- Academic Editor
Uterine fibroids represent the most frequent benign tumor of the uterus in women of reproductive age. They may be discovered accidentally or can be symptomatic. They pose substantial intraoperative challenges due to the increased risk of bleeding. Despite the rising incidence of fibroids during pregnancy, there is no consensus on the optimal strategy to minimize bleeding during cesarean section (C-section). This review aims to synthesize and critically examine the current evidence on medical, surgical, interventional, and innovative experimental approaches for reducing intraoperative bleeding during C-section. We aim to provide an up-to-date, evidence-based overview to guide clinical decision-making and highlight a gap in knowledge for future research.
Three electronic databases were searched using the keywords: “fibroids”, “intraoperative bleeding”, “cesarean myomectomy”, and “pregnancy”, following the inclusion criteria set. Eligible studies were analyzed, and the extracted data were examined for duplication before inclusion in the review. Four major categories of interventions were identified for reducing intraoperative bleeding: medical, surgical, innovative, and experimental methods. For each technique, relevant data were recorded and synthesized into tables.
Medical techniques were effective, although they had side effects, and their efficacy could be enhanced when used in combination. Surgical techniques proved effective when medical approaches failed, especially in complicated cases. Emerging modalities show promising efficacy with minimal impact on future fertility, but they need further validation.
A personalized, multidisciplinary approach is needed to effectively reduce and manage intraoperative bleeding. Current practice should shift toward risk stratification using predictive tools to estimate bleeding preoperatively. Surgical teams should develop personalized bleeding-control strategies incorporating appropriate pharmacological agents. Future research should examine the integration of artificial intelligence (AI)-based risk modeling and three-dimensional (3D)-printed surgical planning to optimize maternal safety while preserving fertility.
Fibroids coexisting with pregnancy are becoming increasingly common in clinical obstetrics due to demographic shifts toward delayed childbearing, rising obesity rates, and higher pregnancy rates after fibroid treatment [1, 2]. Fibroids are the predominant benign tumor of the uterus, arising from the uterus’s smooth muscle cells and primarily occurring during a woman’s reproductive years. Fibroids contribute annually to a substantial proportion of hysterectomies, accounting for more than one-third to half of all cases. Their development is associated with several epidemiological factors, such as age, race, genetics, hormones, and lifestyle. Fibroids may be incidentally discovered during pregnancy, making a conservative approach the first-line management strategy [3]. Nevertheless, their presence classifies the pregnancy as high-risk due to the potential for increased pregnancy-related complications. Earlier studies have associated fibroids with adverse outcomes, such as miscarriage, preterm prelabor rupture of membranes, and preterm labor. However, a systematic review by Pritts et al. [4] found that only preterm delivery remained significantly increased in women with fibroids, whereas other complications showed inconsistent associations. The increased risk of bleeding during cesarean section (C-section) in women with fibroids, especially those located in the lower uterine segment, has been shown to be two- to four-fold higher compared to healthy controls. Zhao et al. [5] retrospectively studied 112,403 cases of C-sections with and without fibroids among Chinese pregnant women. Their analysis showed higher odds for C-section and increased bleeding, with adjusted odds ratios (ORs) of 1.8 and 1.2, respectively [4, 6]. The size and location of uterine fibroids can influence pregnancy and the delivery process, although their presence is not a contraindication to vaginal delivery. Nonetheless, most affected individuals ultimately undergo C-section [7]. Hemorrhaging associated with fibroids during C-section primarily results from disruption of the highly vascular myometrium and uterine vessels surrounding the fibroids, rather than from the fibroids themselves, which are typically avascular. Fibroids located in the lower uterine segment can be challenging by complicating the uterine incision, thus increasing intraoperative bleeding due to distorted vascular anatomy [8]. Fibroid size and location at the lower uterine segment were significant independent predictors (79.3% specificity) of operative hemorrhage [9]. Despite the growing awareness, evidence-based protocols for such a scenario remain limited [2, 10]. This review offers a comprehensive and critical analysis of the multifaceted challenges in reducing intraoperative bleeding during C-section among women with fibroids, encompassing medical, surgical, interventional radiology, and experimental or innovative techniques. It examines the latest evidence, identifies knowledge gaps within the current guidelines, and evaluates the advantages and limitations of each method. The objective is to establish a robust evidence-based framework to enhance preoperative planning, optimize intraoperative decision-making, and ultimately improve patient outcomes through effective bleeding risk reduction.
A thorough online search was conducted up to 1 May 2025, across multiple electronic databases, such as ScienceDirect, PubMed, and Web of Science (WOS). The following keywords were used: “intraoperative bleeding”, “C-section”, “fibroid”, “blood loss”, “hemostasis”, and “pregnancy”. The search included publications from indexed journals that addressed the topic of interest, i.e., strategies to reduce blood loss during C-section with associated fibroid. We included original articles, systematic reviews, and meta-analyses. Articles were included based on the methodological rigor, clinical relevance, recent publication date, and the availability of a quantitative outcome measured. Exclusion criteria included letters to editors, commentaries, and articles with poor methodology or missing outcome data. Finally, for studies with overlapping datasets, the most comprehensive or original dataset was selected. A cross-reference was made between systematic reviewers and original studies based on authors’ names, institutions, sample size, and study time. The findings of meta-analysis reviews were reported separately and referenced when the original data were unavailable. The extracted data were analyzed and sub-categorized into four major categories for controlling bleeding (see Fig. 1): medical methods, surgical methods, interventional radiology, experimental and innovative methods.
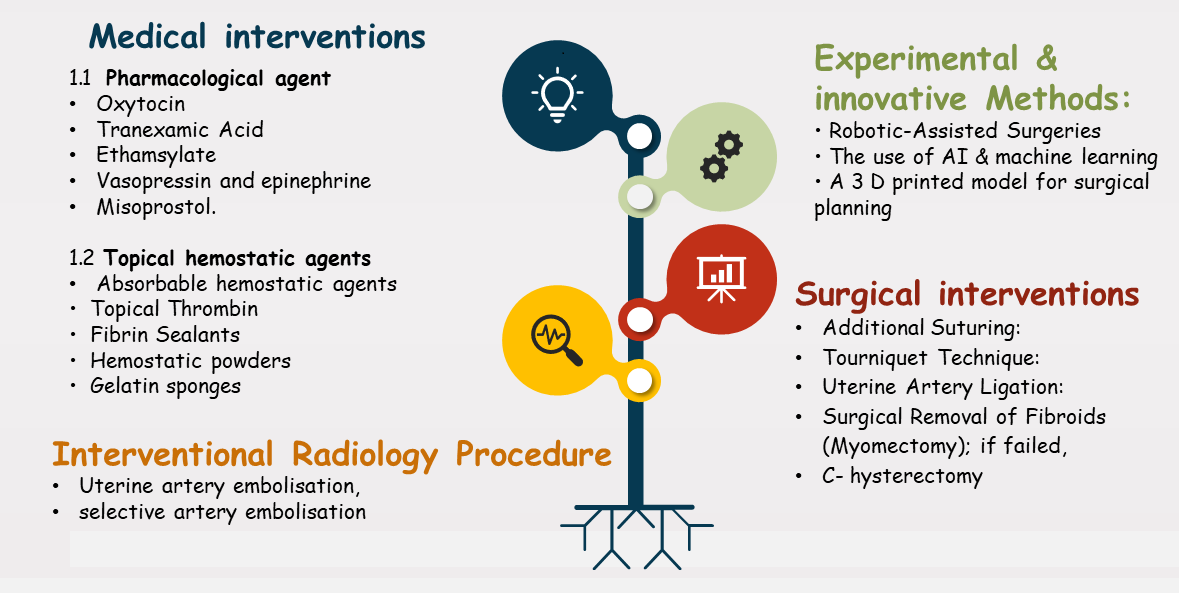 Fig. 1.
Fig. 1.
Suggested mechanisms to reduce intraoperative bleeding for fibroids. AI, artificial intelligence; 3D, three-dimensional.
Many of the pharmacological agents exist and have been used in practice, including:
Oxytocin is a hormone that promotes uterine contractions, which in turn helps to decrease bleeding [11].
Tranexamic acid is an antifibrinolytic drug that lowers bleeding by inhibiting the degradation of blood clots, hence minimizing bleeding, both before and after a C-section; it effectively reduces bleeding [12].
Ethamsylate is a hemostatic drug that is thought to improve the activity of platelets and stabilize blood vessels, resulting in less bleeding [12]. When combined with ethamsylate, the efficacy of oxytocin or tranexamic acid was equally effective in reducing intraoperative blood loss and hysterectomy risk. Nonetheless, tranexamic acid, when combined with ethamsylate, increases the risk of thrombotic accidents [13].
Vasopressin and epinephrine can be directly injected into the myoma to reduce blood loss. However, epinephrine has cardiovascular side effects, while vasopressin appears safe and may be repeated within 1 hour of its application [2, 14].
Misoprostol, a prostaglandin E1 analog, reduces intraoperative bleeding. However, it does not affect the need for intraoperative blood transfusion. According to a Kongnyuy meta-analysis, patients exhibited higher hemoglobin levels than the placebo group postoperatively.
The performance of various medical options is summarized in Table 1. based on the analysis of Kongnyuy [2].
| Intervention | Reduced blood (mean difference) | Effect on transfusion need | Evidence quality |
| Vaginal misoprostol | –97.88 mL (95% CI: –125.41 to –70.35) | No effect | Moderate |
| IV vasopressin | –245.87 mL (95% CI: –292.32 to –199.42) | OR: 0.15 (95% CI: 0.03–0.74) | Moderate |
| Pethidine + Epinephrine (Intravenously) | –243.00 mL (95% CI: –293.00 to –193.00) | No effect | Low |
| IV tranexamic acid | –243.00 mL (95% CI: –593.26 to –46.74) | No effect | Low |
| Gelatin-thrombin matrix | –411.16 mL (95% CI: –493.26 to –329.06) | OR: 0.01 (95% CI: 0.00–0.10) | Low |
| IV ascorbic acid | –411.16 mL (95% CI: –493.26 to –329.06) | OR: 0.17 (95% CI: 0.04–0.81) | Low |
| Vaginal dinoprostone | –265.50 mL | No effect | Low |
| Oxytocin | No effect | No effect | Low |
IV, intravenous; OR, odds ratio; CI, confidence interval.
Topical hemostatic agents: They are defined as substances or materials used to control or stop bleeding. These compounds or materials promote hemostasis by enhancing the body’s endogenous clotting mechanisms or directly facilitating blood clot production. Several hemostatic agents are administered intraoperatively to the bleeding site [15].
Absorbable hemostatic agents: They are such as gelatin sponges, can be directly administered to the bleeding site. They function by creating a framework for platelet adherence and the production of blood clots [16].
Topical thrombin: It is a crucial enzyme involved in blood clotting. They are obtained from either human or bovine sources. When immediately administered to the site of bleeding, it transforms fibrinogen into fibrin, resulting in the development of a blood clot [17].
Fibrin sealants: They are synthetic agents that mimic the natural clotting process. They consist of fibrinogen and thrombin, along with additional compounds that enhance clot formation. Upon application, these components combine to form a fibrin clot, thus attaining hemostasis [18].
Hemostatic powders: They consist of small particles or granules that offer a large surface area for platelet aggregation and clot formation [19]. They effectively reduce bleeding and achieve hemostasis with minimal postoperative adhesion [20]. Hemostatic powders are also recommended for oozing bleeding [21].
Gelatin sponges: These are highly effective hemostatic agents that are user-friendly and quickly absorbed; however, they are not suitable for controlling severe bleeding. They may increase infection risk, can elicit foreign body reactions, and are more expensive in comparison to alternative options [22].
In summary, medical options are used as first-line or adjunctive measures in low-resource settings or when the anticipated bleeding is not severe.
Surgical interventions to reduce intraoperative bleeding include the following options:
Additional suturing: If direct pressure is ineffective in reducing bleeding, additional suturing to the bleeding site may be considered [23].
Tourniquet technique: It temporarily occludes the uterine blood flow, followed by a period of relief to allow reperfusion. The technique permits the effective reduction of intraoperative blood loss. The occlusion site is chosen based on ease of access, as well as the degree to which complete blood flow control is achieved with simultaneous protection of ovarian perfusion [24, 25].
The technique can be single, where only the uterine vessels are occluded, or triple, where both uterine vessels are occluded, plus ovarian vessels [25].
Hangman’s tourniquet, described by Bahall et al. [26], achieved the best bleeding control; it involves per-cervical uterine artery and infundibular-pelvic (IP) ligament occlusion, occluding the blood supply to the ovaries. Their study recommended this method as an effective and safe way to reduce blood loss (the loss was less than 500 cc in over 90% of cases) and the need for transfusion (only 2% need transfusion), with a mean operating time of 45.3 min and reduce overall patient morbidity [26]. Baktiar et al. [25] reported two cases showing the efficacy of the tourniquet technique in myomectomy during C-section; blood loss was limited to 300 and 500 mL for fibroids measuring 12 and 15 cm. Neither case required blood transfusion, and no postpartum hemorrhage occurred, supporting the safety of the technique [25].
Uterine artery ligation (UAL): Ligating the uterine arteries to reduce
blood flow minimizes bleeding that often complicates big intramural fibroids and
reduces hemorrhage risk [27, 28]. Hiratsuka et al.’s case-control study [29]
analyzed 264 cases; the group where ligation was done showed reduced bleeding by
half with a longer operation time (
| Outcome measure | Sanders et al. (2019) [30] | Hiratsuka et al. (2022) [29] |
| Meta analysis | Case control study | |
| Mean intraoperative blood loss | by 103.7 mL (UAO vs. control) | from 158 |
| Hemoglobin drop | by 0.60 g/dL in the UAO group | Not reported |
| Transfusion requirement | by 7.2% absolute reduction | Not reported |
| Operative time | Slightly increased | from 160.1 |
| Hospital stay | Shorter in the UAO group | Not reported |
| Fibroid recurrence | Lower recurrence rates | Not reported |
| Association with massive bleeding | Not directly reported | UAL was negatively associated with bleeding |
UAO, uterine artery occlusion; UAL, uterine artery ligation.
Surgical removal of fibroids during C-section [cesarean-myomectomy]: Myomectomy has many subtypes, which are described and compared in Table 3 (Ref. [31]).
| Type | Definition | Clinical context |
| Cesarean myomectomy | Removal of fibroids during C-section | Controversial; increased hemorrhage risk. |
| Considered for subserosal/pedunculated fibroids; selectively safe in some series (e.g., Kanthi et al. [31]). | ||
| Abdominal myomectomy | Open (laparotomic) myomectomy outside of pregnancy | Elective procedure for symptomatic fibroids or infertility; widely practiced. |
| Laparoscopic myomectomy | Minimally invasive removal of fibroids via laparoscopy | Preferred for fewer/smaller fibroids; lower morbidity; requires surgical expertise. |
| Myomectomy during pregnancy | Removal of fibroids during ongoing pregnancy (not during C-section) | Rare; high-risk for pregnancy loss. Reserved for emergencies (e.g., fibroid torsion or intractable pain). |
C-section, cesarean section.
C-myomectomy remains risky and should be preserved for carefully selected cases and done by experienced surgeons [2]. When conventional measures fail to control intraoperative bleeding, fibroid removal becomes necessary. Historically, myomectomy was discouraged during the C-section and was postponed 3–6 months in a separate procedure setting to allow uterine involution and fibroid shrinkage; currently, many recommend performing both procedures concurrently [8]. The evidence shows mild complications, including hemoglobin drop and increased operation time, with the advantage of reducing cost and avoiding of second surgery [32]. Not all fibroids may be removed during the C-section; this decision must be weighed against multiple factors [8, 33]. This includes the surgeon’s experience, competence, and availability of hemostatic techniques, as well as fibroids’ number, size, location, and proximity to major vessels [34]. Patient selection and associated risk, such as uterine atony. Not all patients may be willing to accept these potential morbidities; therefore, shared decision-making discussing the advantages and disadvantages of concurrent surgery is essential, particularly for patients who have not yet completed their family [35, 36, 37].
Huang et al. [1] and Goyal et al. [32] compared the outcome of C-myomectomy versus C-section alone. Their findings are summarized in Table 4 (Ref. [1, 32]). Other studies discussed impaired wound-healing, higher odds for adhesions, and long-term implications for fertility [38]. A higher risk of abnormal placentation in future pregnancies, preterm deliveries, dehiscence of a scar, and risk of myoma recurrence was also reported [38, 39, 40]. Tinelli et al. [41] described myoma pseudocapsule occlusion, a surgical approach to reduce the bleeding during myomectomy; there was a reduction of 300 cc with a 95% confidence interval (CI): 354.8 to –255.19. Preserving the myoma pseudocapsule enhanced healing and improved fertility outcomes for women [41]. Tinelli et al.’s study [41] reported a favorable outcome with the use of intracapsular myomectomy; however, their study was conducted on non-pregnant women, and the capsule preservation did not reduce the risk of bleeding. Another study by Kanthi et al. [31] reported safe elective C-myomectomy in cases where there is no bleeding. Neither study involves cases with active obstetric hemorrhage, where the hemorrhage morbidity is high.
| Parameter | Huang et al. [1] (2022) | Goyal et al. [32] (2021) | Comment |
| Operative time | Significantly increased (p |
+14.7 minutes (mean increase) | Increase justified if avoiding secondary surgery |
| Hemoglobin drop | Significant (p = 0.007) | Mean drop: 0.27 g/dL | Statistically significant, but often clinically mild |
| Bleeding risk | Increased (p = 0.02) | Higher in fibroids |
Linked to fibroid size, number, and location |
| Blood transfusion | OR: 1.47 (95% CI: 1.09–1.99), p = 0.01 | OR: 1.45 higher in CM group | Risk varies; more common in previa or large/multiple fibroids |
| Postoperative fever | OR: 1.12 (not statistically significant) | Generally comparable between groups | No consistent increase across studies |
| Hospital stay | Significantly longer (p |
Slight increase (mean +0.35 days) | Not clinically significant in most studies |
| Hysterectomy rate | Not specified | 4.1% (51/1242 in CM group) | Often related to emergency bleeding or fibroid complexity |
| Use of hemostatic measures | Not specified | Vasopressin, UAL, tourniquets, oxytocin, electrocautery | Key to minimizing intraoperative bleeding |
CM, cesarean myomectomy.
The advantages of C-myomectomy include less surgery and lower costs, as there is no longer a need for a separate tumor removal surgery, resulting in less total surgery and financial stress [31]. Simultaneous treatment, performing both procedures simultaneously, alleviates patients’ symptoms [1, 6], and offers improved chances of conception as well as better outcomes in future pregnancies [2, 31].
Cesarean hysterectomy may be the last resort if all other techniques fail. In conclusion, surgical techniques are quite effective in controlling active bleeding, but they have greater operative risks. C-myomectomy is beneficial, especially in the hands of skilled surgeons in selected cases. Good pre-surgical planning and patient counseling are crucial elements to optimize the outcome.
They include uterine artery embolization (UAE) and selective artery embolization. The estimated success rate for blocking uterine artery blood flow is high, up to 100%, and clinical success is up to 96%. The procedure has a favorable safety profile, and associated complications are well tolerated, including post-embolization syndrome, which is resolved within one week [42, 43]. While this procedure is mainly done preoperatively, it may be done intraoperatively in some instances where the bleeding cannot be controlled. It carries the risk of reduced fertility potential for the patient, although the evidence is conflicting [43]. Gupta et al.’s analysis [43] shows that fertility potential is reduced among UAE-treated cases. Their study examined 7 randomized controlled trials (RCTs) with 793 participants, comparing UAE with medical and surgical interventions; the results are summarized in Table 5 (Ref. [43]).
| Parameter | Effect (UAE vs. Surgery) | Statistic/OR |
| Intra-procedural complications | No significant difference | OR: 0.91 (95% CI: 0.42–1.97) |
| Major complications (1 Year) | No significant difference | OR: 0.65 (95% CI: 0.33–1.26) |
| Major complications (5 Years) | No significant difference | OR: 0.56 (95% CI: 0.27–1.18) |
| Minor complications (1 Year) | Higher in UAE group | OR: 1.99 (95% CI: 1.41–2.81) |
| Need for blood transfusion | Lower in UAE | OR: 0.07 (95% CI: 0.01–0.52) |
| Hospital stay and recovery | UAE leads to faster recovery and shorter hospital stay | Consistently observed; no pooled OR due to heterogeneity |
| Fertility-live birth | Lower in UAE | OR: 0.26 (95% CI: 0.08–0.84) |
| Fertility-pregnancy | Lower in UAE | OR: 0.29 (95% CI: 0.10–0.85) |
| Re-intervention rate (2 years) | Higher in the UAE (15–32% vs. 7% surgery) | OR: 3.72 (95% CI: 2.28–6.04) |
| Summary | UAE has fewer perioperative risks but higher long-term and re-intervention rates, as well as reduced fertility vs. surgery | |
UAE, uterine artery embolization.
The study by Kohi and Spies [44] reported no significant impact on ovarian reserve or ovarian function over 36 months of follow-up, whereas other studies reported higher odds of primary ovarian failure post UAE, especially when performed during the postpartum period [45]. There is inconsistent evidence regarding fertility potential following the procedures; some reported no adverse effects and achieved spontaneous pregnancies, while others suffered higher rates of abortions and preterm labor compared to surgical myomectomy [44]. Recent evidence by Chatani et al. (2024) [42] raises significant concerns regarding future fertility and pregnancy outcomes. Their study followed cases of those who received UAE, whether following delivery or abortion, and showed significant adverse outcomes. These included higher odds of recurrent hemorrhage, preterm labor, and abnormal placentation. Their findings imply that despite the immediate efficacy in controlling bleeding, it has profound long-term reproductive consequences, which require careful patient selection and counseling when opting for UAE [42]. Therefore, the UAE may be unsuitable for younger ages or those with future reproductive desires. Their use should be preserved for refractory cases when all other measures have failed. It was estimated that 15–33% of treated cases would require additional surgery within 24 months, with one-third of these ultimately undergoing hysterectomies within 10 years [46].
This is a minimally invasive surgical approach that offers reduced risk of intraoperative bleeding due to the use of vasopressin. Additionally, it has a lower risk of hysterectomies, making it an attractive option for younger patients. Nevertheless, their use is hindered by the high cost of robots and technical constraints, as they are not suitable for all fibroid types [47].
While pre-pregnancy myomectomy performed using robotic surgery is well established and associated with favorable fertility odds (70%–85%) and a live birth of 70%–72% [48], evidence regarding its safety during pregnancy remains scarce. There is only one case report for robotic surgery successfully done for a pregnant woman, resulting in an uneventful term delivery via C-section [49]. Although selected cases showed promising outcomes, the emerging data indicate that myomectomy, regardless of the approach used during or before pregnancy, increases preterm delivery and postpartum hemorrhage. It is safe to say that while robotic-assisted myomectomy offers a surgical advantage, its use in women planning for future pregnancy should be weighed against potential obstetrical risks.
Predicting the risk of bleeding during a C-section is an evolving area of research with significant potential to enhance real-time decision-making and predict complications. The predictive value for these methods in general surgeries shows substantial accuracy [50, 51].
However, their accuracy remains limited in C-sections due to external validation issues and a high risk of bias. One of the developed models that shows optimistic results is the model for a second C-section. It can predict the risk of postpartum hemorrhage based on operative timing and placenta previa [52, 53]. Chen et al.’s [54] discussed that fibroids of less than 7 cm responded well to AI-assisted hysteroscopic myomectomy with a significant reduction of intraoperative blood loss, thus emphasizing the role of perioperative planning in improved patient outcomes; their results and related studies are described in Table 6 (Ref. [54, 55, 56]).
| Application | Study details | Operative time | Intraoperative blood loss | Ref |
| AI-assisted MRI segmentation | 120 patients; laparoscopic myomectomy of broad ligament fibroids | 118 min (IQR 112–125) vs. 140 min (IQR 116–161); p |
50 mL (range 50–100) vs. 85 mL (range 50–100); p = 0.01 | [54] |
| AI MRI in hysteroscopic myomectomy | 56 patients with submucosal fibroids | 32.11 |
Median 10 mL (AI) vs. 10 mL (control); ranges: 5–15 mL vs. 6.25–15 mL; p = 0.04 | [55] |
| ML risk prediction: surgical bleeding | A retrospective study reviewed records of N = 9728 and assessed multiple models for bleeding risk prediction in laparoscopy. | – | Bleeding risk models show high predictive accuracy, with AUC 0.933 (87% sensitivity, 85% specificity). | [56] |
ML, machine learning; MRI, magnetic resonance imaging; IQR, interquartile range; AUC, area under the curve.
These models are physical, patient-specific replicas of the pregnant pelvis, uterus, and growing fetus. These are created using advanced imaging techniques such as CT or MRI scans. Through 3D print technology, 3D physical models are generated by converting digital imaging data into tangible structures made from resin or polymers [57, 58]. The information provided by these models offers detailed anatomical references, allowing an early glance at potential complications for the operating surgeon. These models help improve preoperative planning by adjusting hemostatic techniques and anticipating potential blood loss. They enhance surgical precision by improving the understanding of surgical complexity, boosting confidence in surgical plans, and reducing expected procedural difficulty [57, 58, 59, 60]; see Table 7 (Ref. [57, 58, 59]) for detailed results.
| Characteristic | Benefit | Ref |
| Time savings | Up to 50 minutes shorter planning and surgery | [57] |
| Bleeding reduction | About 100–120 mL less blood loss is expected | [58] |
| Enhances decision-making | Surgeons change strategy in |
[57] |
| Confidence boost | High self-reported scores (≈8/10) | [58] |
| Operation alteration | In 60% of cases, the model changed surgical approach | [59] |
3D, three-dimensional.
In summary, these innovative techniques hold promise for minimizing invasive procedures and improving outcomes. However, their use is hindered by high cost, limited availability, and validation gaps. They are best suited for specialized centers and need further validation before routine implementation in clinical practice.
Medical strategies are typically employed as the first-line approach to reduce intraoperative bleeding due to their ease of administration, safety profile, and accessibility. Local hemostatic agents offer targeted control; however, their high cost and increased risk of postoperative infection limit their use. The efficacy of medical interventions significantly varies across agents, and combinations of these agents have demonstrated enhanced effectiveness, albeit with a concomitant increase in thrombotic risk. They are used as primary or adjunctive measures, especially in low-resource settings or when anticipated blood loss is minimal to moderate [2, 15].
C-myomectomy is increasingly accepted for small, pedunculated, or subserosal fibroids in selected cases with well-prepared surgical settings. Postponing the myomectomy for 3–6 months is generally preferable for large intramurally and those with extensive adhesion. The rationale is to allow uterine involution, thereby reducing vascularity and enabling safer surgery. Optimizing surgical outcomes relies on tailored, patient-centered planning based on fibroid type, individual risk factor, and available surgical resources [8, 61].
The tourniquet technique is easily applicable and significantly reduces blood loss (approximately 250 mL vs. 2000 mL in cases without tourniquet use). Additionally, it reduces the need for blood transfusion, which drops to 2.5%–7% with tourniquet use compared to 79% in controls. It is especially effective in cases with multiple fibroids. The choice of tourniquet placement depends on the ease of access and the ability to achieve complete blood flow control while simultaneous preserving ovarian blood flow [26].
Studies have shown that maximal devascularization can be achieved by combining uterine-only ligation with the tourniquet technique; however, this approach requires surgical expertise, may obscure the surgical field if excessively tightened, and its success can vary depending on operator experience. Rare cases of adjacent organ injury and ischemic damage have been reported when the tourniquet was applied for prolonged periods. No long-term effects on fertility have been demonstrated [62].
UAL requires surgical skills, is typically performed in specialized centers, and may be complicated by injury to adjacent structures, hematoma, or infection, and may inversely impact future fertility [30].
Radiological approaches, such as UAE, offer a minimally invasive approach that significantly reduces fibroid size and bleeding. However, they are associated with post-embolization syndrome that includes fever and pelvic pain, as well as risks of infection and ovarian failure, with the latter reported to increase by 15% in older patients. Selective arterial embolization offers a more favorable safety profile and less impact on fertility, but it is technically more demanding [44, 62]. Several interventions have been proposed for hemorrhage control from fibroids during C-section, each with its advantages, efficacy, limitations, and potential impacts on future fertility (see Table 8).
| Category | Medical | Surgical | Interventional radiology | Experimental and innovative |
| Key advantages | Easy to administer; low cost, moderate efficacy in reducing blood loss (≈230 mL) | Immediate flow control; proven effectiveness in major bleeding | Rescue in refractory bleeding targeted vascular control | Those techniques hold promise to minimize invasive procedures and help minimize invasive procedures and improve outcomes |
| Key limitations | Limited efficacy alone has side effects | Requires surgical skill; risk of ischemia if prolonged | Not universally available; may reduce future fertility | Costly, limited availability, training needed |
| Impact on fertility | Preserved (minimal risk) | Low to moderate risk (dependent on method) | High-risk is considered only in non-fertility-desiring patients | Minimal risk (technique-dependent) |
| Estimated efficacy star rating | Good | Good | Variable | Variable |
The choice of intervention should be personalized based on specific factors, such as fibroid size, location, number, and the presence of adhesions. Importantly, the use of appropriate hemostatic techniques by experienced surgeons can lead to favorable outcomes, irrespective of fibroid size or location.
While narrative reviews offer a broad overview of the available interventions, they lack the methodological rigor of systematic reviews and the quantitative synthesis of meta-analyses. The absence of standardized outcome measures and the considerable heterogeneity among included studies further constrain the generalizability of their conclusions. Notably, none of the reviewed studies comprehensively addressed the long-term impacts of these interventions, especially in relation to fertility preservation. This consideration is critical when counseling women who seek fertility-sparing options and should be addressed in future studies. Additionally, some of the blood loss control methods discussed in this review are primarily applicable to non-pregnant surgical and are unsuitable for C-myomectomy, limiting their generalizability in an obstetric setting
This review examined diverse medical, surgical, innovative, and experimental interventions aimed at reducing intraoperative bleeding from fibroids during the C-section. By encompassing a wide range of interventions, this review offers a comprehensive perspective to guide clinical decision-making and support tailored patient care. Furthermore, it addresses practical considerations relevant to healthcare professionals, from limited-resource environments to advanced surgical infrastructure, to enhance its applicability and value for a global audience.
Uterine fibroids during pregnancy are often asymptomatic and may not affect gestation, allowing for a conservative management approach in many cases. However, intraoperative bleeding from a large fibroid is common and can be challenging. Effective management requires a multidisciplinary approach integrating perioperative planning, intraoperative hemostasis, and postoperative monitoring to achieve a successful outcome. Multiple strategies to reduce intraoperative bleeding can be tailored on the clinical scenario, including medical, surgical, interventional radiology, and innovative approaches. Advancements in technology, especially the use of AI and ML to predict complications such as intraoperative bleeding, warrant further evaluation to guide surgical decision-making and patient outcomes. Further research is needed to develop standardized protocols and validate experimental strategies to be incorporated into clinical practice to improve maternal safety.
WN, ZRH: conception, design, and literature review. RMM, EAM, and MAT: analysis, visualization, and validation. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We gratefully acknowledge the assistance and instruction from Professor Wisam Akram for his support during this work.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
