- Academic Editor
Colonization of the lower genital tract by Ureaplasma urealyticum (UU) and Mycoplasma hominis (MH) has been associated with adverse pregnancy outcomes, including preterm birth, but evidence remains inconsistent. This study aimed to assess the association between UU/MH colonization and preterm birth in singleton and twin pregnancies among Korean women.
This single-center observational study reviewed electronic medical records of pregnant women who attended the obstetrics clinic at Gyeongsang National University Changwon Hospital from January 2017 to December 2020. A total of 1614 pregnant women with available UU and MH test results were included. Both singleton (n = 1298) and twin pregnancies (n = 316) were analyzed. Vaginal swab samples were tested using culture methods, and obstetric outcome data were collected. Statistical analyses included Student's t-test and chi-square (χ2) test, with p-values calculated for statistical significance.
The prevalence of UU and/or MH colonization in the lower genital tract was 36.6% (n = 590/1614). In singleton pregnancies, UU/MH positive women had a higher incidence of preterm birth compared to the negative group (24.6% vs. 16.8%, p < 0.001), with an odds ratio (OR) of 1.62. Late preterm birth (34+0 to 36+6 weeks) constituted the majority of preterm births in singleton pregnancies. There was no significant difference in the proportion of small for gestational age (SGA) neonates between the two groups. In twin pregnancies, UU/MH colonization did not significantly affect preterm birth rates (p = 0.443). Bacterial vaginosis was significantly associated with UU/MH colonization (p < 0.001), but was not associated with increased risk of preterm birth (p = 0.189).
UU and MH colonization is prevalent in pregnant women in South Korea and is associated with an increased risk of preterm birth in singleton pregnancies. However, this association is not observed in twin pregnancies. Further multi-center studies utilizing both culture-based and polymerase chain reaction (PCR) methods are necessary to evaluate neonatal outcomes and to refine clinical management strategies.
The genera Ureaplasma and Mycoplasma belong to the family Mycoplasmataceae [1] and are recognized as the smallest free-living organisms [2, 3]. These intracellular bacteria are sexually transmitted pathogens that can cause urogenital infections in humans [3]. The significance of these organisms lies in their potential to adversely affect fertility and pregnancy [4, 5, 6, 7, 8]. They can trigger semen inflammation, reduce sperm quality [9, 10, 11], and induce female infertility [12, 13, 14], spontaneous abortion [15, 16, 17], and preterm labor, often resulting in preterm birth [13, 14, 18, 19, 20, 21]. Furthermore, newborns and immunocompromised hosts are particularly susceptible to infections caused by these intracellular organisms [22, 23, 24]. Notably, Ureaplasma urealyticum (UU) is a common cause of central nervous system and lower respiratory infections in premature infants [22, 23, 24, 25, 26, 27, 28].
The colonization of UU and Mycoplasma hominis (MH) in the human genital tract can be detected using vaginal swab samples via polymerase chain reaction (PCR) tests [29] or culture [30] methods. Although a positive result confirms the presence of the bacteria, detection of these microorganisms does not necessarily warrant treatment in non-pregnant women due to limited evidence supporting their role in causing symptoms [31, 32, 33]. However, in pregnant women, genital infection or colonization by UU and MH is generally considered a risk factor for adverse pregnancy outcomes [15, 16, 17, 18, 19, 20, 21]. Intra-amniotic infection or colonization with UU is associated with chorioamnionitis [34, 35, 36, 37, 38], a condition that can lead to preterm birth. Moreover, vertical transmission of UU occurs in 18%–55% of neonates born to colonized mothers [39, 40].
A key clinical question is whether colonization of the lower genital tract by UU and/or MH increases the risk of adverse pregnancy outcomes, particularly preterm birth. Although this relationship has been studied, findings remain inconclusive, and limited data are available from Korean populations. Therefore, we conducted a study to evaluate the association between UU and MH colonization and the risk of preterm birth in both singleton and twin pregnancies in South Korea. Our objective was to assess whether genital colonization with these organisms is associated with a higher incidence of preterm birth.
This single-center observational study reviewed electronic medical records of pregnant women who attended the obstetrics clinic at Gyeongsang National University Changwon Hospital between January 2017 and December 2020. We included patients with documented UU and MH test results. Both singleton and twin pregnancies were included, while patients without delivery records were excluded.
The detection of UU and MH was conducted using culture methods Vaginal swab samples were transferred to transport media, and identification of the microorganisms was performed using the commercially available Mycoplasma IST-2 kit (bioMerieux, Marcy-l’Etoile, France). Incubation and confirmation of color changes in the media were conducted strictly according to the manufacturer’s instructions. Results for UU and MH were analyzed retrospectively. Additionally, we performed vaginal swab cultures to identify concurrent bacterial vaginosis. Diagnosis of bacterial vaginosis was made according to the Nugent scoring system, which is based on Gram-stained vaginal smears on a scale from 0 to 10, with scores of 7–10 indicating bacterial vaginosis.
Relevant clinical data were collected retrospectively by reviewing electronic medical records. Data included patient age, parity, gestational age at delivery, birth weight, and obstetrical and medical complications. The interval between testing and delivery was calculated, and the appropriateness of neonatal birth weight was determined based on percentile distributions by gestational age from Korean reference data published by Lee JK et al. [40].
Statistical analyses were performed using R software version 4.2.1 (R Project
for Statistical Computing, Vienna, Austria). Continuous variables were expressed
as mean
The association between UU/MH colonization and preterm birth was evaluated by calculating odds ratios (ORs) and 95% confidence intervals (CIs). A p-value of less than 0.05 was considered statistically significant. No multivariate adjustments were applied due to the retrospective and descriptive nature of the study. Missing data were excluded from the analysis.
We collected 1822 test results for UU and MH in pregnant women between January 2017 and December 2020. Of these, 202 were excluded due to the absence of delivery records, 186 cases were lost to follow-up after testing, and 16 were transferred to other facilities before delivery. Additionally, deliveries occurring before 23 weeks’ gestation were excluded (n = 6). Consequently, a total of 1614 cases were included in the analyses.
Table 1 summarizes the general characteristics of the cohort. The prevalence of
UU and/or MH in the lower genital tract of pregnant women was 36.6% out of 1614
pregnant women, including both singleton and twin pregnancies. The presence of UU
and/or MH was slightly higher in singleton pregnancies compared to twin
pregnancies (37.6% vs. 32.3%), although this difference was not
statistically significant (p = 0.078) (Fig. 1). Vaginal swab cultures
revealed no cases of isolated MH growth. The cohort showed significant
differences in parity (p
 Fig. 1.
Fig. 1.
Prevalence of UU and MH colonization in pregnant women in South Korea. UU, Ureaplasma urealyticum; MH, Mycoplasma hominis.
| Characteristic | Singleton (n = 1298) | Twin (n = 316) | p-value | |
| Age (year) | 33.4 |
33.3 |
0.706 | |
| Parity (number)† | 0 (0–4) | 0 (0–3) | ||
| Weeks’ gestation at delivery | 38.1 (37.1, 39.0)† | 36.3 (35.5, 37.0) | ||
| Weeks’ gestation at test | 22.6 (14.1, 32.0) | 23.2 (15.3, 29.2) | 0.561 | |
| UU and MH | ||||
| Both negative | 810 (62.4) | 214 (67.7) | ||
| Positive UU | 488 (37.6) | 102 (32.3) | 0.078 | |
| Positive MH | 18 | 3 | 0.735 | |
| HTN | ||||
| No | 1208 (93.1) | 269 (85.1) | ||
| Yes | 90 (6.9) | 47 (14.9) | ||
| DM | 0.025 | |||
| No | 1081 (83.3) | 280 (88.6) | ||
| Yes | 217 (16.7) | 36 (11.4) | ||
| Hyperthyroidism | 0.518 | |||
| No | 1282 (98.6) | 310 (98.1) | ||
| Yes | 16 (1.4) | 6 (1.9) | ||
| Hypothyroidism | 0.141 | |||
| No | 1197 (92.2) | 299 (94.6) | ||
| Yes | 101 (7.8) | 17 (5.4) | ||
Data were presented as mean
† Data were presented as median and interquartile ranges.
UU, Ureaplasma urealyticum; MH, Mycoplasma hominis; HTN, hypertension; DM, diabetes mellitus.
The relationship between UU/MH colonization in the lower genital tract of
pregnant women with singleton pregnancies and pregnancy outcomes is shown in Fig. 2. As shown in Table 2 (Ref. [40]), preterm birth occurred more frequently in the
UU/MH positive groups with statistical significance (24.6% vs. 16.8%,
p
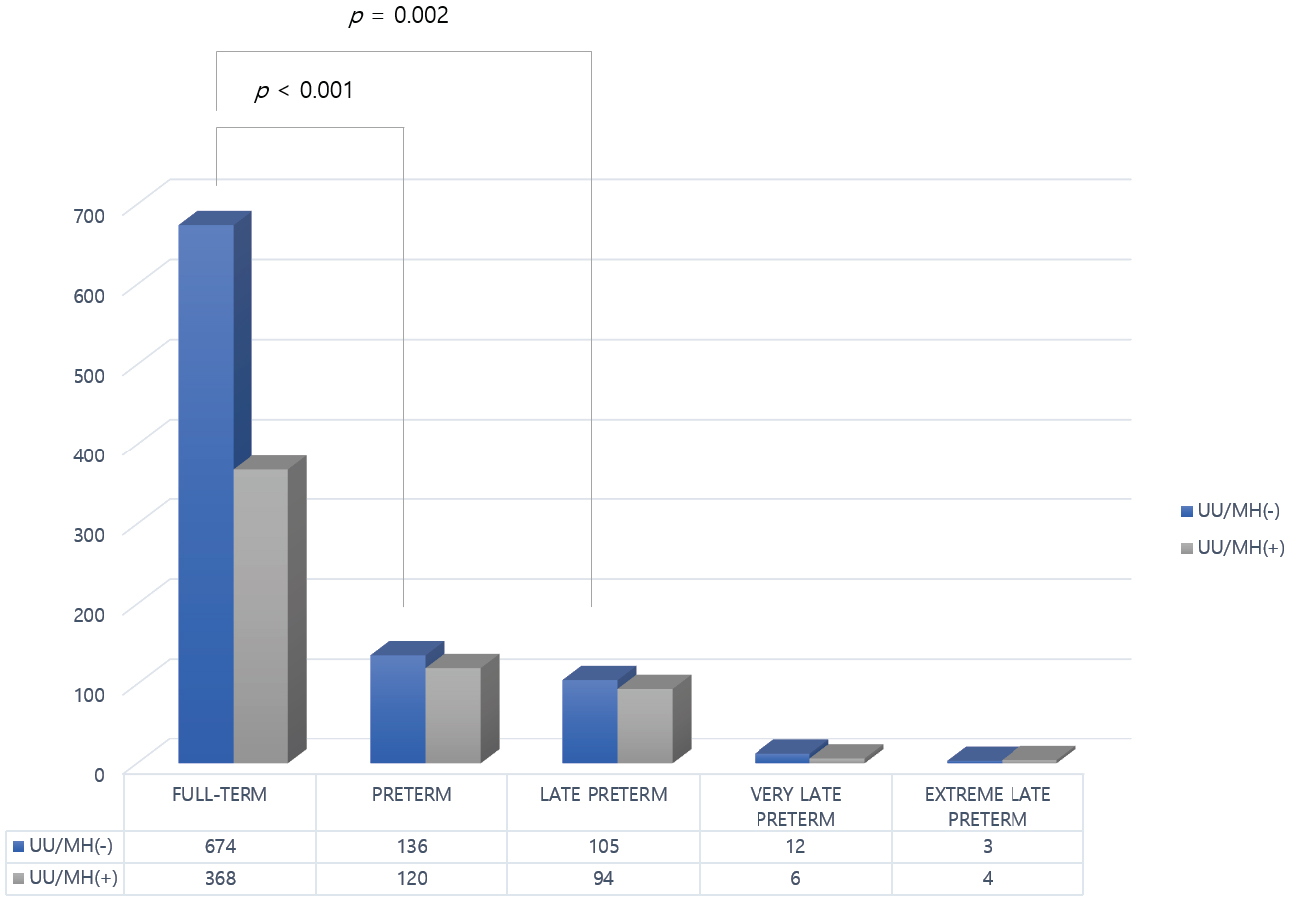 Fig. 2.
Fig. 2.
Association with preterm birth in singleton pregnancies.
| Outcome | Negative for UU/MH (n = 810/1298) | Positive for UU/MH (n = 488/1298) | p-value | |
| Gestational week at delivery | ||||
| 674 (83.2) | 368 (75.4) | |||
| 136 (16.8) | 120 (24.6) | |||
| OR = 1.62 [1.23–2.13] | ||||
| Late preterm birth (between 34+0- and 36+6-weeks’ gestation) | 105 (77.2†) | 94 (78.3†) | 0.002‡ | |
| Very preterm birth (between 28+0- and 31+6-weeks’ gestation) | 12 (8.8†) | 6 (4.4†) | 0.707‡ | |
| Extreme preterm birth (less than 28+0-weeks’ gestation) | 3 (2.2†) | 4 (3.3†) | 0.497‡ | |
| Neonatal body weight at birth | ||||
| SGA* | 27 (3.3) | 21 (4.3) | 0.370 | |
| LGA* | 55 (6.8) | 41 (8.4) | 0.283 | |
| LBW | 97 (12.0) | 70 (14.3) | 0.310 | |
| VLBW | 11 (1.4) | 9 (1.8) | 0.495 | |
| ELBW | 2 (0.2) | 3 (0.6) | 0.372 | |
Data were presented as number and percentile. p-value below 0.05 was considered statistically significant.
† percentile among preterm birth group.
‡ calculated by comparing to full-term birth group.
* The appropriateness of birth weight to gestational age were referred to the Korean neonatal birth weight chart by Lee JK et al. [40].
OR, odds ratio; SGA, small for gestational age; LGA, large for gestational age; LBW, low birth weight; VLBW, very low birth weight; ELBW, extremely low birth weight.
Fig. 2 illustrates the association between UU and MH colonization and preterm
birth in singleton pregnancies. In the UU/MH-positive group, there was a
significantly increased risk of overall preterm birth (24.6% vs. 16.8%, p
In twin pregnancies, the colonization of UU/MH did not result in significant differences in pregnancy outcomes, as shown in Table 3. There was, however, a trend toward a higher incidence of very preterm birth in the UU/MH-positive group compared to the negative group (2.1% vs. 6.8%); however, this difference was not show statistically significant (p = 0.106). Due to the absence of a population-based Korean growth chart correlating neonatal birth weight with gestational age, an evaluation of UU/MH colonization with birth weight was not available.
| Outcome | Negative for UU/MH (n = 214/316) | Positive for UU/MH (n = 102/316) | p-value | |
| Gestational week at delivery | 0.443 | |||
| 70 (32.7) | 29 (28.4) | |||
| 144 (67.3) | 73 (71.6) | |||
| OR = 1.22 [0.73–2.07] | 0.444 | |||
| Late preterm birth (between 34+0- and 36+6-weeks’ gestation) | 129 (89.6†) | 62 (84.9†) | 0.582‡ | |
| Very preterm birth (between 28+0- and 31+6-weeks’ gestation) | 3 (2.1†) | 5 (6.8†) | 0.122‡ | |
| Extreme preterm birth (less than 28+0-weeks’ gestation) | 3 (2.1†) | 1 (1.7†) | 0.707‡ | |
Data were presented as number and percentile. p-value below 0.05 was considered statistically significant.
† percentile among preterm birth group.
‡ calculated by comparing to a group delivered
Conventional culture were performed alongside the vaginal swab for the detection
of UU and MH colonization in pregnant women; results are shown in Table 4. A
significant association was observed between the presence of UU/MH colonization
and positive bacterial vaginosis (p
| Outcome | Negative for UU/MH (n = 1024/1614) | Positive for UU/MH (n = 590/1614) | p-value | |
| Bacterial vaginosis | ||||
| Negative | 1013 (98.9) | 549 (93.1) | ||
| Positive | 11 (1.1) | 41 (6.9) | ||
| Nugent score |
Nugent score |
|||
| Full-term (n = 1141) | 1100 (70.4) | 41 (78.8) | 0.189 | |
| Preterm (n = 473) | 462 (29.6) | 11 (21.2) | ||
| Conventional culture | ||||
| Negative | 765 (74.7) | 378 (64.1) | ||
| Positive | 259 (25.3) | 212 (35.9) | ||
| Positive culture results (n = 476)† | ||||
| GBS | 84 (17.6) | Enterococcus spp. | 20 (4.2) | |
| E. coli | 111 (23.3) | Gardnerella spp. | 22 (4.6) | |
| Candida spp. | 164 (34.5) | Others* | 75 (15.8) | |
Data were presented as number and percentile. p-value below 0.05 was defined to be statistically significant.
† Some culture results were positive for more than two kinds of microorganisms.
* Others include Citrobacter koseri, Enterobacter aerogenes, Klebsiella spp., Kluyvera ascorbata, Morganella korganii, Pseudomonas stutezeri, Staphylococcus spp., Streptococcus spp.
GBS, Group B Streptococcus; E. coli, Escherichia coli.
The main findings of our study indicate that culture-positive UU/MH status was
associated with a 62.0% increased risk of overall preterm birth in singleton
pregnancies compared to the culture-negative group. However, this association is
not observed in twin pregnancies. The prevalence of UU/MH colonization, as
identified by culture from vaginal swabs in the lower genital tract of 1614
pregnant women in the Korean population, was 36.6%. Although UU/MH colonization
did not affect the appropriateness of neonatal birth weight according to
gestational age at birth, it was associated with a higher prevalence of
culture-positivity for other microorganisms and bacterial vaginosis, as defined
by Nugent score
We observed that UU/MH colonization in singleton pregnancies increased the risk of preterm birth, particularly for overall preterm and late preterm births; however, this was not consistent in twin pregnancies. Our findings showed a specifically increased risk in overall preterm and late preterm births in singleton pregnancies. However, since twin pregnancies, especially monochorionic pregnancies, often result in births before 37 weeks of gestation, the risk posed by UU/MH colonization may not significantly impact twin pregnancies as it does with singleton pregnancies.
Hypertensive disorders, including gestational hypertension or preeclampsia, showed a higher prevalence in twin pregnancies. However, diabetes was more common in singleton pregnancies than in twin pregnancies, potentially contributing to a greater proportion of LGA in singleton pregnancies (7.4%) compared to previous reports of 4.3% in the Korean population. In subset analyses, diabetes status did not significantly alter the prevalence of UU/MH colonization (p = 0.230 for the diabetes-negative group; p = 0.101 for the diabetes-positive group).
Preterm birth, defined as delivery before 37 weeks of gestation [41], remains the leading cause of neonatal mortality, with nearly 1 million childhood deaths annually due to complications related to prematurity [42, 43, 44]. Despite the lack of precise prevalence data in many low-income countries [45, 46], the global burden of preterm birth remains substantial, with over 15 million babies born prematurely each year [47]. Approximately 84% of these are late preterm births [46], consistent with our finding that late preterm births constituted 82.5% of all preterm births. The challenge in preventing preterm birth lies in its complex pathophysiology [48].
Numerous factors contribute to preterm birth [48], even when medically indicated cases are excluded [48, 49]. Among the proposed causes, inflammation of the amniotic cavity [49, 50, 51, 52], whether caused by microbes or sterile inflammation, has strong supporting evidence [49, 50, 51, 52, 53]. This has led to numerous studies investigating the role of microbiomes in the female genital tract. A growing body of evidence suggests that UU and other intracellular microorganisms within Mycoplasmataceae family may play a role in preterm birth [13, 14, 18, 19, 20, 21], although some studies show inconsistent findings [18, 54, 55]. A recent meta-analysis on UU, MH, and Ureaplasma parvum (collectively referred to as genital mycoplasmas) concluded that current literature does not clearly define their role in adverse pregnancy and birth outcomes, either independently or in conjunction with bacterial vaginosis [14].
Several mechanisms have been proposed to explain their potential contribution to adverse pregnancy outcomes. These include persistent subclinical endocervical infections [16, 17, 18], which may ascend into the uterine cavity [49, 50, 51, 52], triggering placental inflammation [15], even in the absence of clinically apparent infection. Such inflammation—whether infectious or sterile—can result in chorioamnionitis and subsequent preterm labor. Additionally, co-infection with other vaginal microbiota [20], as seen in our study’s association with bacterial vaginosis, may amplify the inflammatory cascade and further compromise pregnancy outcomes.
Growing evidence, including findings from our study, suggests that genital mycoplasmas such as UU and MH are associated with adverse pregnancy outcomes. However, the effectiveness of treatment against these microorganisms in preventing preterm birth remains controversial. There is a need for clear guidelines on the timing and type of testing for these infections during pregnancy. Additionally, the role of UU and MH should be interpreted within the broader context of vaginal microbiota. Future research should aim to elucidate the interactions between these pathogens and the vaginal microbiome, as well as the impact of targeted treatments on pregnancy outcomes.
The strengths of our study include a relatively large cohort size within a single-center observational design. Our findings are consistent with previously reported higher prevalence rates of hypertensive disorders in multifetal pregnancies compared to singleton pregnancies, as well as a statistically significantly higher proportion of late preterm births among all preterm births.
Limitations of our study include its retrospective design, reliance on culture for UU/MH detection (despite PCR offering several advantages over culture [56, 57, 58, 59]), and lack of distinction between medically indicated and spontaneous preterm births. Additionally, neonatal outcomes, including morbidity and mortality rates, were not included. Since the sequelae of extreme and late preterm births differ significantly, the impact of UU/MH colonization on preterm birth outcomes was not fully addressed in this study.
Our findings demonstrate that UU and MH colonization are prevalent in pregnant women in South Korea and is significantly associated with an increased risk of preterm birth in singleton pregnancies. Although this association was not observed in twin pregnancies, the clinical importance of detecting these microorganisms remains noteworthy, particularly in the context of routine antenatal care. Moreover, UU/MH colonization was also associated with higher rates of bacterial vaginosis, although it was not directly linked to preterm birth. These results underscore the need for further multi-center studies utilizing both culture and PCR techniques to better understand the impact of genital mycoplasmas on pregnancy outcomes and to inform effective screening and treatment strategies.
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
JY and HCJ designed the research study. JY, HCJ and JEP performed the research. JCB, JEP, and IK provided help and advice on the data acquisition and management. JCB and IK analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The Institutional Review Board of Gyeongsang National University Hospital approved this study (IRB No. GNUH-2022-04-035). This study was conducted in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived by the Institutional Review Board of Gyeongsang National University Hospital due to the retrospective nature and minimal risk involved.
The authors would like to thank the staff and patients of Gyeongsang National University Changwon Hospital for their cooperation and support during this study.
This research received no external funding.
The authors declare no conflict of interest.
The authors disclose that artificial intelligence (AI) tools, specifically OpenAI’s ChatGPT, were used to assist in the drafting and editing of this manuscript. The AI was employed for language editing, grammar correction, and the refinement of content to improve clarity and readability. All intellectual contributions, data interpretation, and conclusions presented in this manuscript were made by the authors. The use of AI was strictly for enhancing the manuscript’s presentation and did not influence the scientific content or the integrity of the research. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
