- Academic Editor
†These authors contributed equally.
Brain resting-state functional networks are extensively utilized in research on psychiatric disorders. Meanwhile, pregnancy promotes specific and substantial changes in neural structure and network integration, which are most prominent in the default mode network (DMN). Prior studies have established a relationship between hypertensive disorders in pregnancy (HDP) and mental disorders. Nevertheless, the causal influence of brain resting-state functional networks on HDP is poorly understood.
A bidirectional two-sample Mendelian randomization (MR) framework was applied to estimate the causal effects of 191 resting-state functional magnetic resonance imaging (rsfMRI) phenotypes (sample size: 34,691) on five HDPs, and vice versa. The five HDP conditions were gestational hypertension (GH), pre-eclampsia (PE), eclampsia, chronic hypertension, and PE superimposed on chronic hypertension.
Forward MR estimates identified a potential causal relationship between one rsfMRI phenotype (attention, salience, and motor network) and chronic hypertension in pregnancy. The MR analysis of the reverse direction revealed that chronic hypertension in pregnancy may exert a causal influence on three rsfMRI phenotypes: the motor and subcortical-cerebellum network, the attention, salience, and motor network, and the subcortical–cerebellum and motor network. The causal relationship between the attention, salience, and the motor network and chronic hypertension in pregnancy was found to be bidirectional.
Our findings reveal a potential causal relationship between altered patterns of intrinsic brain connectivity and chronic hypertension in pregnancy. These results provide crucial evidence for an association between chronic hypertension in pregnancy and alterations in functional brain networks.
Hypertensive disorders in pregnancy (HDP), including chronic (pre-existing) hypertension, gestational hypertension (GH), pre-eclampsia (PE), and eclampsia, are some of the most frequent obstetric complications [1, 2]. The global prevalence of HDP is about 116/100,000, but significant geographical disparities exist, with Africa having the highest prevalence (335/100,000) and the Western Pacific region the lowest (16/100,000) [3]. The global incidence of HDP reached 18.1 million in 2019 [4], causing 27,800 maternal deaths. In addition, 200,000 maternal stillbirths occur globally each year due to HDP [5].
HDP induces neuronal structural changes and neuroglial cell proliferation, as well as elevated levels of inflammatory cytokines [6]. Women with a prior diagnosis of HDP have a considerably heightened risk of cognitive impairment and Alzheimer’s disease [7]. Moreover, women with a lifetime history of PE during at least one pregnancy have a 3.5-fold higher risk of vascular dementia, associated with a 50% greater risk of developing Alzheimer’s disease, and a 1.4-fold increased risk of developing other dementias. Those with a history of recurrent PE have a higher risk of dementia compared to women with PE in just one pregnancy [8, 9]. The long-term neurological effects of PE include cognitive impairments such as impaired memory, attention deficits, and motor deficits [10]. Compared to control females with no PE, those with past experiences of PE had greater local efficiencies in the prefrontal cortex and anterior cingulate cortex, but less local efficiencies and measures of local network segregation in the amygdala and parahippocampal gyrus cortex [11]. Furthermore, no statistically significant differences were found in overall functional brain structure between PE women and controls. A Mendelian randomization (MR) analysis found that both PE and eclampsia were inversely correlated with gray matter volume in the brain, and negatively associated with brain volume [12].
Multiple mental disorders increase the risk of HDP [13, 14]. Patients with depression, bipolar disorder, anxiety disorders, bipolar disorder, and schizophrenia spectrum disorders are at higher risk for PE [15, 16, 17, 18]. According to the available research evidence, HDP has impacts on brain function and structure.
Previous investigations have detected structural brain anomalies in psychiatric disorders, implying that cortical structural dysfunction might play a role in psychiatric disorders [19]. Recently, several discoveries with MR have prompted the investigation of multiple causal associations with brain structure and psychiatric disorders [20, 21]. Multiple brain regions synergistically engage in specific brain functions. Functional brain networks are a higher-dimensional organization compared to static brain structures and are associated with many of the higher neurocognitive processes. The pattern of functional connectivity in the brain is the foundation for the complex roles it performs. Connections and transmission among functional webs underpin perception, action, and sentiment. The brain can stay spontaneous during the resting state, and resting-state functional magnetic resonance imaging (rsfMRI) is frequently utilized to image functionally active brain regions [21]. Various intrinsically organized brain networks have consistently been observed during the resting state using fMRI. These include the salience network, the default mode network (DMN), the central executive network, the somatomotor network, and the attention network [22, 23]. Increasing evidence suggests that patients with mental illness have dysfunctional brain function networks. While mental illness is a known high risk factor for HDP, there are few studies on the causal relationship between HDP and rsfMRI phenotypes. Addressing the causal relationship between functional brain networks and HDP will help to understand the pathogenesis of HDP and may provide insights into potential therapeutic targets for HDP, such as specific functional brain networks.
In accordance with Mendel’s laws of inheritance, MR employs genetic variation related to exposure as a tool to infer causal relationships between exposure and outcome [24, 25, 26]. Causal inferences regarding HDP and rsfMRI can be drawn by using MR analysis in conjunction with the genetic findings of HDP and rsfMRI. MR analysis can be regarded as a natural randomized controlled trial that minimizes the impacts of confounding factors [26]. While there is increasing recognition of the associations between HDP, psychiatric risks, and structural brain changes, the causal relationship between HDP and the organization of higher-order, intrinsic functional brain networks remains largely unexplored. Addressing this knowledge gap is critical, as functional networks serve as the foundation for cognition and behavior. Therefore, in the current study we designed a bidirectional, two-sample MR analysis to systematically evaluate the potential causal relationship between 191 brain resting-state functional networks and HDP. This study is the first to specifically examine and provide evidence for causal interplay between functional network phenotypes and HDP. It provides novel insights into the neural procedure underlying these disorders, and identifies possible targets for future research and intervention.
The brain rsfMRI dataset used in this research was derived from an earlier study
[27] in which the relationships between 1777 inherent brain activity phenotypes
and 9,026,427 usual nucleotide variants were explored in a UK Biobank sample (n =
34,691). Among 1777 traits, 191 traits significantly (p
We employed a bidirectional two-sample MR inspection to investigate the causal connection between 191 brain rsfMRI phenotypes and HDP. For forward MR, brain rsfMRI phenotypes were treated as exposures to estimate their causal effect on HDP outcomes. In the reverse MR analysis, HDP was considered as the exposure factor and brain rsfMRI phenotype as the consequence. The instrumental variables (IVs) utilized for MR analysis met the following three fundamental suppositions: (1) a significant connection exists between IVs and exposure factors; (2) IVs are not influenced by potential confounding factors; and (3) IVs affect outcomes solely through exposure factors [28, 29].
A meticulous process was followed to screen qualified IVs for MR analysis.
First, only single nucleotide polymorphisms (SNPs) that were strongly related to
exposure (p
Bidirectional two-sample MR analysis was conducted to investigate the causal connection between HDP and rsfMRI phenotype. In the forward MR analysis, brain rsfMRI was taken as the exposure factor and HDP as the outcome factor. In the reverse MR analysis, HDP was regarded as the exposure factor and brain rsfMRI as the outcome factor. The inverse variance weighting (IVW) method was employed as the main approach for causal inference. The Wald ratio was applied to evaluate causality when there was only one eligible IV. When there were two eligible IVs, the IVW method was utilized. Four other MR methods were also utilized to enhance the reliability of MR estimates: the MR-Egger method, the weighted median method, the simple mode method, and the weighted mode method. A causal connection between exposure and outcome was assumed only when the estimates from the various methods in the MR analysis satisfied the subsequent requirements.
(1) When more than two eligible IVs were incorporated into the analysis, the
estimates for all five causal effect assessment methods converged in the same
direction, and the p-value for the IVW practice was
(2) The p-value was
A set of responsiveness investigations was carried out to examine the robustness
of the MR estimates. First, the MR Pleiotropy Residual Sum and Outlier
(MR-PRESSO) international test was performed to verify the existence of
horizontal multinomiality (p
To investigate a possible causal relationship between resting-state functional networks in the brain and HDP, we carried out a bidirectional, two-sample MR analysis using GWAS pooled data of rsfMRI phenotypes and HDP (Fig. 1). Following a succession of rigorous and exacting screening procedures, qualified IVs were selected for the MR analysis. Sensitivity analyses were performed to assess the validity of forward and reverse MR extrapolation, with the results indicating this bidirectional MR estimate of causality was reliable.
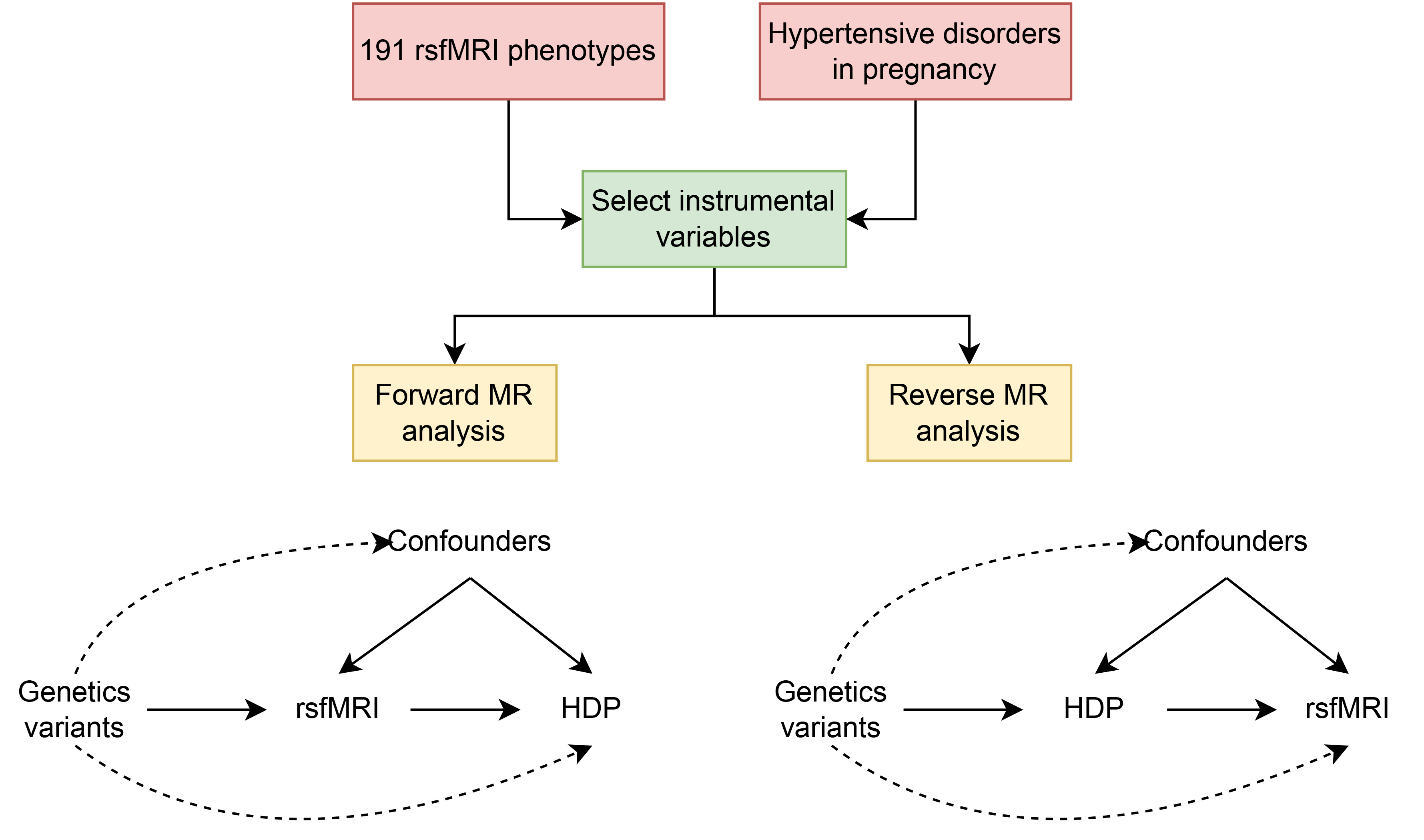 Fig. 1.
Fig. 1.
Flowchart of the study design for bidirectional Mendelian randomization (MR) of resting-state functional magnetic resonance imaging (rsfMRI) and hypertensive disorders in pregnancy (HDP).
In the forward MR analysis, a probable causal relationship was identified
between one rsfMRI phenotype (Attention, Salience, and Motor network) and chronic
hypertension in pregnancy [Odds Ratio (OR): 0.027, 95% CI: 0.005–0.153,
p = 4.57
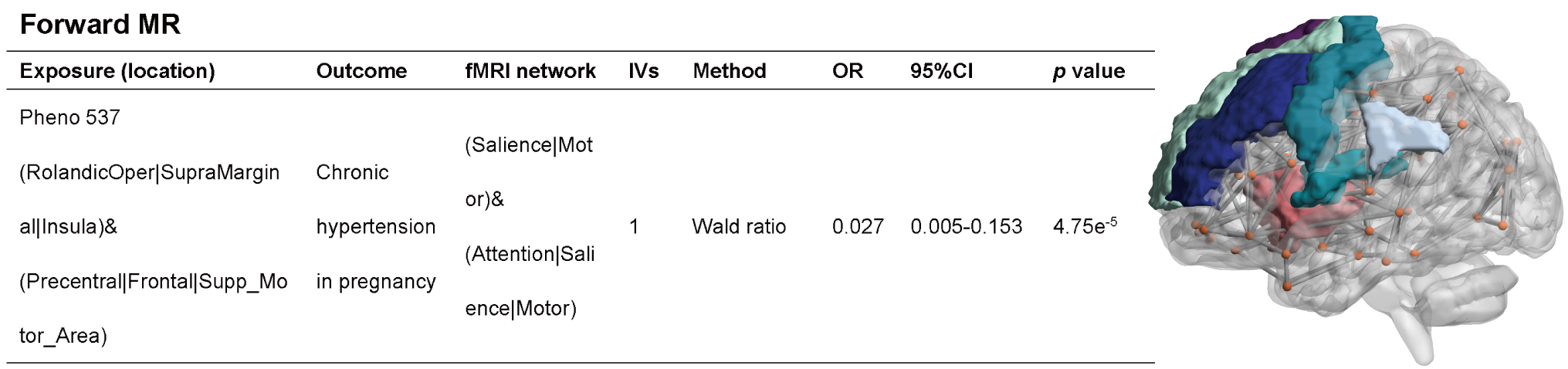 Fig. 2.
Fig. 2.
MR analysis results when rsfMRI is used as the exposure factor and HDP as the outcome factor. The Attention, Salience, and Motor network was significantly associated with chronic hypertension in pregnancy when rsfMRI phenotype was used as an exposure factor and chronic hypertension in pregnancy was used as an outcome factor. OR, Odds Ratio; IVs, instrumental variables.
In the reverse MR analysis, a potential causal relationship was found between
chronic hypertension in pregnancy and three rsfMRI phenotypes: the Motor and
Subcortical-cerebellum network (OR: 0.912, 95% CI: 0.866–0.960, p =
4.50
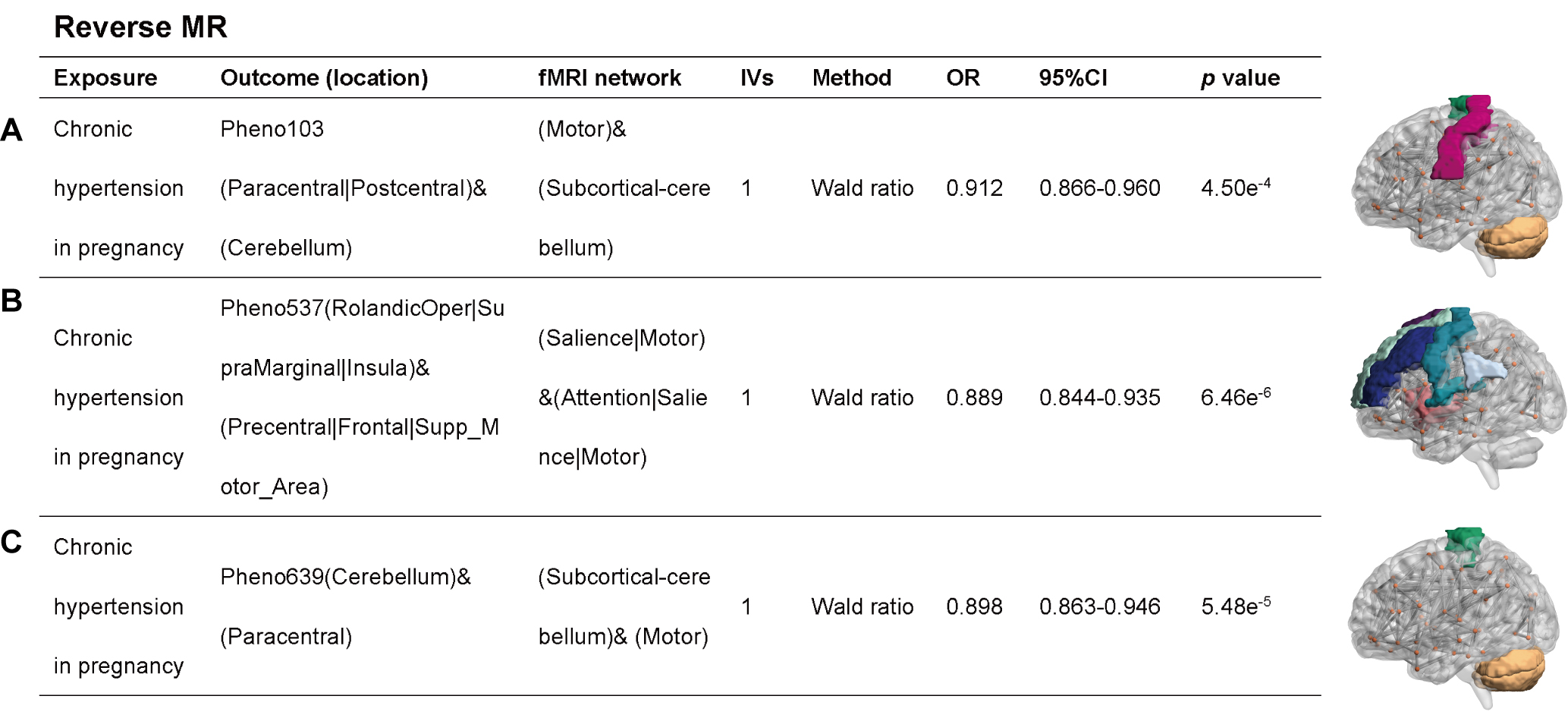 Fig. 3.
Fig. 3.
MR analysis when chronic hypertension in pregnancy was used as an exposure factor and rsfMRI phenotype was used as an outcome factor. (A) Motor and Subcortical-cerebellum network; (B) Attention, Salience and Motor network; (C) Subcortical-cerebellum and Motor network.
Detailed results of all responsiveness analyses, including Cochran’s Q test, MR-Egger intercept test, and MR-PRESSO test, are presented in Supplementary File 1. The Cochrane Q-test indicated that only a small number of forward and reverse MR analyses exhibited heterogeneity. Likewise, only some of the IVs incorporated in the MR analyses showed significant horizontal pleiotropy. MR-PRESSO results demonstrated that virtually no IVs exhibited significant horizontal pleiotropy. These results imply the associations identified in the forward and reverse MR analyses are mostly reliable.
The connection between HDP and psychiatric disorders has been extensively investigated, with MR studies on psychiatric disorders and the resting state network of the brain revealing a causal relationship between the two [21]. However, the relationship between HDP and the resting state network of the brain has yet to be investigated, including an analysis of whether a causal relationship exists. In the current research, we therefore conducted a bidirectional, two-sample MR analysis to examine the causal relationship between 191 brain rsfMRI phenotypes and HDP. The Attention, Salience and Motor network and chronic hypertension in pregnancy were found to be causally related. The very low OR value observed indicates a strong protective effect, meaning that a genetically predicted increase in the functional activity of this specific brain web is related to a substantially lower risk of developing chronic hypertension in pregnancy. However, the large CI and the extreme point estimate should be noted, as these could reflect the statistical imprecision inherent in a single-IV analysis, and may not accurately represent the true biological effect size. In the reverse MR analysis, a potential causal relationship was found between chronic hypertension in pregnancy and three rsfMRI phenotypes: the Motor and Subcortical-cerebellum network; the Attention, Salience and Motor network; and the Subcortical-cerebellum and Motor network. Critically, this causal inference is based on a single genetic instrument. While the F-statistic for this SNP was strong, indicating a low likelihood of weak instrument bias, results from Wald ratio estimators are more susceptible to bias from horizontal pleiotropy compared to methods utilizing multiple instruments. These results should therefore be interpreted with caution. Compared with the multi-SNP method, the Wald ratio method offers less protection against horizontal pleiotropy. However, the strength of this association and its biological rationality provide credibility to the findings.
MR analysis of structural alterations in specific brain regions due to HDP found that chronic hypertension combined with PE can result in increased cortical thickness in the supramarginal gyrus. PE and eclampsia led to cortical thinning in the lingual gyrus, increased hippocampal volume, and parietal lobular surface area. Chronic hypertension was associated with reduced cortical thickness in the caudal and rostral anterior cingulate gyrus, increased cuneate lobe surface area, and orbital cortical thickness. No significant changes in brain regions were found in GH [33]. These insights clarify the neural and cognitive impacts of HDP by identifying the affected brain regions. The connectivity patterns in hypertension mainly involve the cerebellum, prefrontal lobes, anterior insula, anterior cingulate cortex, superior marginal gyrus and precuneus, which are crucial regions of the central autonomic network engaged in cognitive processing [34]. Chronic hypertension disrupts the integrity of the blood-brain barrier, promotes neuroinflammation, and may lead to amyloid deposition and Alzheimer’s disease [35]. Hypertensive rats exhibit behavioral changes, as well as impaired motor and cognitive function [36, 37]. An MRI study of the brain of pregnant women with chronic hypertension and PE revealed a significantly smaller volume of gray matter of the right middle temporal gyrus (MTG) cluster compared to nonpregnant healthy controls [38]. The authors suggested this alteration might be due to pregnancy, and that chronic hypertension combined with PE was not the primary cause of the change. The MRI of the head in untreated hypertensive women shows edematous changes in the brainstem, comprising the subcortex, cerebellum, and pons. Right MTG affects speech motor and cognitive flexibility [38]. The rsfMRI results in patients with hypertension combined with fundus lesions indicated higher degree centrality values in the left posterior cerebellar lobe, left medial occipital gyrus, and bilateral precuneus compared to the control group, while the average degree centrality values were lower in the right medial frontal gyrus/bilateral anterior cingulate cortex. The degree centrality values mirrored changes in spontaneous brain activity [39]. Another study examined changes in brain activity through the amplitude of low-frequency fluctuations (ALFF) in brain regions [40]. Compared to controls, ALFF values in the left medial superior frontal lobe and left middle frontal lobe were lower in hypertension combined with fundopathy, but higher in the cerebellum (left inferior lobe, right superior lobe, etc.) and left inferior temporal gyrus. These associations were partly attributed to insufficient cerebral perfusion. MRI findings revealed that chronic hypertension in non-pregnant patients can lead to multiple low-signal lesions involving deep gray matter nuclei, such as the basal ganglia, thalamus, corona radiata, brainstem, and cerebellum [41]. Alterations in brain structure and function in non-pregnant hypertensive patients might be related to two rsfMRI phenotypes because of chronic hypertension and concomitant pregnancy.
Pregnancy is known to result in long-term alterations in the human brain structure. Nevertheless, only limited knowledge exists regarding the altered topological organization of functional networks. Compared to non-pregnant control women, the network pivot node of first-time mothers shifted from the left inferior temporal gyrus to the right precentral gyrus. Moreover, the network of first-time mothers showed enhanced global efficiency, greater local efficiency, increased clustering coefficients, shortened characteristic path lengths, decreased normalized clustering coefficients, and shortened normalized characteristic path lengths [42]. In the later stage of pregnancy, pregnant women had smaller cortical volumes than controls in all functional networks, although these differences were reduced in the early postnatal period [43]. A comprehensive preconception cohort study examined whether pregnancy is related to changes in resting-state brain activity, white matter microstructure, neurometabolite concentrations, and gray matter structure. Pregnancy causes selective and intense alterations in neural structure and neural network organization that are most prominent in the DMN. These neural changes are associated with pregnancy hormones, mainly late gestational estradiol, rather than with other factors such as osmotic effects, stress, or sleep. The reductions in gray matter volume in the brain were concentrated bilaterally at the superior temporal sulcus and temporoparietal junction, and in the anterior and posterior midline of the brain. Reductions were also found in clusters situated in the anterior cuneate and posterior cingulate cortex, as well as in the medial prefrontal cortex that extends to various lateral frontal regions. Strong structural changes in the DMN were observed throughout pregnancy, with significant clustering in the comparison of DMN in the bilateral cuneate lobes. Correlation analyses revealed an association between the observed changes in gray matter volume and estradiol levels [44].
Changes in brain function can influence chronic hypertension in pregnancy primarily through the association between psychiatric disorders and the subsequent onset of hypertension [45]. Women with anxiety or depression may exhibit significantly elevated blood pressure during pregnancy [46]. Reduced intrinsic connectivity within the frontoparietal network has been observed in depression, accompanied by weakened connections between the frontoparietal system and parietal regions of the dorsal attention network [47]. Conversely, anxiety disorders are associated with functional decline in both the frontoparietal network and the DMN [48]. This evidence suggests that psychiatric disorders linked to alterations in brain functional connectivity may play a significant role in the relationship between functional brain changes and chronic hypertension complicating pregnancy.
There are several limitations to this study. Firstly, the GWAS dataset we employed was mainly from a European population. Consequently, the genetic instruments and the estimated causal effects may not be directly generalized to other groups because of potential differences in genetic architecture, LD patterns, and environmental exposures. Therefore, the applicability of our findings to non-European populations remains to be established and warrants further study in large-scale, multi-ancestry cohorts. Secondly, the causal relationship between chronic hypertension in pregnancy and rsfMRI phenotypes was analyzed based on only one eligible IV, potentially influencing the reliability and stability of the results. The F-statistic for this SNP was strong, indicating a low likelihood of weak instrument bias. However, results from Wald ratio estimators are more susceptible to bias from horizontal pleiotropy compared to methods utilizing multiple instruments. Although the sensitivity analyses and the consistency of the direction of effect across related phenotypes lend credibility to our findings, these results should be interpreted with caution and further validation in larger studies is needed. Thirdly, despite the multiple sensitivity analyses, the results of this study may have been affected by potential confounders due to the lack of original baseline characteristics (e.g., age, history of prior HDP disease). While the MR design reduces confounding by leveraging genetic instruments, residual bias from unmeasured confounders cannot be entirely ruled out. Importantly, our analysis was limited by the lack of individual-level data on key obstetric factors such as gestational age at diagnosis, disease severity, parity, and the timing of rsfMRI assessment relative to the hypertensive pregnancy. These factors could potentially influence both the genetic predictors of brain function and the risk of HDP. Their absence limits our ability to fully disentangle the precise temporal and clinical context of the observed associations. Consequently, while the strength of the association and the biological plausibility lend credibility to our findings, they must be considered preliminary. Further validation is required with larger GWAS summary statistics, enabling the identification of more IVs for these specific phenotypes. Finally, although our MRI analyses identified several potential causal associations between chronic hypertension in pregnancy and rsfMRI phenotypes, these results are largely statistical extrapolations that require validation in longitudinal clinical studies.
Further studies are needed to overcome these limitations. Firstly, large-scale, multi-ancestry GWAS for HDP subtypes and rsfMRI phenotypes are crucial to determine whether our findings can be generalized beyond European populations. Secondly, dedicated longitudinal studies that acquire rsfMRI data prospectively in women before, during, and after pregnancies affected by HDP are essential. Such designs would allow direct control over confounders such as parity and gestational age. They would also help to establish the exact temporal sequence of changes in functional networks relative to the onset of hypertension, providing deeper insights into causality and the potential mechanisms involved.
We are currently unable to fully explain the basis for the observed correlation between chronic hypertension in pregnancy and rsfMRI phenotypes. However, an in-depth understanding of the effects of hypertension and pregnancy on brain structure and function suggests that both factors are potential contributors to brain resting-state function. In conclusion, this is the first study to systematically and comprehensively analyze the relationship between HDP disease and functional brain networks using MR methods. Our findings highlight the potential of specific functional brain network features to serve as candidate biomarkers for risk stratification of chronic hypertension in pregnancy. Moreover, they suggest potential directions for the development of predictive models and for the identification of novel therapeutic targets.
rsfMRI, resting-state functional magnetic resonance imaging; MR, Mendelian randomization; GH, gestational hypertension; PE, pre-eclampsia; DMN, default mode network; IVs, instrumental variables; SNPs, single nucleotide polymorphisms; IVW, inverse variance weighting; MR-PRESSO, MR Pleiotropy Residual Sum and Outlier; MTG, middle temporal gyrus; ALFF, amplitude of low-frequency fluctuations; GWAS, Genome-wide Association Study; LD, linkage disequilibrium; FDR, false discovery rate; HDP, hypertensive disorders in pregnancy.
All GWAS data used in this study are publicly available and can be obtained independently. The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
TZ and HW were responsible for study design, data curation, formal analysis, visualization and original draft. YW and YT were responsible for conceptualization, data curation, visualization and original draft. MC and RJ were responsible for study design, original draft, supervision and review. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We thank Dr. Sicong Liu for her guidance and all the peer reviewers for their valuable opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
During the preparation of this work the authors used DeepSeek in order to check spell and grammar. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG44610.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
