- Academic Editor
†These authors contributed equally.
Endometriosis (EMS), which affects >10% of women, is characterized by painful symptoms, such as dysmenorrhea. Meanwhile, the gut microbiota is linked to EMS; however, the relationship between the gut microbiota and EMS-related pain remains unclear.
This study conducted 16S rRNA sequencing on fecal samples from 20 patients with EMS and dysmenorrhea, 13 patients with EMS but not dysmenorrhea, and 12 healthy controls.
Carbohydrate antigen 125 (CA125) levels were significantly higher in patients with EMS and dysmenorrhea. No significant differences in α- and β-diversity values were observed among the three groups (p > 0.05). However, Proteobacteria and Subdoligranulum showed increased abundance trends in patients with EMS and dysmenorrhea. Acinetobacter and Colidextribacter were less abundant in patients with EMS and dysmenorrhea than in those without dysmenorrhea (p < 0.05). Faecalicoccus pleomorphus and its genus also showed consistent depletion. At the genus level, a correlation analysis of the differential microbes revealed that UCG_005 and UCG_002 were central nodes in the correlation network (p < 0.05). Functional prediction indicated significant enrichment in cofactor and vitamin metabolism pathways in the EMS group with dysmenorrhea (p < 0.05). Several differential microbes, such as Faecalicoccus, Colidextribacter, and Acinetobacter, were negatively correlated with pain severity. Receiver operating characteristic curve analysis identified 10 microbial species with moderate diagnostic potential (area under the curve >0.7) for discriminating patients with EMS and dysmenorrhea, notably Ligilactobacillus.
These results highlight distinct microbial alterations and functional pathways associated with EMS-related pain, suggesting potential diagnostic biomarkers for this condition.
Endometriosis (EMS) is a chronic estrogen-dependent disorder affecting
The gut microbiota has been implicated in EMS, and emerging evidence positions EMS within the gut-microbiota-disease axis, which demonstrated microbial dysbiosis in EMS patients [7, 8]. Mechanistic studies suggest that the microbiota influences EMS progression through estrogen metabolism, immune modulation, and cytokine regulation [9]. Gut-derived lipopolysaccharides may activate macrophage-mediated inflammatory cascades, promoting EMS lesion angiogenesis and proliferation [10]. In addition, gut microbiota was found to be related to pain. Notably, the gut microbiota modulates pain perception via central and peripheral pathways, balancing pro-/anti-inflammatory mediators and visceral hypersensitivity [11]. Moreover, a study has suggested that probiotic intake may alleviate stress-induced visceral hypersensitivity [12]. In particular, butyrate produced by Roseburia intestinalis was found to attenuate neuropathic pain [13]. In mouse models of primary dysmenorrhea, the gut microbiota is significantly altered. Specifically, the abundance of Lactobacillus is reduced coupled with an increase in Romboutsia [14]. However, relatively few studies have examined the gut microbiota in EMS-related secondary dysmenorrhea.
This study aimed to identify microbial markers associated with EMS-related dysmenorrhea. A comparative analysis of the gut microbiomes of women with EMS was conducted, distinguishing between those who experienced dysmenorrhea and those who did not and comparing them with a group of healthy controls to discover potential avenues for reducing pain symptoms in EMS through gut microbiome regulation.
Fecal specimens were prospectively collected from June to December 2023 at The Sixth Affiliated Hospital of Sun Yat-Sen University, comprising three patient cohorts: (1) EMS with dysmenorrhea (n = 20; median age, 31.5 years), (2) EMS without dysmenorrhea (n = 13; median age, 28.0 years), and (3) healthy controls (n = 12; median age, 28.5 years).
Ethical approval was obtained from the institutional review board (2024ZSLYEC-020), and written informed consent was obtained from all participants. Pain severity was assessed using the 11-point numerical rating scale, where participants rated their average pain during the past three menstrual cycles on a 0–10 scale.
The specific inclusion and exclusion criteria for the study were as follows:
Inclusion criteria for patients with EMS:
1. Aged 18–45 years, enrolled during the nonmenstrual, nonpregnant, and nonpuerperal periods, with regular menstrual cycles.
2. Diagnostically confirmed by ultrasonography or pelvic magnetic resonance
imaging (MRI, both with sensitivity and specificity
3. No hormone therapy in the past six months and did not take antibiotics or probiotics within the past eight weeks.
4. Non-smokers.
5. Absence of inflammatory or nerve-related pain conditions.
6. No history of intestinal surgery, no intestinal treatments within the past six months, and no significant family history of major intestinal diseases (e.g., colorectal cancer or inflammatory bowel disease).
7. Not in the acute stage of vaginitis, with a negative cervical human papillomavirus test.
8. No special dietary habits (e.g., vegan or vegetarian diet, preference for spicy foods, or highly acidic diet).
The diagnosis was based on characteristic findings on transvaginal ultrasonography (unilocular cyst without vascularity and homogeneous ground-glass echogenicity) and/or MRI (high T1 signal, T2 shading, T2 dark spots, and evidence of adhesions), which are widely recognized as reliable alternatives to laparoscopy for this EMS subtype.
Inclusion criteria for healthy controls:
Healthy controls were recruited with the same eligibility criteria as patients with EMS, except that they had regular menstrual cycles without a history of EMS or dysmenorrhea.
The exclusion criteria were as follows:
1. Have other chronic abdominal or kidney issues, ovarian/uterine tumors, liver diseases, inflammatory or immune conditions, or heart/coronary artery diseases.
2. Received hormone therapy within six months before surgery.
3. Regularly use painkillers.
4. Have a history of headaches or other neurological disorders.
5. Experienced severe pelvic inflammation during surgery.
6. Have severe chronic or acute inflammatory diseases.
7. Have adenomyosis.
DNA was extracted using a DNA extraction kit according to the manufacturer’s
protocol. The highly variable V3–V4 region of the bacterial 16S rRNA gene,
identified by the primer set 338F–806R, was then targeted for amplification by
polymerase chain reaction (PCR). The primer sequences employed were as follows:
338F 5′-ACTCCTACGGGAGGCAGCA-3′ and 806R
5′-GGACTACHVGGTWTCTAAT-3′. The PCR-amplified products were visualized on
agarose gels and then purified. The purified PCR amplicons were then pooled in
equimolar concentrations for paired-end sequencing (2
Fecal specimens were initially collected on dry ice and subsequently stored at –80 °C until sequencing to ensure sample integrity. The raw sequencing data were initially processed based on an individual nucleotide quality assessment. The raw data were rigorously filtered using Trimmomatic (version 0.33: http://www.usadellab.org/cms/?page=trimmomatic) [16]. Subsequently, primer sequences were identified and precisely excised with Cutadapt (version 1.9.1; https://cutadapt.readthedocs.io/en/stable/) [17]. The paired-end reads obtained from previous steps were assembled by USEARCH (version 10; https://www.drive5.com/usearch/) [18]. To ensure the reliability of the assembled sequences, chimeras were detected and removed by employing UCHIME (version 8.1; https://www.drive5.com/usearch/manual8.1/uchime_algo.html) [19].
By employing a 97% sequence similarity threshold, operational taxonomic units
(OTUs) were derived from nonrepetitive sequences, except for single sequences,
and clustered using USEARCH (version 10.0; http://www.drive5.com/usearch/). This stringent clustering approach
resulted in a comprehensive OTU abundance table. OTUs were meticulously
classified and annotated, leveraging the SILVA database (version 138.1;
https://www.arb-silva.de/) [20] and employing the Naive Bayes classifier
integrated within QIIME2 (v.2020.6; https://qiime2.org/) [21]. This classification was
conducted with a confidence level set at 70%. To assess the
Statistical analyses were conducted using GraphPad Prism 10 (GraphPad Software,
San Diego, CA, USA). The distribution of continuous variables was assessed using
the Shapiro-Wilk test and visual inspection of quantile-quantile (Q-Q) plots (Supplementary
Fig. 1). For normally distributed variables (age and body mass index [BMI]), the
homogeneity of variances between groups was evaluated using the Brown-Forsythe
test. Given that both variables satisfied the assumption of equal variances, they
were compared between groups using the one-way analysis of variance. Non-normally
distributed continuous variables with three groups (age at menarche [AAM],
gravidity, parity, miscarriages, pain level, pain duration, anti-Müllerian
hormone [AMH], carbohydrate antigen 125 [CA125], CA19-9, neutrophil count [N],
lymphocyte count [L], and neutrophil-to-lymphocyte ratio [NLR]) were compared
using the Kruskal-Wallis test, whereas volume of chocolate cysts (VOCC), which
involves two groups, was compared using the Mann-Whitney U test. Categorical
variables, such as the distribution of endometriotic cysts (unilateral vs.
bilateral), were analyzed using Fisher’s exact test. Continuous data are
presented as mean
This study enrolled 45 women categorized into three groups: 20 patients with EMS
and dysmenorrhea, 13 patients with EMS but without dysmenorrhea, and 12 healthy
controls. Their baseline characteristics are shown in Table 1. No significant
differences were observed among the three groups in terms of age, BMI, AAM, gravidity,
parity, or miscarriage frequency (all p
| Characteristics | Control | EMS without dysmenorrhea | EMS with dysmenorrhea | p value | |
| N | 12 | 13 | 20 | - | |
| Age, years (Mean |
28.42 |
29.38 |
31.35 |
0.2336 a | |
| BMI, kg/m2 (Mean |
20.53 |
19.77 |
19.45 |
0.4329 a | |
| AAM, years | 14.00 [13.00, 14.75] | 13.00 [13.00, 13.00] | 13.00 [12.00, 14.00] | 0.3932 | |
| Gravidity | 0.00 [0.00, 0.75] | 0.00 [0.00, 1.50] | 0.00 [0.00, 1.75] | 0.6384 | |
| Parity | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.50] | 0.00 [0.00, 1.00] | 0.5099 | |
| Miscarriages | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.4951 | |
| Pain level | - | - | 5.00 [3.25, 7.00] | - | |
| Pain duration, years | - | - | 6.00 [3.25, 10.00] | - | |
| Unilateral/Bilateral endometriotic cysts (%) | 0.4311 b | ||||
| Unilateral | - | 11 (84.6) | 14 (70.0) | ||
| Bilateral | - | 2 (15.4) | 6 (30.0) | ||
| VOCC, mm3 | - | 43,952.00 [2529.00, 128,658.00] | 7683.00 [2457.00, 58,702.00] | 0.2081 c | |
| AMH, ng/mL | 4.74 [3.54, 6.16] | 3.95 [1.50, 5.65] | 2.34 [1.88, 4.07] | 0.0616 | |
| CA125, U/mL | 15.70 [12.18, 24.35] | 30.20 [23.35, 47.05] | 48.80 [32.05, 100.80] | 0.0012 | |
| CA19-9, U/mL | 11.20 [4.93, 21.63] | 11.03 [6.21, 18.50] | 21.24 [11.37, 31.26] | 0.1879 | |
| N, (×109/L) | 3.28 [2.63, 4.52] | 3.60 [3.01, 5.01] | 3.54 [2.55, 4.27] | 0.4407 | |
| L, (×109/L) | 2.01 [1.79, 2.36] | 1.82 [1.56, 2.47] | 1.89 [1.34, 2.29] | 0.3709 | |
| NLR | 1.58 [1.15, 2.31] | 1.97 [1.50, 2.50] | 1.90 [1.45, 2.35] | 0.3756 | |
Data are represented as median [interquartile range]. N, number; SD, standard deviation; EMS, endometriosis; BMI, body mass index; AAM, age at menarche; VOCC, volume of chocolate cysts; AMH, anti-müllerian hormone; CA125, glycoconjugate antigen 125; CA19-9, glycoconjugate antigen 19-9; N, neutrophil; L, lymphocyte; NLR, neutrophil/lymphocyte ratio. Data were statistically analyzed using Kruskal-Wallis test, with the exception of the data labeled as a, b, and c. a indicates One-Way Analysis of Variance (ANOVA) tests, b indicates Fisher’s exact test, and c indicates Mann-Whitney test.
To explore potential relationships between dysmenorrhea and the gut microbiome
in EMS, 16S rRNA sequencing was performed on 45 stool samples, identifying 5535
OTUs. Samples were categorized into three groups: control (healthy controls),
EMS–Pain (EMS without dysmenorrhea), and EMS+Pain (EMS with dysmenorrhea).
Rank-abundance and species accumulation curves indicated sufficient sequencing
depth and adequate OTU coverage across the groups (Fig. 1A,B). The
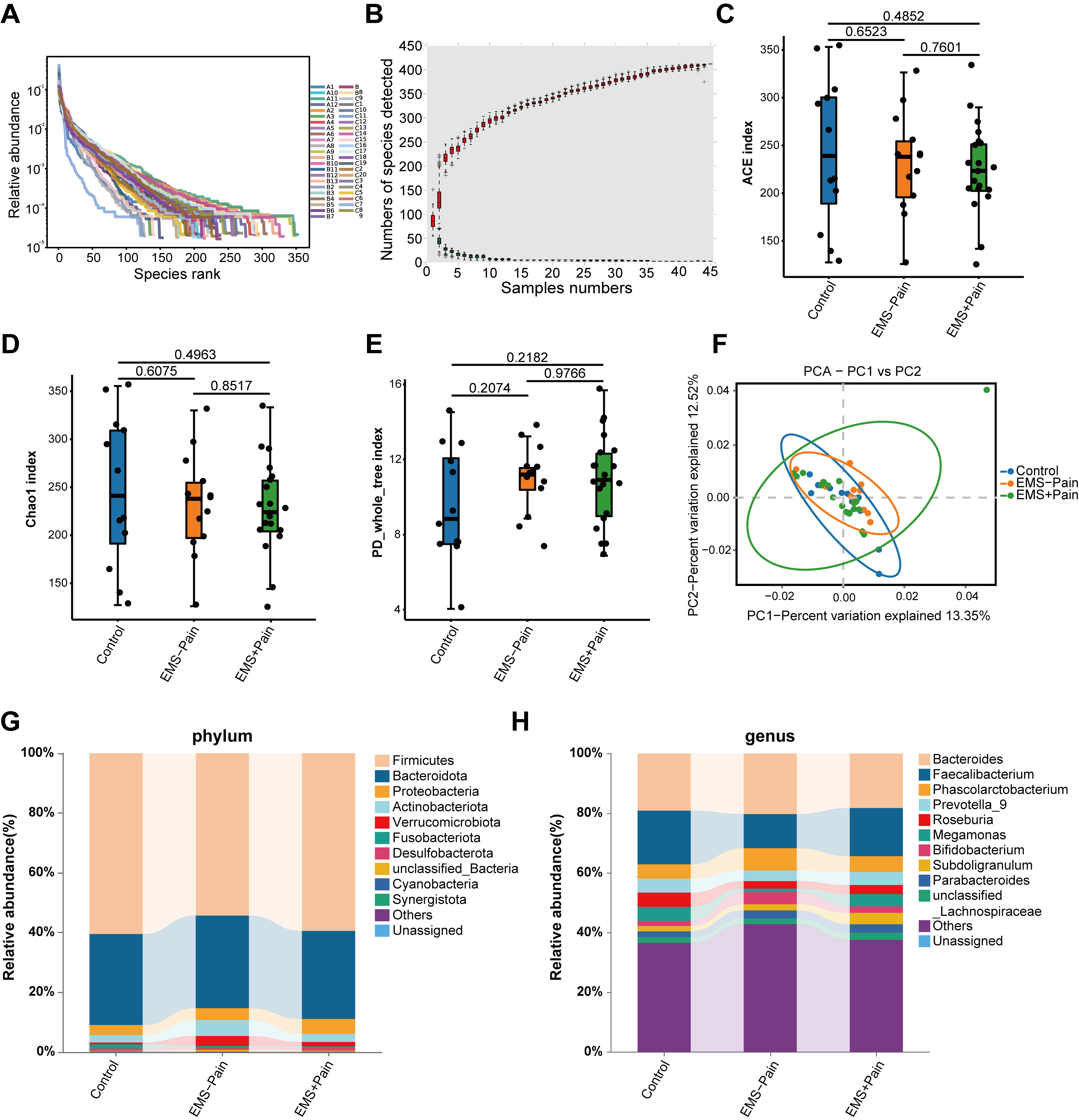 Fig. 1.
Fig. 1.
Gut microbial diversity and structural analysis. (A) Rank–abundance curves. (B) Species accumulation curves. (C) Abundance-based coverage estimator (ACE) indexes. (D) Chao1 indexes. (E) PD_whole_tree indexes. (F) Principal component analysis (PCA) score plot based on the relative abundance of operational taxonomic units (97% similarity). (G) Phylum-level abundance ranking. (H) Genus-level abundance ranking. Control, healthy control group; EMS–Pain, EMS group without dysmenorrhea; EMS+Pain, EMS group with dysmenorrhea.
Then, composition analyses were conducted at both phylum and genus levels. At the phylum level, Firmicutes and Bacteroidetes were dominant across all groups. The relative abundance of Proteobacteria displayed a trend toward an increase in the EMS group with dysmenorrhea, whereas Fusobacteriota showed relatively lower abundance in the EMS group with dysmenorrhea compared with the other groups (Fig. 1G). At the genus level, Bacteroides and Faecalibacterium were relatively abundant (Fig. 1H). Specifically, the relative abundance levels of Subdoligranulum and Parabacteroides were higher in the EMS group with dysmenorrhea. Healthy controls exhibited higher Roseburia abundance than the EMS group (Fig. 1H). However, none of these microbial abundance variations reached significance. In summary, although overall diversity remained comparable, subtle group-specific shifts in the relative abundance of certain taxa may be associated with the dysmenorrhea status in EMS. Thus, further validation is necessary to confirm these potential associations.
To discern the gut microbes specifically implicated in EMS and EMS-related pain, differential species analysis was conducted using taxonomically annotated data from the five most abundant phyla and other phyla. The ternary diagram showed uniform abundance and distribution of Bacteroidota and Firmicutes across three cohorts (Fig. 2A). Wilcoxon rank-sum testing identified 12 genera with the top smallest p-values, with Colidextribacter exhibiting the highest relative abundance. Compared with the EMS group without dysmenorrhea, the abundances of Colidextribacter and Acinetobacter were significantly decreased in the EMS group with dysmenorrhea. Conversely, compared with the EMS group without dysmenorrhea, the abundance of uncultured_Bacteroidales_bacterium in the EMS group with dysmenorrhea significantly increased (Fig. 2B).
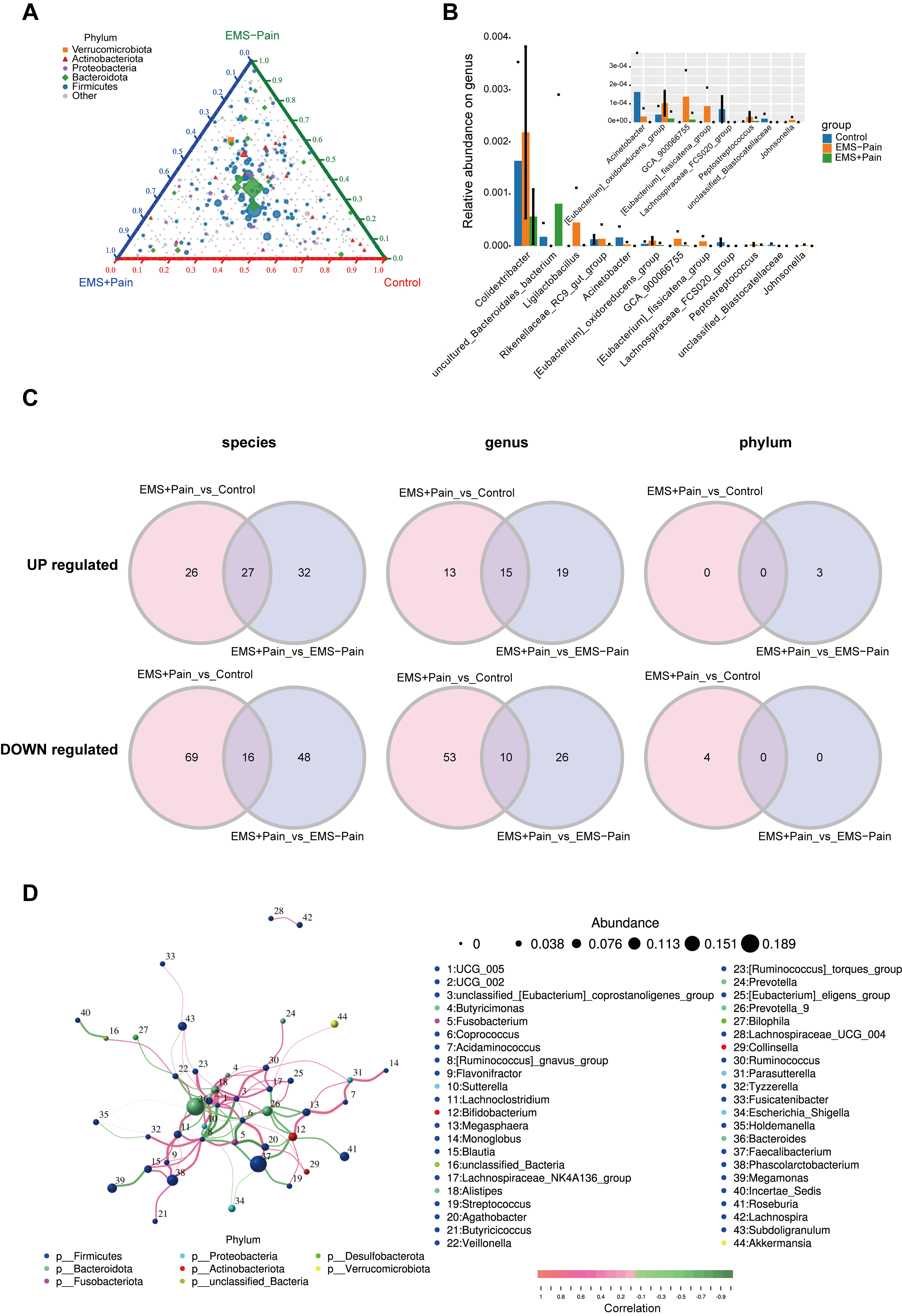 Fig. 2.
Fig. 2.
Screening for differential microorganisms in EMS with pain. (A) Ternary plot showing phylum-level abundance distributions. Pie charts represent phyla, with the size indicating relative abundance; the distance from the vertex reflects group-specific ratio. (B) Relative abundance of the top 12 genera with the smallest p-values. (C) Differential microbes at the phylum, genus, and species levels. (D) Correlation analysis of gut microbiota in the EMS group. The dot size indicates relative abundance, and the line color denotes correlation: red for positive and green for negative. Control, healthy control group; EMS–Pain, EMS group without dysmenorrhea; EMS+Pain, EMS group with dysmenorrhea.
To identify core microbial alterations specifically associated with dysmenorrhea
in EMS, “Control vs. EMS+Pain” and “EMS–Pain vs. EMS+Pain” were compared, and
the differential microorganisms shared in both comparisons were identified
(p
Additionally, Spearman’s rank correlation analysis of the top 50 genera revealed a robust co-occurrence network, with UCG_005 and UCG_002 as the central hub taxa (Fig. 2D). The high abundance levels of Bacteroides and Faecalibacterium highlighted their potential regulatory roles in EMS-related phenotypes. Collectively, these exploratory findings implicate multilevel gut microbiota alterations in EMS-related dysmenorrhea, though mechanistic roles must be experimentally validated.
Community-wide gut microbiota functions were predicted using PICRUSt2 based on clusters of orthologous groups (COG) family data to explore potential roles in EMS and associated pain. Analysis revealed significant functional enrichment across 24 distinct COG categories, particularly in signal transduction mechanisms and amino acid transport and metabolism (Supplementary Fig. 2A). Furthermore, intergroup comparisons of COG functional profiles exhibited trends of variation in predicted functions; however, no significant differences were observed (Supplementary Fig. 2B).
Gut microbiota-derived KEGG pathway analysis highlighted enrichment in pathways
related to amino acid metabolism, metabolism of cofactors and vitamins, signal
transduction, and endocrine and metabolic diseases (Fig. 3A). Pairwise KEGG
pathway comparisons among the three groups revealed significantly higher
enrichment in pathways related to cofactor and vitamin metabolism (p
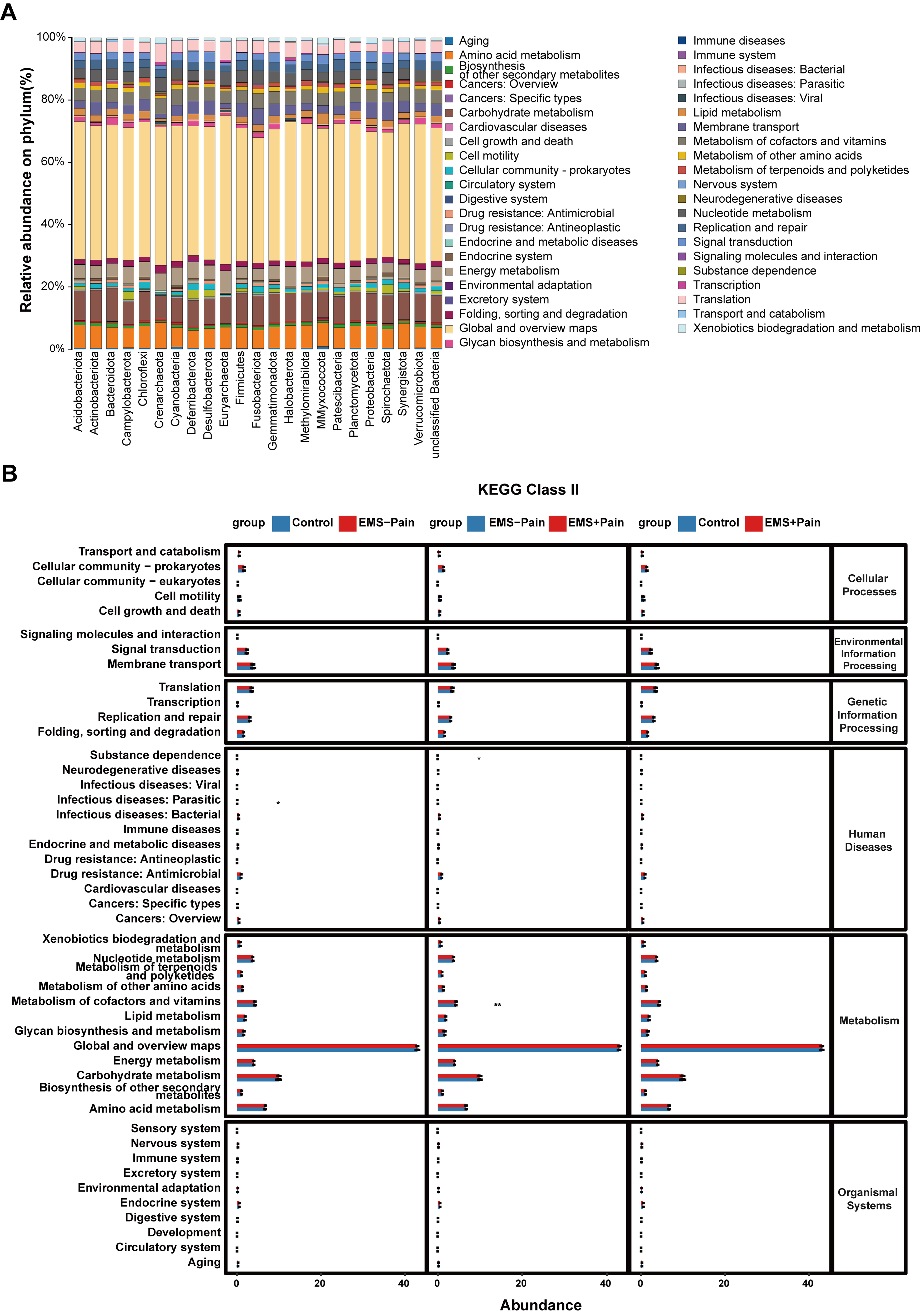 Fig. 3.
Fig. 3.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
predictive analysis. (A) KEGG pathway prediction analysis at the phylum level.
(B) KEGG pathway prediction analysis among the groups. Control, healthy control
group; EMS–Pain, EMS group without dysmenorrhea; EMS+Pain, EMS group with
dysmenorrhea. * means p
To investigate the relationship between differential gut microbes in EMS and clinical indicators of pain and inflammation, correlation analyses were conducted across phylum, genus, and species levels. Clinical parameters included age, BMI, pain severity, AAM, VOCC, AMH, CA125, CA19-9, and NLR.
At the phylum level, Spirochaetota and Deferribacterota showed negative and positive correlations with NLR, respectively, in the comparison between the healthy control group and EMS group without dysmenorrhea (Fig. 4A). In the comparison between the EMS group without dysmenorrhea and the group with dysmenorrhea, Crenarchaeota and Cyanobacteria showed significant positive correlations with pain severity (Fig. 4B). Conversely, in the comparison between the healthy control group and the EMS group with dysmenorrhea, Spirochaetota, Deferribacterota, and Halobacterota were negatively associated with pain severity (Fig. 4C).
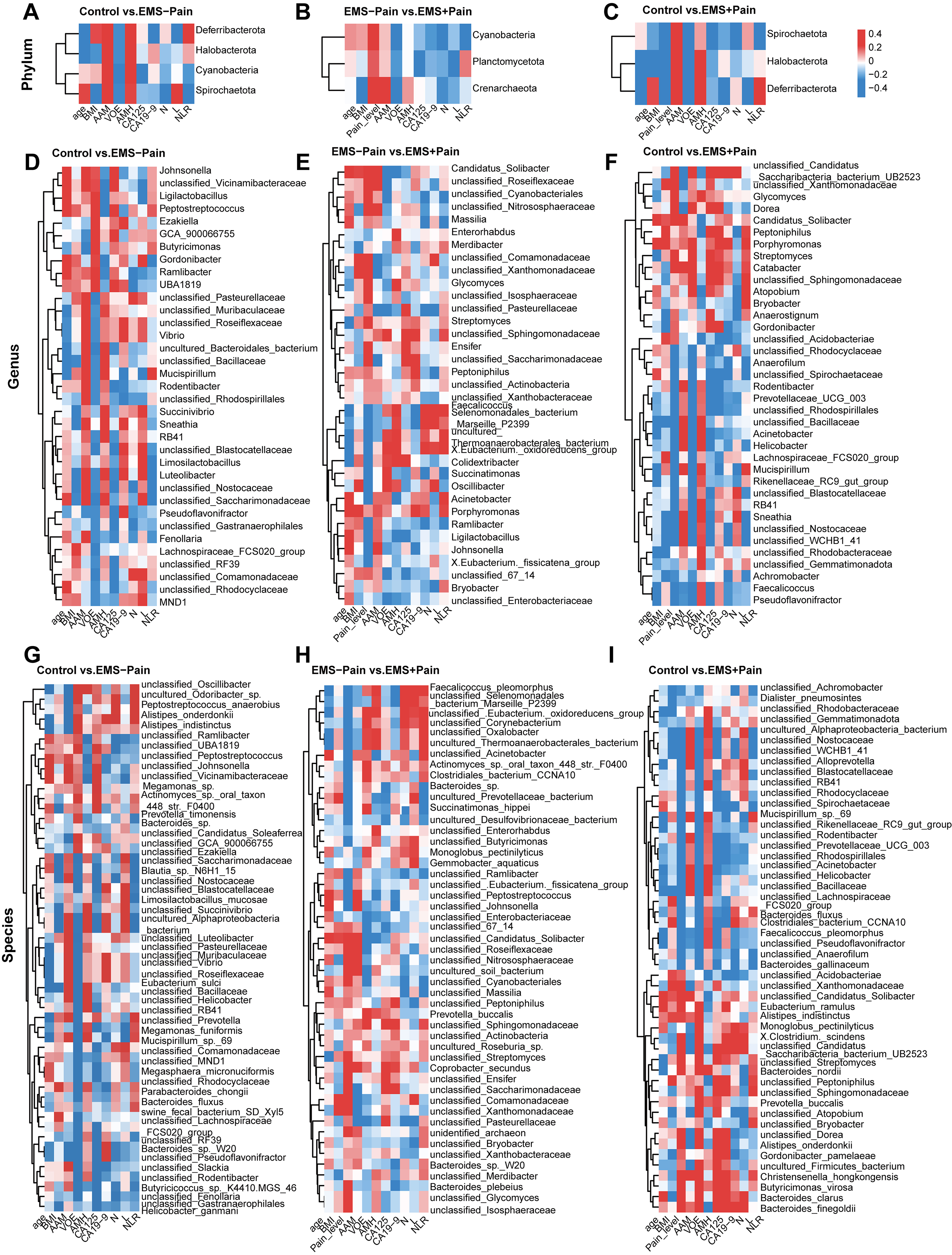 Fig. 4.
Fig. 4.
Correlation analysis of differential microorganisms at different levels of classification with clinical indicators. (A–C) Phylum level: EMS–Pain vs. control and EMS+Pain vs. EMS–Pain, and EMS+Pain vs. control. (D–F) Genus level: EMS–Pain vs. control, EMS+Pain vs. EMS–Pain, and EMS+Pain vs. control. (G–I) Species level: EMS–Pain vs. control, EMS+Pain vs. EMS–Pain, and EMS+Pain vs. control. Control, healthy control group; EMS–Pain, EMS group without dysmenorrhea; EMS+Pain, EMS group with dysmenorrhea.
At the genus level, Gordonibacter and Luteolibacter showed a negative correlation with NLR, whereas Mucispirillum and Butyricimonas demonstrated a positive correlation in the comparison of the healthy control group with the EMS group without dysmenorrhea (Fig. 4D). In the analysis between the EMS group without dysmenorrhea and the dysmenorrhea group, Colidextribacter, Faecalicoccus, and Acinetobacter were significantly negatively correlated with the pain severity of dysmenorrhea in EMS. Oscillibacter, Streptomyces, and Candidatus_Solibacter exhibited a significant positive correlation with the degree of pain in EMS (Fig. 4E). In the analysis between the healthy control group and the EMS group with dysmenorrhea, Acinetobacter and Faecalicoccus showed a significant negative correlation with the degree of dysmenorrhea of EMS, whereas Candidatus_Solibacter and Streptomyces demonstrated a positive correlation (Fig. 4F).
At the species level, in the comparison between the healthy control group and the EMS group without dysmenorrhea, Limosilactobacillus_mucosae and Bacteroides sp. showed a negative correlation with NLR, whereas Megamonas funiformis and unclassified_Prevotella demonstrated a positive correlation with NLR (Fig. 4G). In the analysis between the EMS group without dysmenorrhea and the dysmenorrhea group, Faecalicoccus_pleomorphus showed a negative correlation with pain, whereas unclassified_Candidatus_Solibacter and unclassified_Streptomyces showed a positive correlation with pain (Fig. 4H). In the analysis between the healthy control group and the EMS group with dysmenorrhea, unclassified_Acinetobacter and Faecalicoccus_pleomorphus displayed a negative correlation with pain, whereas Alistipes_onderdonkii, Alistipes_indistinctus, and unclassified_Streptomyces showed a positive correlation with pain (Fig. 4I). These findings identify microbiota–clinical indicator correlations that indicate potential roles of specific taxa in EMS-related inflammation and pain.
To validate discriminatory microbial markers, ROC curve analysis was performed
using an area under the curve (AUC)
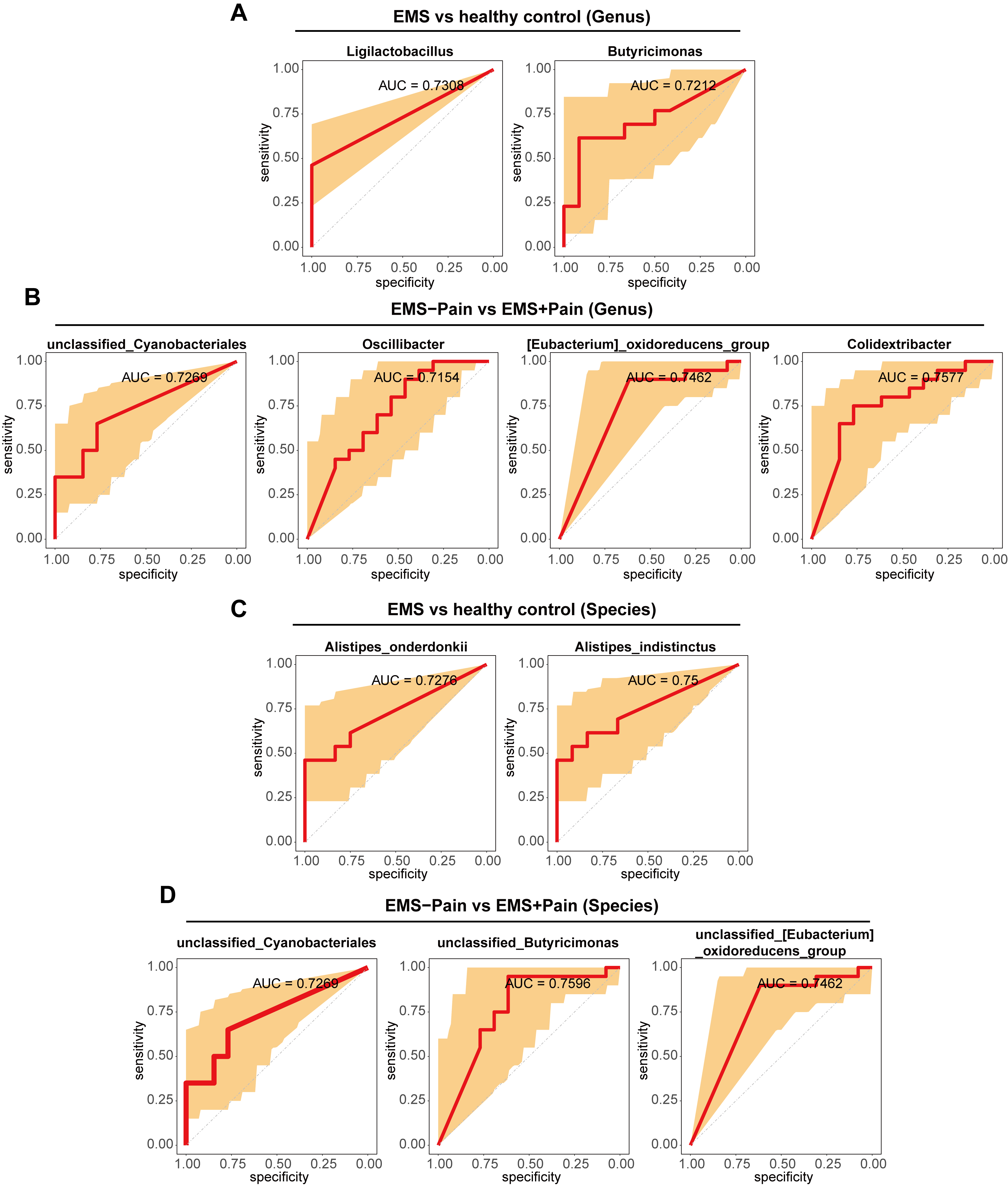 Fig. 5.
Fig. 5.
ROC analysis of differential microorganisms. (A) ROC analysis of differential microorganisms at the genus level between the EMS group and the healthy control group. (B) ROC analysis of differential microorganisms at the genus level between the EMS group with dysmenorrhea and the EMS group without dysmenorrhea. (C) ROC analysis of differential microorganisms at the species level between the EMS group and the healthy control group. (D) ROC analysis of differential microorganisms at the species level between the EMS group with dysmenorrhea and the EMS group without dysmenorrhea. ROC, receiver operating characteristic; Control; healthy control group; EMS–Pain, EMS group without dysmenorrhea; EMS+Pain, EMS group with dysmenorrhea.
EMS is a complex chronic inflammatory disease that affects 10%–15% of women of childbearing age [22]. It is characterized by a series of painful symptoms, particularly dysmenorrhea. In recent years, scientific researchers have shown great interest in the interrelationship between the gut microbiota and human health. A study found that the gut microbial composition was disrupted in the EMS patients [19]. In the present study, although no significant differences were observed in the overall microbial diversity or community structure among the healthy control group, EMS group without dysmenorrhea, and the EMS group with dysmenorrhea, specific taxa that exhibited significant differences in relative abundance were identified between the groups. Additionally, several of these differential microorganisms showed correlation with clinical parameters, such as CA125 levels. Moreover, correlations were established between these differential microorganisms and clinical indicators in the EMS group, and specific microorganisms with high predictive value for EMS and EMS pain symptoms were identified.
Previous studies have identified early menarche as a risk factor for both EMS
and the severity of dysmenorrhea. A recent cohort analysis indicated that early
menarche (
Notably, fecal samples from patients with EMS exhibited significant gut
microbial dysbiosis, characterized particularly by an altered
Firmicutes/Bacteroidetes ratio and increased Bacteroides abundance [26]. In this
study, although no significant overall differences were found in microbial
community structure, a higher relative abundance of Proteobacteria was observed
in the EMS group with dysmenorrhea than in the other groups. Proteobacteria, one
of the most common phyla in the human microbiome, is closely related to various
inflammatory conditions [27].
Accumulating evidence presents that the gut microbiota is involved in the
pathogenesis of EMS through mechanisms such as immune regulation, estrogen
metabolism, and inflammatory signaling [31]. Among these, metabolite-mediated
immune regulation has gained particular attention. Short-chain fatty acids
(SCFAs), particularly butyrate, are critical microbial metabolites that influence
host physiology, and several studies have highlighted their roles in EMS onset
and progression. In line with this, this study identified Roseburia and
Butyricimonas, both of which are established SCFA producers, including butyrate
and propionate [32, 33]. Mechanistic studies have provided further support for
this link; for example, Xu et al. [34] demonstrated that microbiota-derived
acetate ameliorates EMS by modulating M1 macrophage polarization through the
JAK1/STAT3 pathway, whereas Gou et al. [35] reported that butyrate enhances
ferroptosis sensitivity in EMS via FFAR2/PPAR-
At the functional level, COG analysis shows that the COG categories of the three groups of samples were mainly concentrated in fields, such as signal transduction mechanisms and amino acid transport and metabolism. A previous study showed that the balance of the progesterone- and estrogen-related signaling pathways is disrupted in EMS and that this imbalance can worsen inflammation and potentially increased pain from the disease [37]. KEGG pathway prediction showed that EMS was related to pathways, such as the immune system, amino acid metabolism, and cofactor and vitamin metabolism. Notably, vitamin B are critical in maintaining normal neural function, and vitamin B deficiencies have been linked to neuropathic pain symptoms [38]. Recent studies have also reported that vitamin B5 acts as a context-dependent dietary regulator of nociception [39], whereas altered vitamin D status contributes to chronic inflammatory pain in fibromyalgia [40]. These findings advise that alterations in the functional capacity of the gut microbiota may constitute mechanistic links between gut dysbiosis and EMS-associated dysmenorrhea, thereby offering potential targets for therapeutic intervention.
This study identified four microorganisms with predictive value for EMS and seven microorganisms with predictive value for EMS accompanied by dysmenorrhea. Among them, Alistipes_onderdonkii and Butyricimonas had excellent predictive ability for EMS. Studies have shown that oral administration of the commensal bacterium Alistipes onderdonkii can prolong the survival time of allografts and can be used as a probiotic for transplant recipients to reduce inflammation in a stable state following transplantation [41]. Butyricimonas has shown negative correlation with inflammation parameters [42].
These findings have several potential clinical implications. Specific gut microbial taxa and their metabolites may serve as noninvasive biomarkers for diagnosing EMS and distinguishing patients with dysmenorrhea. Moreover, the microbiota–metabolite axis represents a promising therapeutic target, with potential interventions, such as probiotics, dietary modulation, or supplementation with key microbial metabolites such as SCFAs. These strategies could complement existing hormonal or surgical therapies, potentially mitigating disease progression and alleviating pain. Further multi-omics and longitudinal studies may validate these translational applications and assess their clinical feasibility.
Although laparoscopy with histological confirmation remains the gold standard for EMS diagnosis, the exclusive inclusion of patients with imaging-confirmed ovarian endometriomas provided robust diagnostic certainty in our cohort. Transvaginal ultrasonography and MRI demonstrate pathognomonic features for ovarian endometriomas, supporting the reliability of the imaging-based diagnosis in this study. Despite these promising findings, several limitations should be acknowledged. First, the relatively small sample size may limit statistical power and generalizability. Second, the lack of metagenomic or metabolomic data constrains the depth of functional interpretation. Third, the cross-sectional design precludes causal inference between microbial alterations and EMS or its pain-related symptoms. Additionally, the ROC analysis was based on the presence or absence of specific taxa, with presence defined as a relative abundance above a minimal detection threshold to reduce sequencing noise. Future studies incorporating larger cohorts, longitudinal sampling, and multi-omics approaches are warranted to validate these results and further elucidate microbe–host interactions in EMS pathogenesis.
This study reveals that alterations in specific microbial taxa, rather than
changes in the overall community composition or diversity, are associated with
EMS-related dysmenorrhea. Functional prediction analyses showed enrichment in
cofactor and vitamin metabolism pathways in EMS-associated pain. The abundances
of Acinetobacter and Colidextribacter showed a negative correlation with pain
severity. Moreover, 10 microbial species were identified, including
Colidextribacter, with moderate diagnostic value (AUC
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
WS, MYW, and SQC conceived and designed the experiments. HLL and XCC conducted the collection and processing of sequencing samples and performed bioinformatics analysis. ZJ and QYZ analyzed the results with the assistance of XL. WS and MYW authored the manuscript, with revisions provided by SQC and XL. XL, JYM, and LBQ contributed to data interpretation, manuscript drafting, and critical review of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki. The research protocol was approved by the Ethics Committee of The Sixth Affiliated Hospital of Sun Yat-sen University (2024ZSLYEC-020), and all of the participants provided signed informed consent.
We are grateful to the anonymous reviewers for their constructive critiques and insightful recommendations. We also acknowledge the helpful input from our colleagues during the preparation of this paper.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG45531.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
