinical pregnancy rates were 16.67% in PRP-1 group and 42.85% in the control group; (p = 0.790 and p = 0.585, respectively). However, the cancellation rate was significantly higher in the PRP-2 group (77.78%, p = 0.015).
Single intrauterine PRP instillation on D8 prior to ET in a frozen-thawed cycle may improve endometrial thickness, although without statistical significance. In contrast, repeated PRP administration was associated with a higher cycle cancellation rate.
The study has been registered on https://clinicaltrials.gov/ (registration number: NCT06234540; registration link: https://clinicaltrials.gov/study/NCT06234540?cond=NCT06234540&rank=1).