- Academic Editor
This review aims to provide an overview of the factors contributing to central obesity, particularly in postmenopausal women, who are affected at a global rate of 26%. It emphasizes the heterogeneity of adipocytes, the impact of prenatal genetic factors, and the role of estrogenic neuroendocrine regulation. Additionally, the review explores the paradoxical functions of visceral fat and identifies the primary depots that may contribute to its overall function.
Estrogen deficiency is a key factor in central adiposity among postmenopausal women, leading to a reduction in subcutaneous adipose tissue (SAT) and an increase in visceral adipose tissue (VAT) compared to premenopausal women. This deficiency deactivates pro-opiomelanocortin (POMC) neurons and steroidogenic factor-1 (SF1) neurons via estrogen receptor alpha (ERα), desensitizes vagal cholecystokinin-A (CCK-A) receptors, and hyperactivates the hypothalamic-pituitary-ovary (HPO) axis, resulting in increased food intake and decreased energy expenditure. The differences between VAT and SAT, such as expandability, anatomic location, free fatty acid (FFA) mobility, facilitate energy transfer from SAT to VAT, thereby contributing to central obesity. VAT also compensates for estrogen deficiency by releasing estradiol, inflammatory and anti-inflammatory adipocytokines, and increasing 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) activity, which potentiates glucocorticoid functions and ultimately leading to the development of metabolic syndromes. VAT is heterogeneous, including distinct depots such as mesenteric, gonadal, and perirenal fat. Mesenteric fat may play a significant role in body weight regulation and insulin resistance, while other fat depots interact more closely with surrounding organs to regulate various physiological functions. Understanding VAT heterogeneity is crucial for identifying adiposopathy markers associated with various metabolic syndromes. This knowledge can inform holistic, personalized therapeutic and bodybuilding approaches, helping patients to mitigate the risks associated with current hormone therapies.
The ratio of SAT to VAT is shaped by a combination of prenatal genetics, neuroendocrine regulation, and postnatal epigenetic factors influenced by environmental energy availability and estrogen deficiency. VAT accumulation exhibits paradoxical roles, aiding adaption to energy surplus stress while simultaneously contributing to postmenopausal syndromes. Within VAT, heterogeneity exists, with mesenteric fat depots playing a key role in its overall function. Long-term protective strategies during the perimenopausal and menopausal periods may include energy restriction and the maintenance of normal estrogen levels. Personalized diets and estrogen supplementation hold promise in alleviating associated syndromes. Further exploration of the relationship between mesenteric fat, VAT accumulation, and menopausal syndromes could help clarify existing contradictory evidence and position mesenteric fat as a potential target for effective interventions aimed at alleviating postmenopausal symptoms with fewer side effects.
Visceral fat accumulation in postmenopausal women is a consequence of energy stress due to estrogen deficiency, followed by the energy transfer from SAT to VAT. The heterogeneity of VAT suggests that its components may have different roles in body weight regulation. Mesenteric fat may play a major role among the depots.
Body fat can be classified into essential fat and storage fat. Storage fat refers to the fat accumulated in adipose tissue and is primarily composed of triacylglycerols [1]. Essential fat, on the other hand, is found in lipid-rich tissues, including the liver, heart, lungs, central nervous system in both men and women. In women, additional essential fat in found in the mammary glands and pelvic region. Reference values indicate that men have approximately 12% storage fat and 3% essential fat, whereas women generally have around 15% storage fat and 12% essential fat [2]. Storage fat constitutes around 86% of total body fat and is the most variable component among individuals [1]. Body fat can also be categorized based on its location, such as trunk fat and appendicular fat, the latter being found in the arms and legs [3].
Functionally, mammals, including humans, possess different types of adipocytes—white, beige, and brown adipocytes—distributed throughout various regions of the body. WAT is the predominant form of fat in adults [4]. It can be further divided into three main anatomical categories: SAT, VAT, and ectopic fat [5, 6]. Ectopic fat, which accumulates in organs such as muscles, pancreas, liver, and heart [7], is typically present in small quantities and is beyond the scope of this review. This manuscript will primarily focus on SAT and VAT. Additionally, WAT can be further subdivided into three groups based on its depth relative to the skin: superficial SAT, deep SAT, and VAT [8]. Additionally, specific terminology is often used to describe fat in particular anatomical regions, such as abdominal SAT [9].
VAT surrounds intra-abdominal organs, including abdominal and intra-abdominal fat [10, 11]. This encompasses fat surrounding internal organs, such as epicardial fat, perivascular fat, and fat surrounding gastrointestinal structures [6, 7]. VAT is a component of android fat, which also includes abdominal SAT.
Excessive accumulation of VAT is now recognized as adiposopathy due to its strong association with increased risks of insulin resistance, type 2 diabetes, and cardiovascular diseases [5, 12, 13, 14, 15, 16, 17, 18, 19]. For example, individuals with Crohn’s disease or intestinal tuberculosis exhibit significantly higher ratios of VAT to SAT or total fat [20]. VAT plays a key role in the secretion of pro-inflammatory cytokines, including interleukin-6 (IL-6), as well as hormones like leptin and adiponectin, particularly in the context of systemic inflammation [21, 22]. Reducing VAT through interventions such as fasting has been shown to alleviate low-grade chronic inflammation linked to excessive visceral adiposity [23]. Additionally, VAT is closely associated with conditions such as sleep apnea, certain tumors, and other disorders [11].
SAT lies just beneath the skin and is distributed across various body regions, including the thighs, hips, and gluteal region. It is further classified into deep and superficial SAT. SAT distribution varies by anatomical site, age, and sex [24, 25, 26]. Unlike other types of fat, SAT plays protective roles by shielding delicate organs (such as the eye) and cushioning body areas exposed to high mechanical stress (such as the heel and toe pads) [13]. Additionally, it contributes to the sexually dimorphic appearance of human faces [27]. Prior to menopause, women tend to accumulate more SAT, which may offer protection against the adverse effects of obesity and metabolic syndrome [12]. However, with aging, SAT can infiltrate the dermal layer, impairing skin elasticity and promoting wrinkle formation [28]. Additionally, SAT can adapt to changes in nutrient status, age, and sex [26, 29]. As a result, SAT is often considered a beneficial tissue, with its subcutaneous accumulation generally being less harmful, particularly in female [6].
However, SAT is not always beneficial. Excessive SAT, for instance, can contribute to biomechanical disorders associated with increased fat mass, such as ulcers, immobility, and sleep apnea. Moreover, when caloric intake exceeds the storage capacity of SAT, excess energy may be redirected toward VAT accumulation [11]. The benefits of SAT can also vary by anatomical location. In humans, upper-body SAT is responsible for most systemic free fatty acids (FFAs) and is more insulin-resistant than lower-body SAT [6, 15].
Emerging research suggests that, rather than acting as a passive storage site for excess energy, WAT is considered a dynamic and diverse organ, dispersed throughout the body and capable of sensing and responding to various physiological signals from its microenvironment. It, in turn, functionally and morphologically adapts to changes in physiological status. The plasticity of WAT is further evident in the sexual dimorphism of fat mass between females and males [12, 30, 31, 32], as well as in the differences in fat distribution observed between perimenopausal and postmenopausal women [33]. Therefore, WAT is now considered as a heterogeneous organ with a remarkable degree of plasticity [34, 35]. Understanding the factors that drive WAT heterogeneity, including its prenatal and postnatal genetic influences, hormonal status, and regional variations, is essential for gaining deeper insights into the characteristics of this newly recognized organ, as well as the pathological consequences of postmenopausal obesity.
Men aged 20–24 years typically have a body fat percentage of around 15%, while women of the same age have about 27% body fat, with a higher proportion of SAT [1]. The primary factor driving the differences in fat mass and distribution between sexes is the presence and dosage of the X chromosome, as observed in individuals with Klinefelter syndrome (KS) (47, XXY; mosaicism 47, XXY/46, XY; 48, XXXY; 49, XXXXY) [36, 37]. The supernumerary X chromosome significantly influences VAT, as indicated by measures like epicardial fat thickness and truncal body fat [38, 39, 40]. However, KS patients treated with testosterone tend to exhibit lower abdominal fat [41].
Similarly, mouse models with various combinations of gonads and sex chromosomes (XX female, XX male, XY female, and XY male) demonstrate that the presence of two X chromosomes leads to increased adiposity compared to XY mice, regardless of the effects of ovaries or testes [42]. In both intact and gonadectomized animals, XX mice consistently exhibit higher levels of high-density lipoprotein cholesterol (HDL-C) than XY mice, regardless of sex [43]. Additionally, a quantitative trait locus (QTL) on X chromosome, near D10Mit174 (DXMit174, a microsatellite marker), has been associated with adiposity, body weight, and the size of individual fat depots [44]. The connection between the X chromosome and central obesity is further supported by studies indicating that low-birth-weight males tend to have lower overall and central adiposity, while low-birth-weight females more frequently show increased central fat accumulation [45]. The X-inactive specific transcript (Xist) is expressed specifically in female adipose tissue, which may help explain the observed sex dimorphism in fat accumulation [46]. Moreover, sex differences in adipose tissue gene expression and development may be modulated by differential miRNA expression [47].
In addition to sex chromosomes, autosomes also play a significant role in the regulation of fat mass and distribution. Quantitative genetic analyses have revealed that the traits of VAT and SAT are heritable [48]. For example, two single nucleotide polymorphisms (SNPs) in the fat mass and obesity-associated protein (FTO) gene, located on chromosome 16, have been linked to SAT accumulation in humans [49]. Several QTLs on mouse chromosome 9 contribute to strain-specific differences in overall fatness, with at least four regions at or near adipocyte fat-associated gene 5 (Adip5) exerting a stronger influence on specific fat depots, such as the gonadal fat depot weight [50, 51].
Interestingly, gene expression in adipocytes may exhibit sex-specific
differences. For example, DEAD-box Y RNA helicase (DBY) and eukaryotic initiation
factor 2 gamma (eIF2
Estrogens play a central role in regulating and exerting sex-biased effects on specific genes, such as reprimo, a putative p53-dependent tumor suppressor gene. This regulation occurs in the ventromedial hypothalamus (VMH), a key regulatory node for energy expenditure that demonstrates sexual dimorphism. This regulation contributes to higher core temperatures in male mice compared to females [53]. Furthermore, estrogen-mediated transcriptional and epigenetic regulation in adipocytes plays a crucial role in the sexual dimorphisms observed in body fat distribution and the associated risk of obesity-related diseases. This topic has been extensively reviewed by Bjune et al. [54].
Furthermore, estrogens regulate gene expression in a site-specific manner,
influencing the expression of genes such as monocyte-chemoattractant protein-1
(MCP-1), androgen receptor (AR), estrogen receptor
The aforementioned genetic factors collectively establish the prenatal and premenopausal set points for balanced body weight through various pathways, contributing to sex-based differences in fat distribution and related traits [56, 57, 58]. Hormonal fluctuations, environmental chemicals, drugs, and diets can modify these homeostatic metabolic set points through epigenetic mechanisms, thereby influencing individual fat redistribution.
Postmenopausal women typically have a higher percentage of body fat and lower
lean tissue mass compared to premenopausal and perimenopausal women, with 40% of
postmenopausal women experiencing visceral obesity [33, 59, 60, 61]. Excessive
VAT accumulation is particularly prevalent in perimenopausal women [33, 62, 63].
Both visceral and gynoid (gluteal-femoral regions) fat increase during the
menopause transition, with yearly changes of 6.24% and 2.03%, respectively
(both p
VAT is particularly metabolically active, more sensitive to lipolysis, and more insulin-resistant than SAT, releasing FFAs from lipolysis directly into the liver through the portal circulation [5]. As shown in Table 1 (Ref. [5, 18, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89]), visceral adipocytes have a lower capacity for preadipocyte differentiation and a higher percentage of larger adipocytes, which promotes adipocyte expansion [5, 65]. This contributes to central abdominal fat accumulation when there is an excess of energy or low estrogen levels. In other words, estrogen deficiency is a key causative factor driving significant central and VAT redistribution in postmenopausal women [90]. Additionally, VAT depots are responsive to various stimuli during the menopause transition, including aging, positive energy balance [90], physical inactivity, stress [65], and local hormonal influences such as cortisol production [66]. Comparing the cellular characteristics of VAT and SAT can offer valuable insights into the mechanisms underlying fat redistribution (Table 1).
| SAT | VAT | Consequence | Reference | |
| Cell division and differentiation | ++ | + | More SAT cells | [67] |
| Expandability capacity | + | ++ | Larger VAT cells | [68] |
| Absorption of FFA | + | ++ | SAT: energy storage |
[5] |
| Absorption of TG | + | ++ | [69] | |
| Lipolytic activity | + | ++ | VAT is more metabolically active; gluconeogenesis |
[5] |
| Glucose uptake | + | ++ | [70] | |
| Insulin resistant | + | ++ | [65] | |
| Plasma lipoprotein-lipid | + | ++ | ||
| Plasma triglyceride | + | ++ | [70] | |
| 11 |
+ | ++ | Cortisol response to stress |
[71] |
| 11 |
+ | Unchanged or decreased | Compensation for central obesity; lipid mobilization |
[71, 72, 73] |
| GR | Fructose or/and stress activate glucocorticoid signaling with |
Response to glucocorticoids and stress |
[5, 65, 74, 75] | |
| AR | Adipocyte AR KO increases susceptibility to visceral obesity | Fat mass |
[76] | |
| Testosterone low | Visceral obesity and metabolic syndrome in males | Waist circumference |
[66] | |
| Testosterone | Testosterone |
Metabolic syndrome |
[77, 78] | |
| ER |
++ | + | Lipid storage |
[65] |
| ER |
+ | ++ | Anti-ER |
[79, 80] |
| Growth hormone response | + | ++ | Lipolysis |
[81, 82] |
| + | ++ | Lipolytic sensitivity |
[83] | |
| ++ | + | Lipolytic sensitivity |
||
| Capillary density | + | ++ | VAT hypoxia |
[18, 84, 85] |
| Proinflammatory cytokines | + | ++ (mostly mesenteric fat) | Adipocytokines |
[18, 86] |
| Adiponectin content | ++ | + | [87] | |
| VAT-resident Treg cells | + | ++ | VAT caused inflammation in male mice | [88] |
| Macrophage | + (least infiltration) | ++ (mostly omental fat) | [89] | |
+: normal level; ++: higher level. VAT, visceral adipose tissue; SAT,
subcutaneous adipose tissue; FFA, free fatty acid; TG, triglyceride;
11
In summary, during menopause, characterized by an altered ER
Beyond fat redistribution, it is important to recognize that the characteristics of VAT, as outlined in Table 1, may reflect its roles extending beyond merely being a marker of adiposopathy or an indicator of obesity-related metabolic complications. Adiposopathy, defined as the pathological dysfunction of adipose tissue, is often a primary cause of metabolic syndrome. In fact, these characteristics are often oversimplified to the idea that “obesity protects obesity” [57, 58, 94]. This concept is particularly evident in the expansion of VAT.
VAT has a higher density of glucocorticoid and ARs compared to SAT. Moreover,
VAT adipocytes exhibit increased activity of 11
Another key factor is that VAT is considered a primary driver of adiposopathy, largely due to the release of adipokines that regulate both adaptive and acquired immune responses, contributing to the development of metabolic and immune diseases [11, 19]. For instance, increased leptin secretion from adipose tissue suppresses appetite and promotes energy expenditure, thereby contributing to the maintenance of energy balance and the mitigation of lipodystrophy. Paradoxically, leptin also stimulates the renin-angiotensin system (RAS), which plays a role in regulating fluid balance, but can lead to hypertension [97], systemic sclerosis, and other disorders [98].
In addition, adipose tissue secretes a combination of pro-inflammatory cytokines
(such as leptin, interleukin-1 beta (IL-1
Another notable paradox is that, during menopause, adipose tissue becomes the primary source of estrogen, with VAT often referred to as a “third ovary”—a concept central to the “obesity paradox” [94]. Although the total amount of estrogen produced after menopause is significantly lower than during a woman’s reproductive years [12], this shift has crucial implications. Estrogen derived from adipose tissue can help mitigate certain cardiovascular risks, but it also promotes further adipose tissue expansion, thereby contributing to obesity. This, in turn, increases the risk of developing estrogen-sensitive breast cancer, highlighting the complex role of adipose-derived estrogen in both protective and harmful processes [101, 102].
Consistent evidence suggests that VAT may not be the primary cause of metabolic complications [5, 15, 103]. Several loci on human chromosomes have been identified that paradoxically exert beneficial cardiometabolic effects, either by promoting favorable fat distribution or by having no adverse cardiometabolic impacts at all [104]. Additionally, individuals with metabolic syndrome often exhibit a borderline negative correlation with abdominal SAT mass [105]. The paradox may stem from the underlying fat mass—whether it is present in appropriate amount or in excess.
Emerging clinic evidence has shown that the percentage of VAT is inversely associated with total bone mineral density and osteoporosis [106, 107], as well as with lower levels of HDL-C [108]. In contrast, in postmenopausal women with normal lean mass, VAT does not appear to correlate with bone mineral density or markers of bone formation and resorption. VAT, rather than age, is strongly linked to an increased risk of breast cancer [109], elevated fasting glucose, insulin levels, and cardiovascular risk factors such as triglycerides, apolipoprotein B [108], and blood pressure in postmenopausal women [110, 111, 112]. Notably, peri-coronary epicardial adipose tissue is strongly associated with vascular risk factors and coronary calcification [113]. In contrast, diet-induced weight loss has been shown to significantly improve fasting insulin levels and glucose metabolism [114]. Supplementation with Equol, an ER agonist, can notably alleviate postmenopausal syndromes and reduce VAT mass in postmenopausal women following three months of treatment [115].
Paradoxically, higher adiposity has also been associated with fewer physiological hot flashes among older women experiencing this common postmenopausal symptom, as indicated by age-adjusted models [116]. Furthermore, subcutaneous abdominal fat, but not VAT, appears to be associated with an increased likelihood of hot flashes [117]. These findings suggest that VAT may be a more reliable indicator of metabolic syndrome in menopausal or postmenopausal women, compared to other symptoms such as hot flashes.
VAT, also known as abdominal fat, refers to the fat that surrounds internal organs [7]. Commonly referred to as VAT, it includes a range of fat depots, such as mesenteric, omental, retroperitoneal, intraperitoneal, gonadal, peri-renal, epididymal fat, and others, depending on their anatomical locations [118, 119]. As such, visceral fat is not a homogeneous tissue; it surrounds various intra-abdominal organs and may perform distinct functions depending on the specific abdominal microenvironments. Gene expression studies have also identified validated markers for these fat depots, suggesting a relationship between anatomical location, both adipose tissue identity, and function [120].
VAT depots may differentially contribute to various physiological processes. Regression analyses examining body weight changes in relation to the weights of three visceral fat depots—peri-renal fat, gonadal fat, and mesenteric fat—in C57 mice and nerve-specific receptor-activity modifying protein 1 (RAMP1) overexpressing lean mouse models [121] reveal that mesenteric fat exhibits the largest coefficients compared to the other two fat pads in both strains (unpublished data). Moreover, mesenteric adipocyte size has been associated with a 79% higher likelihood of developing metabolic syndrome compared to other fat depots [18]. Visceral fat, particularly due to its distinct anatomical characteristics and its circulation draining into the portal vein and liver, has been implicated in the development of insulin resistance. Consistent with this, mesenteric fat has been shown to exert more than a two-fold greater influence on improvements in insulin sensitivity in young rats [122].
Other VAT depots also exhibit distinct characteristics, with some being more responsive to dietary changes. For instance, very low energy diets significantly reduce VAT mass, particularly mesorectal fat, while having a lesser impact on total pelvic fat [123]. Among the visceral depots, mesenteric and omental depots contain the fewest macrophages and adipokines [18]. On the other hand, pancreatic fat mass, has been negatively associated with cognition and brain volumes in middle-aged individuals [124]. In contrast, periaortic adipose tissue is characterized by the smallest adipocytes, the highest capillary density, and an elevated secretion of adipokines [18]. There may also be heterogeneity within individual fat depot. For example, in mice, epididymal fat, a component of visceral fat, is divided into distal and proximal regions, each with distinct histochemical and gene expression profiles [125]. This variability may serve an adaptive function, allowing each depot to fulfill more specialized roles in response to subtle nutrient differences within the microenvironment.
The observations outlined above are primarily based on studies in humans and commonly used animal models. To our knowledge, however, no comparative analysis of depot-specific characteristics has been conducted specifically in the context of peri- or post-menopause. Such research could identify the specific fat depots responsible for central adiposity, enhance our understanding of body shape changes during this life stage, and guide the development of targeted therapeutic strategies for related metabolic syndromes.
Fat redistribution due to estrogen deficiency is supported by comparisons of plasma estrogen levels, which range from 100–250 pg/mL in premenopausal women to approximately 10 pg/mL in postmenopausal women [126]. Total estradiol levels are inversely associated with body shape, with the lowest levels observed in postmenopausal women exhibiting an “apple” phenotype and the highest levels found in women with a “pear” phenotype, including both premenopausal women and postmenopausal women using hormone replacement therapy (HRT) [127].
The role of estrogen in regulating fat distribution is further supported by observations in individuals undergoing cross-sex hormonal therapy. Trans women undergoing estrogen therapy exhibit a gynoid pattern of body fat distribution and a lower waist-to-hip ratio, while trans men receiving testosterone display an android pattern of body fat distribution with a reduced hip circumference [128]. These findings suggest that, in the context of estrogen deficiency, fat deposition shifts toward the visceral depot, contributing to the development of an android body shape [12].
The impact of estrogen on energy metabolism is further demonstrated by studies
using ER genetic mouse models, in which activation of ER
Estrogens reduce appetite and suppress food intake by activating ER
Estrogens promote weight loss and reduce visceral fat deposition through
ER
Estrogens suppress food intake and potentiate CCK-induced satiety by increasing
the activity of NTS neurons in the brainstem via ER
Clinical diagnostics for menopausal women typically measure six hormones: estradiol, follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone, progesterone, and prolactin. As ovarian aging progresses, declining levels of ovarian steroids and peptides result in a 15-fold increase in FSH, signaling the cessation of reproduction at menopause, and a 10-fold increase in LH in postmenopausal women [87, 141].
Estrogen deficiency leads to hyperactivation of the HPO axis due to the loss of estrogen’s negative feedback inhibition. This enhanced neuroendocrine pathway, characterized by elevated FSH levels, coincides with increased visceral fat accumulation, higher cellular mitochondrial density, and enhanced thermogenesis [87]. Additionally, progesterone has been shown to be inversely correlated with premenstrual food cravings in both humans and rodents [142].
Furthermore, estrogen deficiency desensitizes the satiety centers in the brain, leading to increased food intake, reduced energy expenditure, and a positive energy balance. This energy imbalance further stimulates the hypothalamic-pituitary-adrenal (HPA) axis, resulting in excess glucocorticoid exposure and promoting visceral obesity [143]. The HPA axis not only orchestrates the body’s stress response but also plays critical role in the endocrine regulation of appetite, particularly in the regulation of energy metabolism during the peri- and postmenopausal periods.
At the cellular level, estrogens regulate a variety of functions by binding to
cytosolic ER
Estrogens also downregulate enzymes involved in fatty acid and triglyceride
synthesis in adipocytes, such as acetyl-CoA carboxylase (ACC1), fatty acid
synthase (FAS), and diacylglycerol acyltransferase (DGAT1 and DGAT2) [145].
Simultaneously, they upregulate enzymes that promote fatty acid
Estrogens further modulate cholesterol metabolism by regulating the expression of 3-Hydroxy-3-Methylglutaryl-Coenzyme A (HMG-CoA) reductase, which is anchored in the endoplasmic reticulum membrane [147]. Moreover, estrogens interact indirectly with peripheral mediators, such as glucagon-like peptide-1 (GLP-1), in the hypothalamus to cooperatively regulate energy homeostasis [148]. In this way, estrogens coordinate brain and body metabolism, orchestrating both endocrine and paracrine effects. Consistently, during menopause, the decline in plasma estrogen levels coincides with a reduction in bioenergetic function across various tissues and organs [149], contributing to postmenopausal phenotypes. The underlying mechanism and its consequences in the context of postmenopause are summarized as Fig. 1.
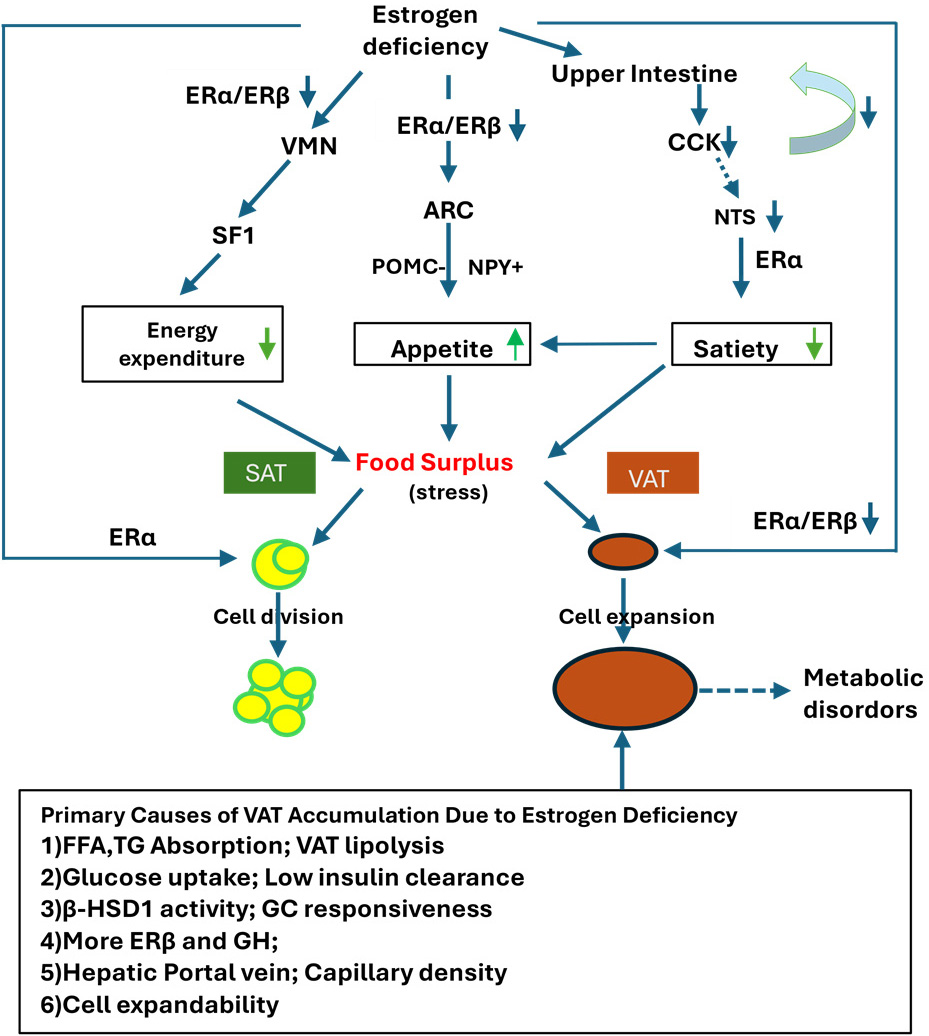 Fig. 1.
Fig. 1.
Impact of estrogen deficiency on central energy regulation,
appetite, and adipose tissue remodeling. Estrogen deficiency reduces the
expression of ER
Emerging evidence highlights the heterogeneity of adipose tissue in terms of its composition, distribution, and function. These characteristics are prenatally determined by sex chromosomes and SNPs in autosomes and are further influenced by epigenetic factors such as estrogen and energy intake. SAT and VAT are two key representatives that define sex dimorphism in adipose tissue distribution and function.
In the context of menopause, estrogen deficiency leads to a positive energy
balance by increasing appetite and food intake through the inhibition of POMC
neurons in the ARC, decreasing energy expenditure by inhibiting SF1 neurons in
the VMN of the hypothalamus, and reducing satiety while promoting food intake
through the NTS in the brainstem. These regulatory processes are primarily
mediated by ER
The distinct characteristics of VAT, including high expandability, elevated FFA
mobility, and increased high 11
Although VAT is often termed the “sick fat” associated with postmenopausal syndromes, it is heterogeneous, comprising various anatomically distinct depots such as mesenteric, peri-renal, omental, retroperitoneal, intraperitoneal, and gonadal fat. Recent studies suggest that these depots differ morphologically and functionally [18, 48, 123]. For instance, mesenteric fat within VAT may play a significant role in regulating body weight, insulin resistance, and inflammation.
Further investigation into the specific characteristics of VAT depots in the context of menopause is crucial for a deeper understanding of postmenopausal syndromes. For instance, examining SNPs in autosomes, glucocorticoid receptors (GRs), HSD enzymes, and adipocyte-specific expression of glucose transporter type 4 (GLUT4), along with associated phenotypes in animal models, could shed light on the mechanisms driving VAT accumulation after menopause. Additionally, personalized treatments such as hormone replacement therapies (including phytoestrogens and estrogen-progesterone combinations), non-hormonal medications, and lifestyle interventions (such as regular exercise, healthy diets, and caffeine to boost plasma brain-derived neurotrophic factor (BDNF) levels) could offer tailored therapeutic approaches for patients [150]. In contrast, behaviors such as high-fat diets, smoking, and alcohol consumption, which may exacerbate postmenopausal symptoms, should be avoided.
To conduct this study, a systematic search of electronic databases, including PubMed and ResearchGate, was performed in June 2024 and updated in November 2024. Studies in English published before these dates were considered. Screening was performed using predefined search terms such as obesity and X chromosome (225 results), postmenopausal and SAT (91), postmenopausal and VAT (109), postmenopausal, VAT, and SAT (41), postmenopausal, VAT, SAT, and metabolism (35), and VAT, SAT, and metabolism (845). After removing duplicates, 912 publications were identified. Of these, 150 were included in the references based on their relevance to the manuscript and its sections, as well as their status as classic works or recent impactful studies. Publications not meeting these criteria were excluded.
The research study was designed by all authors, with ZZ responsible for manuscript writing, and ZH, HY, DL, PD, XW contributing to editorial revision. All authors contributed to the manuscript’s editorial changes, read and approved the final version, and are accountable for all aspects of the work.
Not applicable.
The authors would like to express their gratitude to Stephanie Zhang at UCLA for her editorial assistance with this manuscript. They also extend their thanks to all the peer reviewers for their valuable feedback and suggestions.
This work was supported by grants from Henan Natural Science Foundation (242300421302).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
