- Academic Editor
†These authors contributed equally.
Macrosomia is a significant perinatal complication with potential risks for both mother and child. Although diabetes is a known major risk factor, specific clinical and metabolic factors contributing to macrosomia in non-diabetic pregnancies are not fully understood. Therefore, this study aimed to explore the clinical characteristics and potential metabolic risk factors associated with non-diabetes-related neonatal macrosomia. Additionally, this study aimed to examine the relationship between metabolic dysregulation and the presence of exosomes in umbilical cord blood.
A retrospective analysis of 356 non-diabetic pregnant women (170 non-diabetic pregnant women with macrosomic infants and 186 normal pregnant women) was conducted. Additionally, umbilical cord blood plasma samples were collected from 16 participants (8 macrosomia and 8 normal deliveries). After separating exosomes from plasma, RNA was extracted and sequenced. Weighted gene co-expression network analysis (WGCNA) was used to explore the correlation between clinical characteristics and gene expression.
Among the baseline characteristics, the pre-pregnancy body mass index (BMI) and overall weight gain in non-diabetic mothers with macrosomic infants were significantly higher than those in the normal group (p < 0.05). The lipid profiles revealed that triglyceride (TG) and low-density lipoprotein (LDL) levels were significantly elevated, whereas the high-density lipoprotein (HDL) levels were significantly decreased (p < 0.05). Logistic regression analysis showed that pre-pregnancy BMI, gestational weight gain, LDL levels, and alkaline phosphatase (ALP) levels in the third trimester were risk factors for macrosomia, while primiparas and HDL levels were protective factors. WGCNA analysis revealed that the expression of the mRNA royalblue module and the lncRNA darkgrey module presented a significant positive correlation with gestational weight gain (p < 0.05). Compared to the normal group, the expressions of transmembrane protein 175 (TMEM175) and HIF1A antisense RNA 2 (HIF1A-AS2) were downregulated, whereas the expressions of phosphoglycerate kinase 1 (PGK1) and methionine adenosyltransferase 2B (MAT2B) were upregulated in the exosomes derived from the umbilical cord blood plasma in the macrosomic group.
Messenger RNAs (mRNA) (TMEM175, PGK1, MAT2B) and long non-coding RNAs (lncRNA) (HIF1A-AS2) may potentially contribute to the development of fetal macrosomia in non-diabetic pregnancies.
Macrosomia is a perinatal complication characterized by the birth of a full-term infant weighing 4000 grams or more. This condition is associated with increased risk of complications during delivery for both mother and child, and its prevalence varies across different countries. Maternal obesity and diabetes, including pre-gestational and gestational diabetes, are significant risk factors [1, 2]. Studies have shown that the etiology of macrosomia includes both non-modifiable factors, such as genetics, fetal sex, parity, and maternal age, as well as modifiable factors, including maternal nutrition intake, pre-pregnancy body mass index (BMI), and gestational weight gain [3, 4, 5]. Additionally, the physiological and pathological mechanisms of macrosomia are related to the excessive supply of nutrients by the mother to the fetus and/or the inability of the fetus to regulate the metabolism of nutrients effectively or efficiently [6]. In a study involving 115,097 singleton live births, the prevalence of macrosomia among women with gestational diabetes mellitus (GDM) decreased between 2012 and 2021. However, the incidence of macrosomia in women with GDM remained significantly higher than that in non-GDM women. Furthermore, the study found no significant decline in the incidence of macrosomia in non-GDM women during the same period [7]. Macrosomia is also known to increase the risk of adverse maternal outcomes and long-term health problems in the newborn [8, 9]. Although there are various interventions for the treatment of diabetic macrosomia, the accurate prenatal prediction and treatment of macrosomia in non-diabetic pregnancies remain a significant hurdle, as the diagnosis is often only confirmed retrospectively [10]. Currently, macrosomia in mothers with GDM is relatively well-researched. However, there is a significant knowledge gap concerning macrosomia in non-diabetic mothers, necessitating more research to clarify the possible etiology and mechanisms [11].
Exosomes are transporters of diverse bioactive molecules, including RNAs [such as long non-coding RNAs (lncRNAs) and messenger RNAs (mRNAs)], DNAs, and proteins that facilitate cell-to-cell communications and the exchange of substances and information between the mother and the fetus [12, 13, 14, 15]. As a major organ during pregnancy, the placenta secretes exosomes into the maternal circulation as early as 6 weeks of gestation [16]. Changes in the release of exosomes, their composition, and bioactivity, along with altered patterns of exosomal non-coding RNAs, particularly microRNAs and lncRNAs, can lead to several pregnancy-related complications [17, 18]. Yuan et al. [19] discovered that exosomal RNAs (including mRNAs, lncRNAs, and circRNAs) were expressed at abnormal levels in the umbilical cord blood of patients with GDM-related macrosomia. The altered expression patterns of these exosomal RNAs suggest that they may participate as biomarkers in predicting macrosomia in patients with GDM. The study evaluated the predictive performance of the individual exosomal RNAs, and they determined that growth differentiation factor 3 (GDF3) demonstrated good predictive performance with an area under the curve (AUC) of 0.78. In another study, lncRNAs were also found to be differentially expressed in umbilical cord blood exosomes of patients with pregnancy complications compared to healthy ones [20]. Lu et al. [21] also reported that lncRNAs may participate in the pathogenesis of placental development and may influence fetal growth. They found that lncRNA ubiquitin specific peptidase 2 antisense RNA 1 (USP2AS1) was involved in the pathogenesis of nondiabetic fetal macrosomia by regulating cell function. Furthermore, Ren et al. [22] reported that lncRNA H19 was significantly correlated with the birth weight of fetuses with intrauterine hyperglycemia. While research has shown an association between exosomal RNAs and macrosomia in women with GDM, the role of these RNAs in non-GDM pregnancies needs further investigation. Therefore, addressing the current research challenges will be crucial to leveraging the full potential of exosomal RNA biomarkers in optimizing clinical decision-making and improving the outcomes of both mother and baby in non-GDM-related macrosomia.
Weighted gene co-expression network analysis (WGCNA) is an R package that constructs and analyzes gene co-expression networks. It identifies groups of genes with similar expression patterns (modules) and assesses their relationship to biological processes or phenotypes. Relevant networks facilitate network-based gene screening methods to determine genes associated with specific phenotypes, potentially identifying biomarkers or therapeutic targets [23]. Therefore, we can combine the gene expression and clinical indexes to explore the intrinsic molecular mechanism of macrosomia. Currently, research on the risk factors for macrosomia in non-diabetic pregnancies and their association with exosomal RNA is relatively limited. This study aims to investigate the maternal clinical risk factors related to neonatal macrosomia in non-diabetic mothers and to identify key regulatory factors for its occurrence using the WGCNA method.
A retrospective study was conducted involving pregnant women who received
prenatal examinations and gave birth in the Department of Obstetrics, Maternity
and Child Health Care of Guangxi Zhuang Autonomous Region, from January 1, 2016,
to September 30, 2019. This study was designed and reported in strict accordance
with the strengthening the reporting of observational studies in epidemiology
(STROBE) checklist. Utilizing the hospital’s electronic medical record system,
two participant groups were established: the non-diabetic macrosomia group (n =
170), which included pregnant women whose newborns had a birth weight
During the third stage of labor, 10 mL of umbilical venous blood was collected
from non-diabetic pregnant women and transferred into dipotassium
ethylenediaminetetraacetic acid (K2EDTA) vacuum blood collection tubes
(BH1359, Bioroyee, Beijing, China), each. All blood samples were
centrifuged at 1000
To eliminate cellular debris and large vesicles, 2 mL of plasma was first
centrifuged at 2000
The microstructure of the exosome was observed using a JEM-1200EX transmission electron microscope (TEM) (JEOL Ltd., Tokyo, Japan). The observation was carried out using an accelerating voltage ranging from 40–120 kV. Image data were acquired and exported. The particle size distribution and concentration of exosomes were measured via nanoparticle tracking analysis (NTA) (Nanosight, Wiltshire, UK). Each sample was analyzed in triplicate, and the mean particle size, modal particle size, and particle size distribution were calculated using NTA software (v3.2, Malvern Panalytical Ltd., Malvern, UK).
PBS (100 µL) was added to the resuspended exosomes, which were then incubated with 10 µL Fluorescein Isothiocyanate (FITC)-conjugated anti-CD63 antibody (557288, BD Biosciences, San Jose, CA, USA) or FITC-conjugated anti-CD81 antibody (551108, BD Biosciences, San Jose, CA, USA). Flow cytometry analysis was performed using an Accuri C6 flow cytometer (AccuriC6, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions.
Plasma samples (3 mL) were extracted from umbilical venous blood using the Qiagen exoRNeasy/Plasma Midi Kit (77044, Qiagen, Hilden, North Rhine-Westphalia, Germany) following the manufacturer’s instructions. RNA sequencing was conducted by LC-Bio Technologies Co., Ltd. (Hangzhou, Zhejiang, China) on the Illumina HiSeq 2500 platform. Total RNA extracted from the samples was utilized to construct strand-specific whole-transcriptome libraries with the SMARTer® Stranded Total RNA-Seq Kit v2 - Pico Input Mammalian (Takara Bio USA, San Jose, CA, USA). After verifying the sequencing libraries met the required standards through quality control, paired-end 150-bp (PE150) sequencing was performed. Raw sequencing data were initially processed using Cutadapt (v3.4, Marcel Martin, Stockholm, Sweden, https://cutadapt.readthedocs.io/) to remove adapter sequences introduced during library preparation and filter out low-quality reads, obtaining high-quality valid data. These clean reads were then aligned to the human reference genome GRCh38 using Hisat2 (v2.2.1, Johns Hopkins University, Baltimore, MD, USA, https://daehwankimlab.github.io/hisat2/). Subsequently, transcript-level expression profiles for mRNA and lncRNA were generated based on the corresponding genome annotation file (general transfer format, GTF).
The Batch effect was eliminated using the R package limma (https://bioconductor.org/packages/limma/). Co-expression
networks of mRNA and lncRNA were constructed with the WGCNA package (v1.47,
https://cran.r-project.org/web/packages/WGCNA/) in R (v4.4.3, Foundation for
Statistical Computing, Vienna, Austria). The WGCNA analysis workflow strictly
followed the methodology described by Huang et al. [24], which
encompasses critical steps for the selection of soft-thresholding parameters,
module identification, and screening of key genes. Specifically, the
pickSoftThreshold function was employed to determine the appropriate soft
threshold parameter (
Statistical analyses were performed using SPSS software (v25.0, IBM Corp.,
Armonk, NY, USA). The Shapiro-Wilk test was employed to evaluate the normality of
continuous variables. Continuous variables with a normal distribution were
presented as mean
Maternal age (p
| Patient characteristics | Macrosomia (n = 170) | Normal (n = 186) | p | |
| Maternal age (years) | 31.27 |
29.59 |
||
| Ethnicity | 0.904b | |||
| Han | 109.00 (64.12) | 115.00 (61.83) | ||
| Zhuang | 54.00 (31.76) | 63.00 (33.87) | ||
| Others | 7.00 (4.12) | 8.00 (4.30) | ||
| Blood pressure (mmHg) | ||||
| SBP | 114.91 |
116.48 |
0.154a | |
| DBP | 73.00 (66.00–78.00) | 75.00 (69.00–81.00) | 0.012c | |
| Primiparous | 0.008d | |||
| Yes | 52.00 (30.59) | 83.00 (44.62) | ||
| No | 118.00 (69.41) | 103.00 (55.38) | ||
| Mode of delivery | ||||
| Vaginal | 76.00 (44.71) | 165.00 (88.71) | ||
| Caesarean section | 94.00 (55.29) | 21.00 (11.29) | ||
| Height (cm) | 160.18 |
159.78 |
0.330a | |
| Pre-pregnancy BMI (kg/m2) | 22.91 |
20.86 |
||
| Pre-pregnancy BMI categories (kg/m2) | ||||
| Underweight ( |
12.00 (7.06) | 42.00 (22.58) | ||
| Normal (18.5–24.9) | 116.00 (68.24) | 129.00 (69.35) | ||
| Overweight (25–29.9) | 37.00 (21.76) | 14.00 (7.53) | ||
| Obese ( |
5.00 (2.94) | 1.00 (0.54) | ||
| Overall weight gain (kg) | 15.36 |
13.70 |
||
| Weight gain | ||||
| First trimester | 0.00 (–0.53 to 1.00) | 0.00 (–1.00 to 1.00) | 0.199c | |
| Second trimester | 9.00 (7.50–11.00) | 8.50 (7.00–10.00) | 0.006c | |
| Last trimester | 6.00 (4.00–7.80) | 5.35 (4.00–6.50) | 0.009c | |
| TC (mmol/L) | 5.53 |
5.46 |
0.748a | |
| TG (mmol/L) | 2.69 |
2.06 |
||
| HDL (mmol/L) | 1.79 |
1.90 |
0.004a | |
| LDL (mmol/L) | 3.03 |
2.75 |
0.002a | |
| ALP (U/L) | ||||
| 12 weeks | 50.78 |
48.96 |
0.500a | |
| 32 weeks | 164.82 |
149.08 |
0.006a | |
a, t-test; b, Fisher’s Exact Test; c, Mann-Whitney U test; d, Chi-square test. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ALP, alkaline phosphatase.
| Macrosomia (n = 170) | Normal (n = 186) | p | ||
| Neonatal gender | ||||
| Male | 125.00 (73.53) | 96.00 (51.61) | ||
| Female | 45.00 (26.47) | 90.00 (48.39) | ||
| Neonatal | ||||
| Birth weight (kg) | 4.14 |
3.19 |
||
| Birth length (cm) | 53.22 |
50.29 |
||
| Chest circumference (cm) | 36.20 |
33.06 |
||
| Head circumference (cm) | 35.49 |
32.96 |
||
| Placenta | ||||
| Length (cm) | 22.39 |
20.02 |
||
| Breadth (cm) | 20.81 |
18.55 |
||
| Thick (cm) | 2.57 |
2.38 |
||
| Weight (kg) | 0.77 |
0.60 |
||
a, Chi-square test; b, t-test.
This study utilized logistic regression analysis to identify factors
independently associated with macrosomia in non-diabetic mothers. The chosen
method of delivery was excluded from the analysis as it was based on fetal weight
estimation. Although DBP showed a statistically significant difference in univariate
analysis (p
| Variables | Adjust OR (95% CI) | p | |
| Maternal age (years) | 1.068 (0.999–1.142) | 0.054 | |
| Pre-pregnancy BMI (kg/m2) | 1.235 (1.124–1.358) | ||
| Primiparous | 0.524 (0.287–0.954) | 0.035 | |
| Overall weight gain (kg) | 1.330 (1.177–1.503) | ||
| Weight gain (kg) | |||
| Second trimester | 1.218 (1.004–1.477) | 0.046 | |
| Last trimester | 1.189 (1.008–1.402) | 0.039 | |
| TG (mmol/L) | 1.204 (0.967–1.499) | 0.097 | |
| HDL (mmol/L) | 0.180 (0.080–0.407) | ||
| LDL (mmol/L) | 1.827 (1.298–2.572) | 0.001 | |
| 32 weeks ALP (U/L) | 1.007 (1.003–1.012) | 0.002 | |
OR, odds ratio; CI, confidence interval.
Exosomes were successfully extracted from the umbilical venous blood. First, we analyzed the size and shape of the exosomes isolated from plasma (Fig. 1A). Next, we recorded the median size of the vesicles, the majority of which were between 60 nm and 100 nm (Fig. 1B). Flow cytometry analysis confirmed that the isolated vesicles were exosomes, as indicated by the specific biomarkers CD63 and CD81 (Fig. 1C).
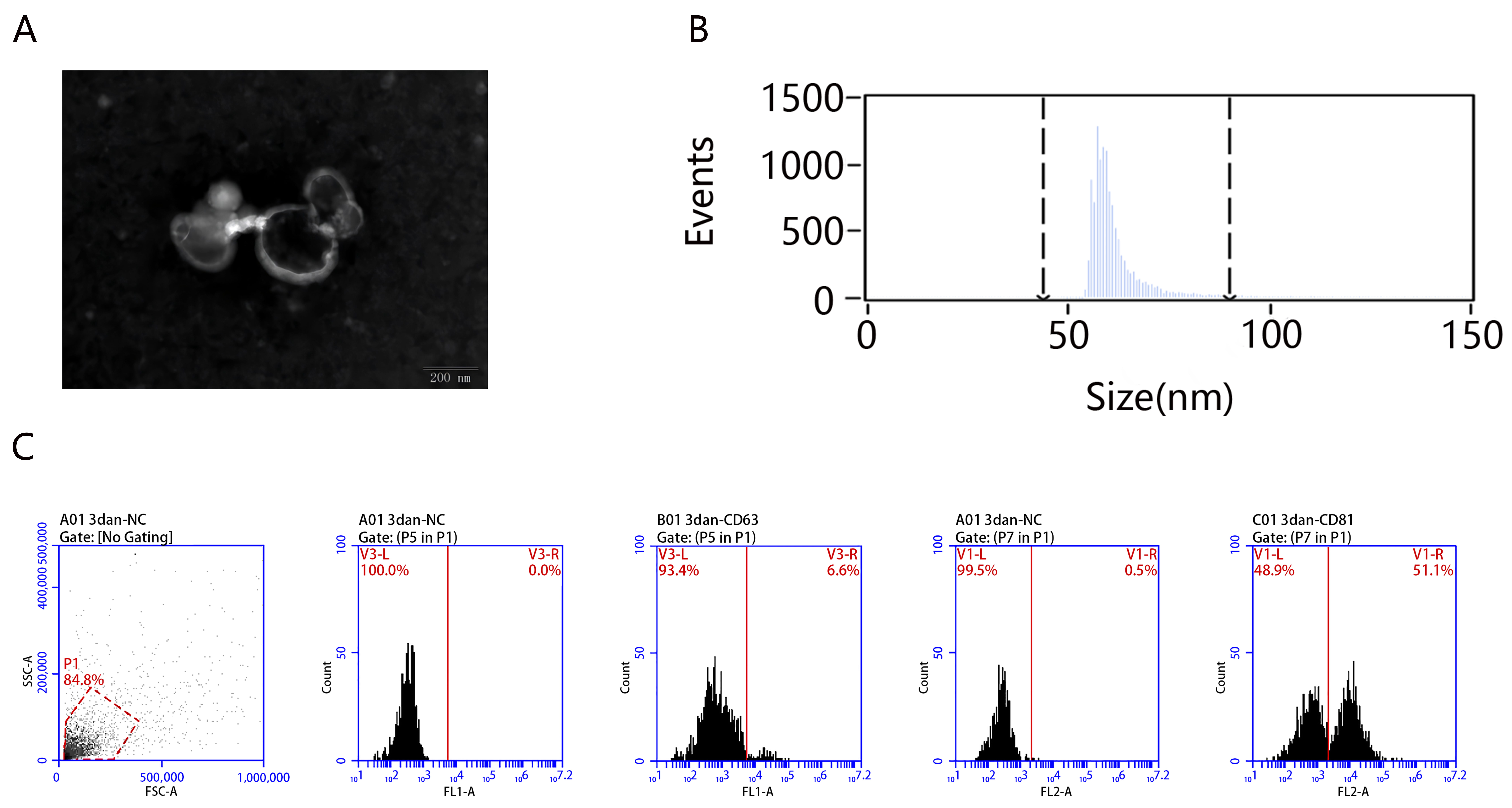 Fig. 1.
Fig. 1.
Characterization of isolated exosomes. A representative TEM image of isolated exosomes. (A) Exosomes ranged in size from 20–80 nm (scale bar: 200 nm). (B) Exosomes isolated from the plasma derived from the cord blood of pregnant women with macrosomic neonates were evaluated by NanoSight. The plot shows a broad size distribution. (C) Flow cytometry of exosomes expressing CD63 and CD81. TEM, transmission electron microscope; FSC-A, Forward Scatter-Area; FL1-A, FLuorescence 1-Area.
We eliminated batch effects in two batches of the sequenced mRNA and lncRNA data
using the R limma package. The datasets, containing 19,349 mRNAs and 9625
lncRNAs, were selected for analysis after removing the missing values of gene
expression from the raw data, and the WGCNA package was filtered. Firstly, Soft
threshold power values were selected in the mRNA and lncRNA groups. When
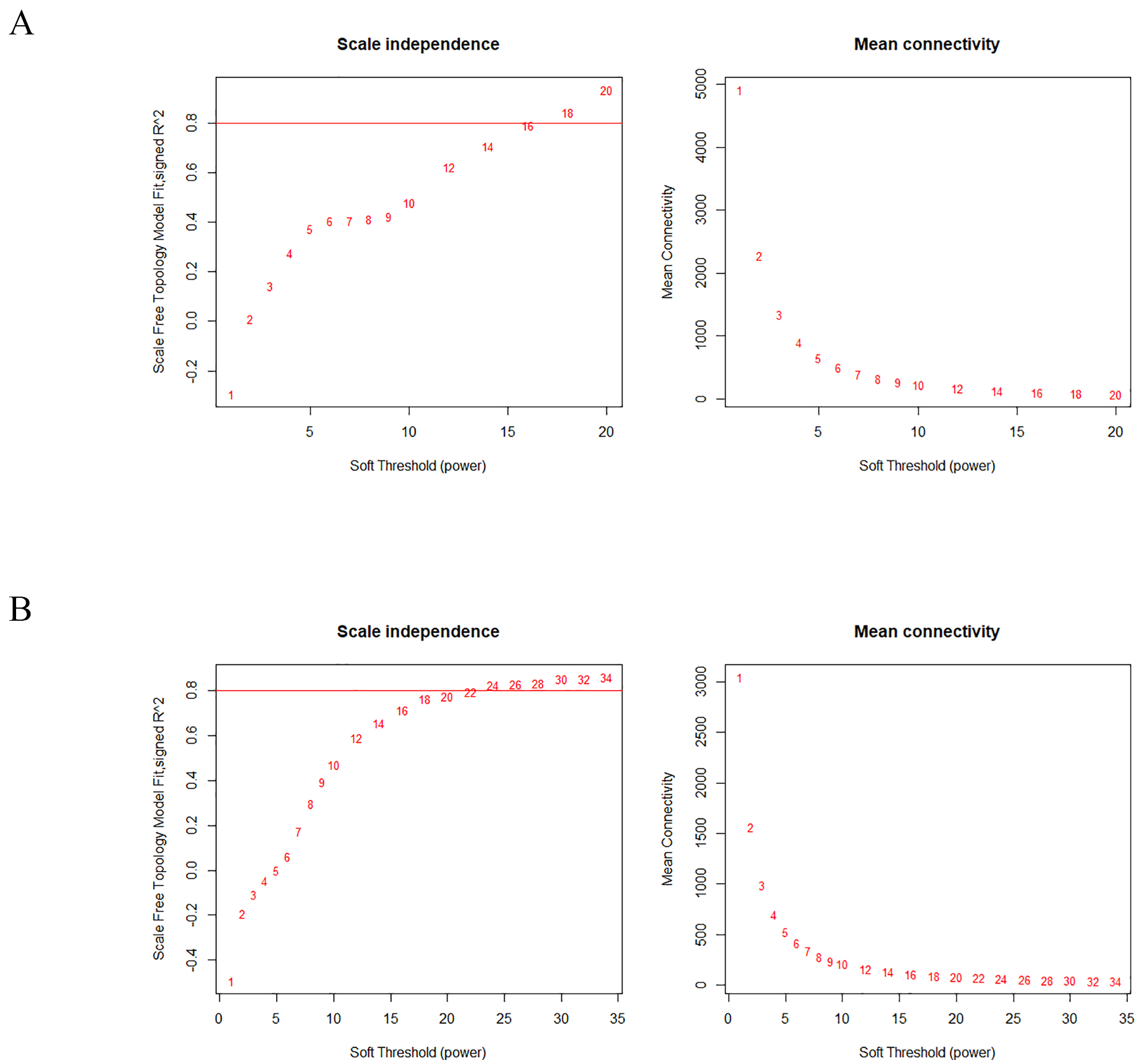 Fig. 2.
Fig. 2.
Analysis of network topology of various soft-thresholding powers. mRNA (A) and lncRNA (B) power value filter. The left panel shows the scale-free fit index (y-axis) as a function of the soft-thresholding power (x-axis). The right panel displays the mean connectivity (degree, y-axis) as a function of the soft-thresholding power (x-axis). mRNA, messenger RNAs; lncRNA, long non-coding RNAs.
This study performed correlation analysis between the eigengene patterns within the co-expression modules and the clinical phenotype dataset (including continuous variables such as maternal age, pre-pregnancy BMI, overall weight gain, weight gain during first/second/last trimesters, and neonatal weight) (Fig. 3A,B). Key modules were identified by calculating the correlation coefficient “r” and p-value between module eigengenes and clinical traits. The mRNA module-trait relationship heatmap (Fig. 3A) revealed that lightyellow module was significantly positively correlated with pre-pregnancy BMI (“r” = 0.56, p = 0.020), green module was significantly positively correlated with weight gain during first trimester (“r” = 0.57, p = 0.020), and royalblue module exhibited a significant positive correlation with overall weight gain (“r” = 0.65, p = 0.006). The lncRNA module-trait relationship heatmap (Fig. 3B) showed that the darkgrey module was significantly correlated with weight gain during the first trimester (“r” = 0.60, p = 0.009). Based on the “r” and p values, the mRNA royalblue module and lncRNA darkgrey module, which have the highest correlation with clinical phenotypes, were selected as key modules for further investigation into their association with macrosomia occurrence in non-diabetic pregnancies.
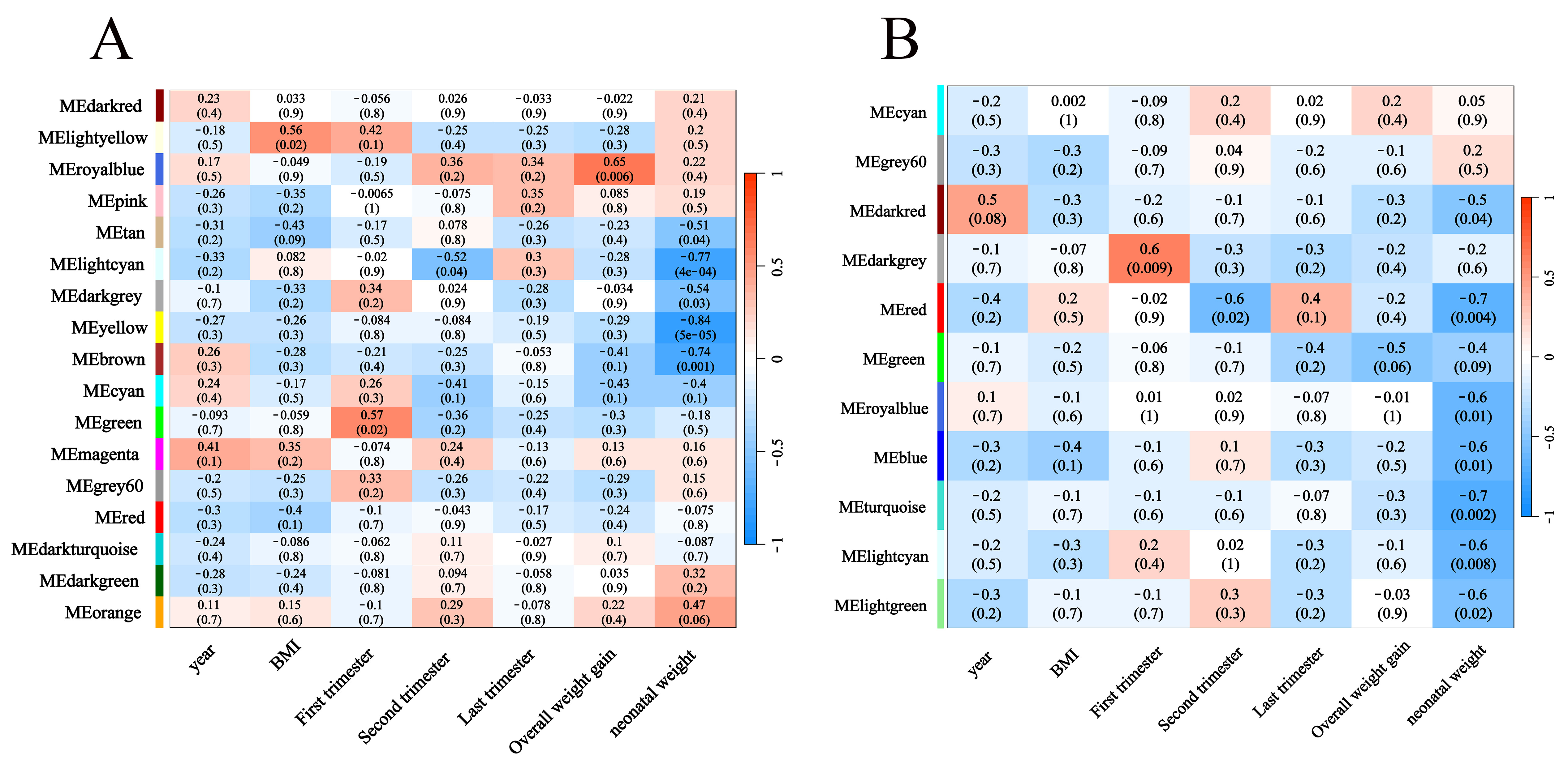 Fig. 3.
Fig. 3.
Module-trait associations for mRNA (A) and lncRNA (B). Each row corresponds to a module eigengene, and the columns correspond to clinical indexes. Red indicates a positive correlation, and blue indicates a negative correlation. Each cell contains the corresponding correlation and p-value.
The analysis of KEGG pathways revealed various important signaling pathways corresponding to the clinical traits, including metabolic pathways such as the oxytocin signaling pathway, aldosterone synthesis and secretion, and cortisol synthesis and secretion (Fig. 4).
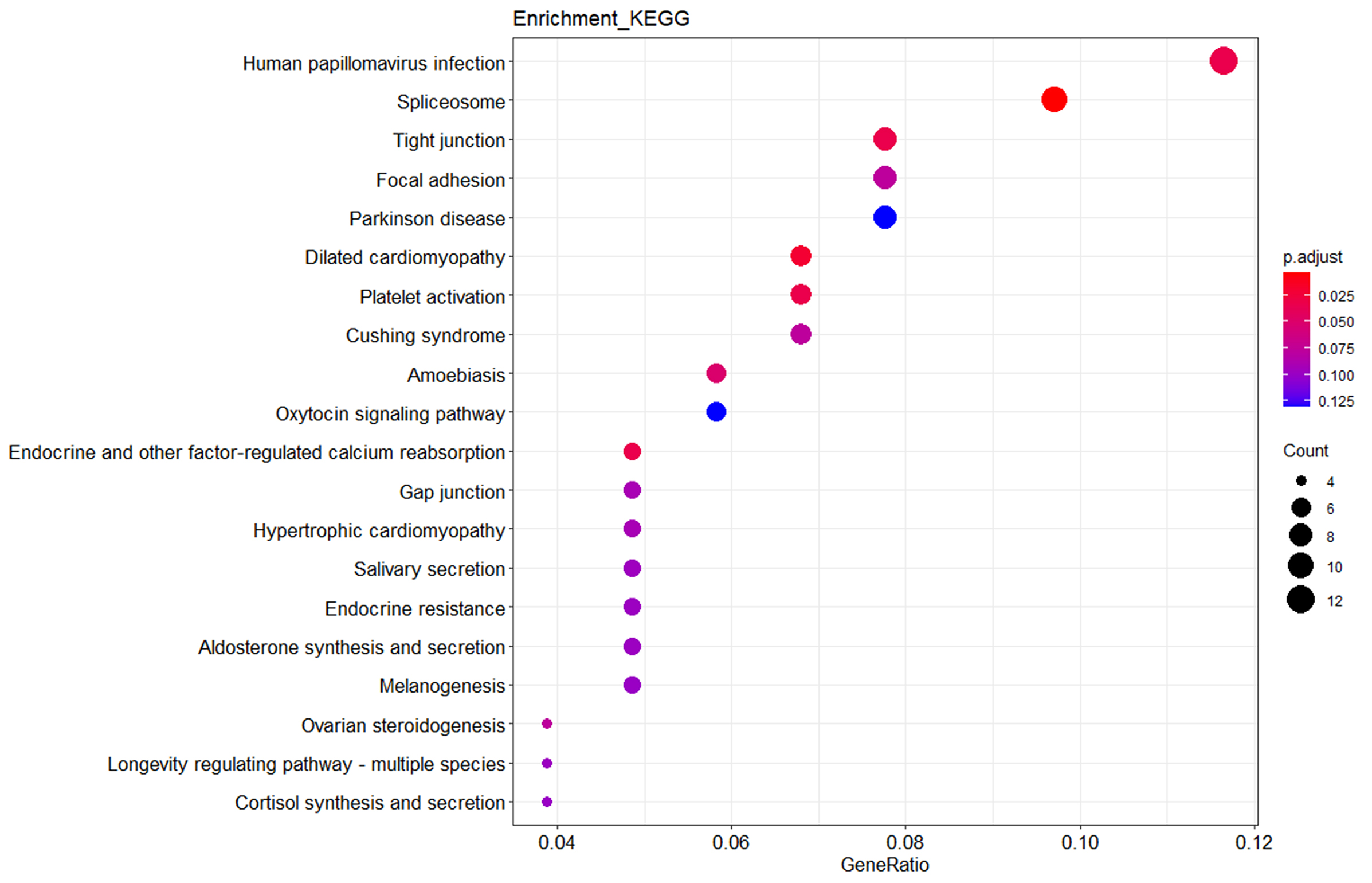 Fig. 4.
Fig. 4.
KEGG enrichment. KEGG analysis revealed that the enriched pathways were the oxytocin signaling pathway, aldosterone synthesis and secretion, and cortisol synthesis and secretion. KEGG, Kyoto Encyclopedia of Genes and Genomes.
Hub gene interaction networks were constructed for the mRNA royalblue
module and the lncRNA darkgrey module, respectively (Fig. 5). Scatter
plots depicting GS versus MM were also generated (Fig. 6). The criteria
for screening hub genes in this study were set as
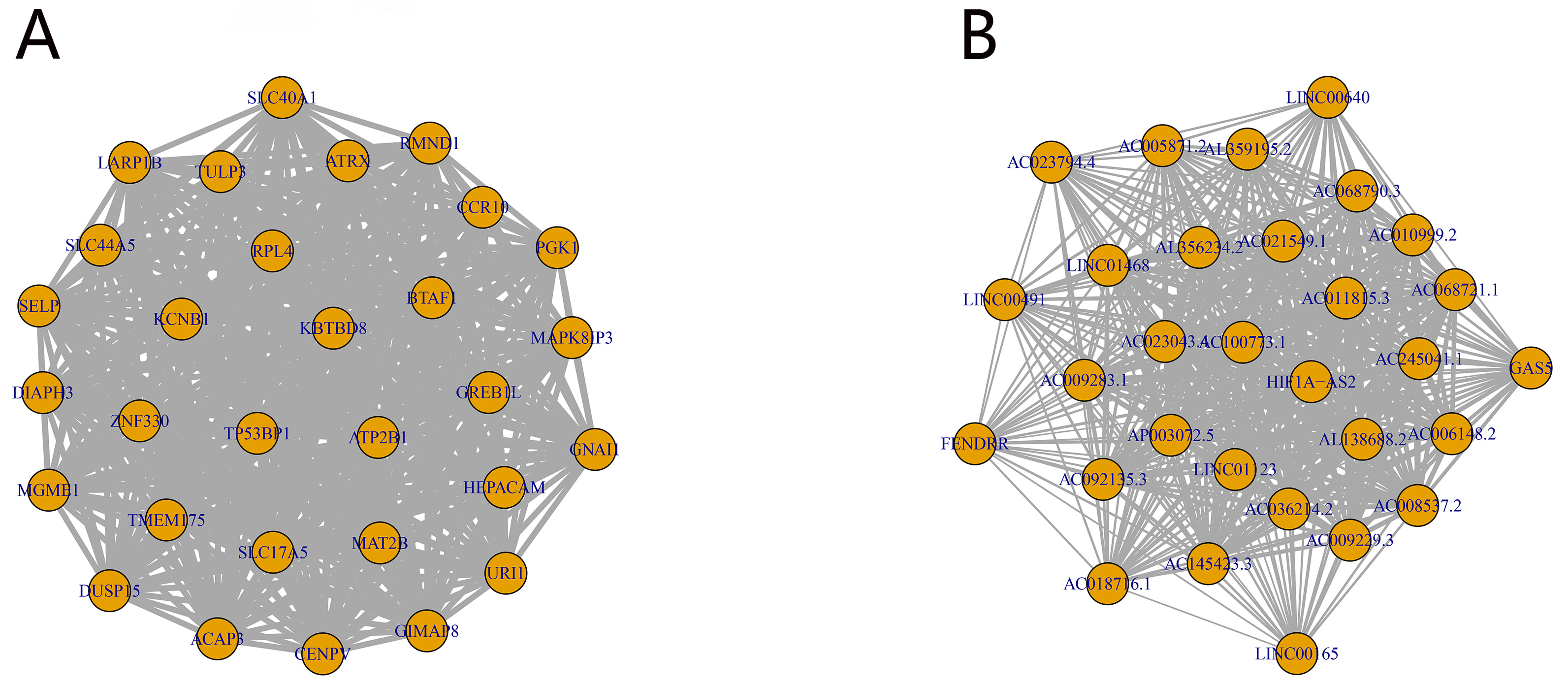 Fig. 5.
Fig. 5.
The core gene interaction network of mRNA royalblue (A) and lncRNA darkgrey (B) modules.
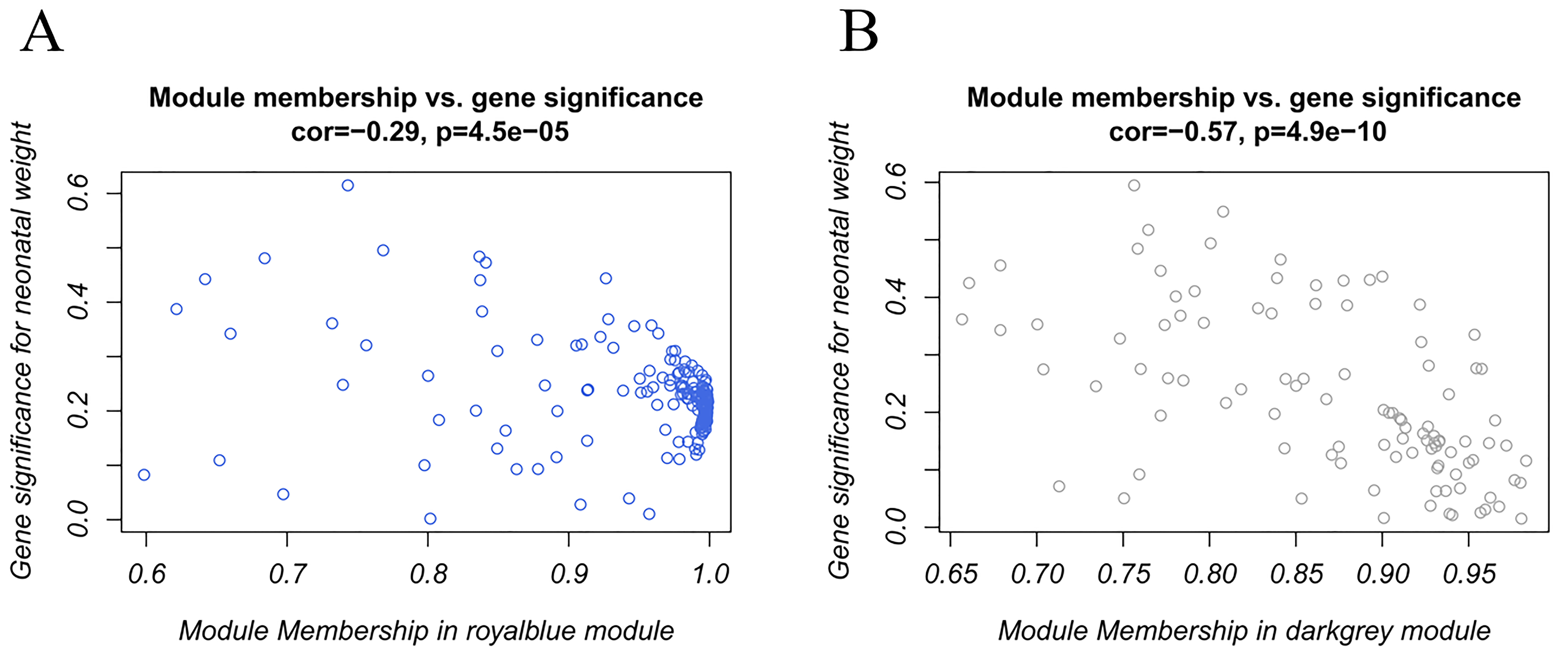 Fig. 6.
Fig. 6.
Scatter plots of GS for neonatal weight and MM in mRNA royalblue (A) and lncRNA darkgrey (B) modules. There was a significant correlation between GS and MM in this module. MM, module membership; GS, gene significance.
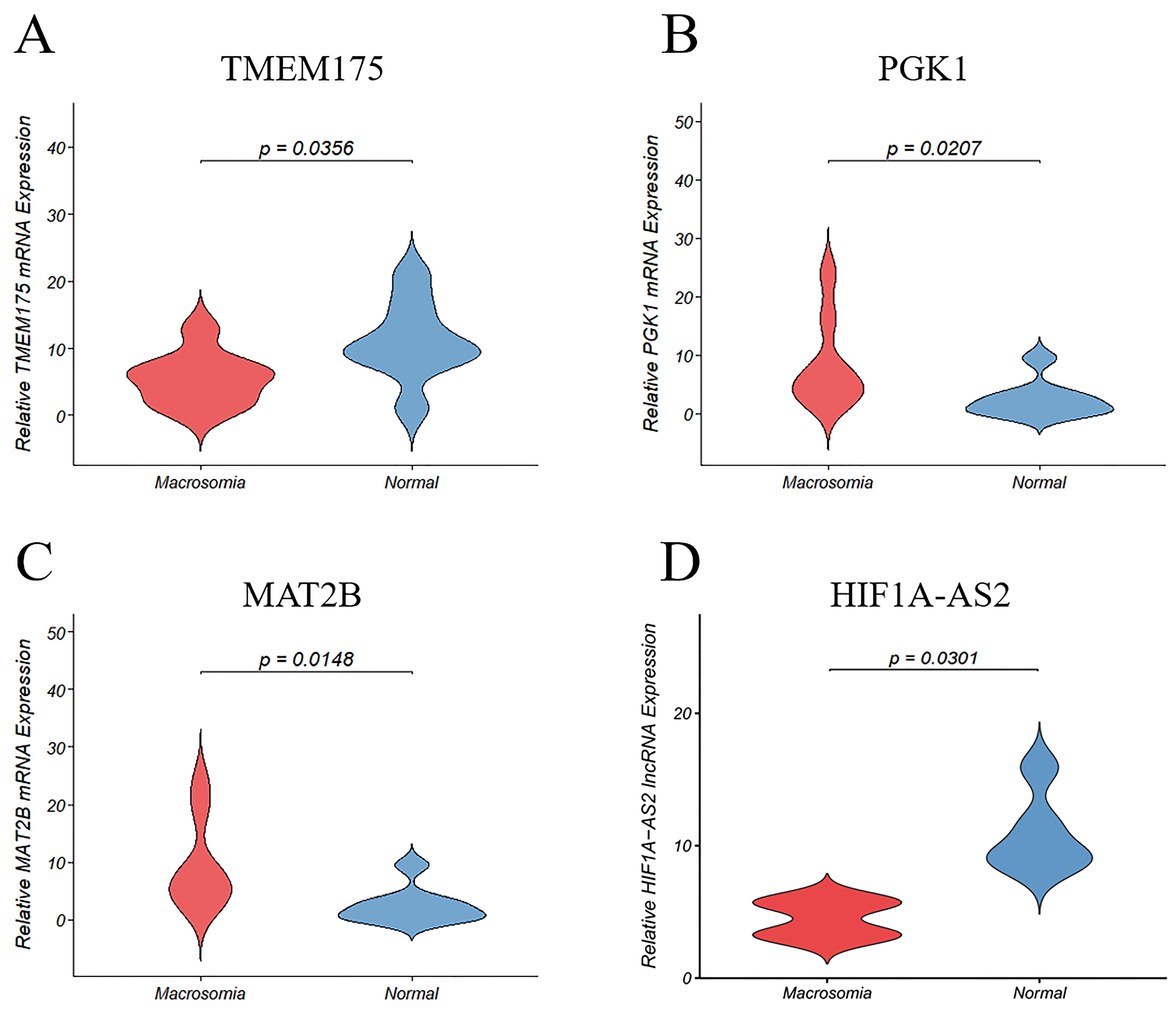 Fig. 7.
Fig. 7.
The expression levels of TMEM175 (A), PGK1 (B), MAT2B (C) in the mRNA royalblue module and HIF1A-AS2 (D) in the lncRNA darkgrey module in umbilical cord blood.
| Gene symbol | |GS| | p | |MM| | p | |
| mRNA royalblue | |||||
| TMEM175 | 0.369 | 0.005 | 0.997 | ||
| PGK1 | 0.357 | 0.007 | 0.999 | ||
| MAT2B | 0.356 | 0.007 | 0.998 | ||
| lncRNA darkgrey | |||||
| HIF1A-AS2 | 0.387 | 0.922 | |||
TMEM175, transmembrane protein 175; PGK1, phosphoglycerate kinase 1; MAT2B, methionine adenosyltransferase 2B; HIF1A-AS2, HIF1A antisense RNA 2.
By integrating clinical risk factors with exosome transcriptome analysis, our study confirmed that elevated pre-pregnancy BMI, excessive gestational weight gain, and dyslipidemia were closely related to the occurrence of macrosomia in non-diabetic pregnancies. The core genes TMEM175, PGK1, MAT2B, and HIF1A-AS2 may remodel the nutrient transport patterns at the maternal-fetal interface by regulating mitochondrial metabolism, glucose metabolism, lipid metabolism, and placental development, ultimately leading to fetal overgrowth.
Our study found that excessive gestational weight gain was a high-risk factor for macrosomia, aligning with the findings of earlier studies [26, 27]. A meta-analysis indicated that excessive gestational weight gain was an independent risk factor for macrosomia compared with moderate weight gain [28]. A retrospective study also found that accelerated weight gain rate in obese pregnant women before 24 weeks of gestation was associated with an increased risk of macrosomia [29]. Our study also confirmed that the pre-pregnancy BMI of the macrosomia group was significantly higher than that of the control group, verifying the protective effect of lower pre-pregnancy BMI against macrosomia [30]. Research has reported that multiparous women were more likely to give birth to macrosomic infants [31], which was also observed in our study. Our study also found that the cesarean section rate is significantly higher in non-diabetic macrosomic infants, further validating that fetal weight is a key factor in the decision-making process for delivery methods [32]. Omaña-Guzmán et al. [33] indicated that elevated DBP during pregnancy may be negatively correlated with fetal growth. Although this study found that maternal DBP was lower in the macrosomia group with a statistically significant difference, the DBP values remained within the normal physiological range. Therefore, the specific impact of blood pressure changes on the occurrence of macrosomia in non-diabetic pregnant women requires further validation through larger-scale clinical studies and mechanistic investigations. Additionally, the intrauterine metabolic environment shaped by maternal glucose and lipid profiles may also affect fetal growth [34]. Our research revealed that lower levels of maternal HDL and higher levels of LDL during the last trimester were significantly associated with an increased risk of macrosomia in non-diabetic pregnancies, indicating the importance of maternal lipid metabolism [35]. This is consistent with the results presented by Li et al. [36], who also reported that elevated LDL in the last trimester increased the risk of macrosomia. Peng et al. [34] also found that increased HDL levels in the last trimester were associated with lower neonatal weight. However, Wang et al. [37] observed no association between HDL-C levels and neonatal birth size. These differences may be attributed to regional variations in study populations, lifestyle factors, or underlying genetic background, warranting further exploration of the complex relationship between maternal lipid metabolism and fetal growth. Notably, although a previous study reported an association between elevated ALP levels and large for gestational age (LGA) infants in non-diabetic mothers [38], our findings showed no significant correlation between ALP levels in the first trimester and macrosomia in non-diabetic mothers. However, elevated ALP levels in the last trimester were associated with macrosomia, potentially as a result of the effects of placental and skeletal isoenzymes [39]. These conflicting results may show that the relationship between ALP levels and adverse effects on fetal outcomes can be complex and remain controversial. A study has found that pregnant women with extremely high levels of serum ALP levels gave birth to normal-weight infants [38]. This may imply that ALP alone does not affect macrosomia in infants, and it should be used together with other indicators to dictate clinical management [40]. These findings highlight the significance of managing pre-pregnancy weight, monitoring gestational weight gain, and regulating lipid levels for preventing macrosomia in non-diabetic mothers. In particular, lipid level regulation can be accomplished through a balanced diet rich in unsaturated fats and dietary fiber, along with the moderate physical activity advised by healthcare providers, to mitigate the effects of maternal obesity on the child [41]. Moreover, neonatal gender seems to be related to macrosomia in non-diabetic mothers. Placental samples showed higher methylation levels in males compared to females [42]. Studies indicated a higher proportion of male neonates among macrosomic births in non-diabetic pregnancies [1, 4, 43], consistent with our findings. Nevertheless, the specific regulatory mechanisms involved require further investigation.
Fetal macrosomia is more prevalent in mothers with pre-gestational diabetes and GDM compared to non-diabetic mothers; thus, mothers with GDM carry a higher risk. This increased risk is primarily due to the effects of maternal hyperglycemia, which leads to excess glucose transfer to the fetus and subsequent fetal hyperinsulinemia and fat storage [44, 45]. In non-diabetic mothers, fetal macrosomia is a complex condition resulting from the interplay of multiple factors such a dyslipidemia and gestational weight gain [35, 45, 46]. Moreover, an increase in maternal glucose metabolism in non-diabetic mothers can contribute to macrosomia [47]. A significant number of patients with GDM may also suffer from obesity, a factor associated with fetal macrosomia. The combination of maternal obesity and GDM appears to have a synergistic effect, increasing the risk even further [48]. However, obesity is an independent risk factor, and research indicates that a significant portion of infants with macrosomia are born to women who are obese without diabetes [49]. This suggests that BMI is a major contributor to macrosomia in both diabetic and non-diabetic mothers.
Currently, the application of exosomes in the research of the mechanism of pregnancy complications remains quite limited, and related explorations have not been extensively conducted [16]. Notably, the feasibility of exosomal RNA as a non-invasive biomarker also deserves attention. Although the initial equipment investment for isolation techniques (e.g., ultracentrifugation) is relatively high, the popularization of commercial kits has significantly reduced the cost of single detection. Moreover, exosomes are widely present in body fluids such as blood, urine, and milk, making sample collection convenient and non-invasive, which is suitable for large-scale population screening [50]. Additionally, combined with multi-omics analysis (such as small RNA sequencing), its specificity and sensitivity can be improved, providing a multi-dimensional solution for the early diagnosis and assessment of pregnancy complications [51].
In our study, RNA sequencing of umbilical venous blood exosomes was used to construct a co-expression network of mRNA and lncRNA. By analyzing continuous variables such as maternal age, pre-pregnancy BMI, and gestational weight gain, we found that the mRNA royalblue module and lncRNA darkgrey module were highly correlated with gestational weight gain. These modules may participate in physiological processes such as adipocyte differentiation and energy metabolism balance through cooperative regulation of gene expression [52]. As key mediators of intercellular communication, RNA molecules carried by exosomes may affect gene expression patterns in the placenta and maternal metabolic organs via blood circulation [53]. Furthermore, KEGG enrichment analysis revealed associations between macrosomia-related genes and the oxytocin signaling pathway, aldosterone synthesis and secretion, and cortisol synthesis and secretion pathways. As a study has shown, the oxytocin pathway may be involved in weight regulation [54]. Aldosterone is found to be elevated in obese individuals, and it is positively associated with BMI [55, 56], while cortisol promoted weight gain by enhancing fat storage and slowing down metabolism [57]. Therefore, we speculate that these three pathways may increase the risk of macrosomia by influencing maternal weight changes during pregnancy. However, the specific role of exosome RNA remains unclear, and future studies using animal models are needed to verify the causal relationship between exosomes in weight regulation during pregnancy and the occurrence of macrosomia, providing a theoretical basis for early intervention in pregnancy complications.
WGCNA analysis identified TMEM175, PGK1, and MAT2B as
hub genes in the mRNA royalblue module, and HIF1A-AS2
as the hub gene in the lncRNA darkgrey module. Moreover, all these genes
were closely related to gestational weight gain. As a key channel protein
regulating the lysosome-mitochondria metabolic axis, TMEM175 mutations
can lead to abnormal lysosomal acidification, which in turn impacts the
lysosomal-mediated autophagy process and nutrient-sensing pathways (e.g., the
mechanistic target of rapamycin (mTOR) pathway). Consequently, this can lead to cellular metabolic disorders
[58, 59]. The mTOR pathway has been proven to play a significant role in
regulating early stages of embryonic development [60], while TMEM175 is
also capable of modulating mitochondrial function and facilitating mitophagy
[59]. Lin et al. [61] observed reduced placental mtDNA copy numbers in
macrosomic infants born to non-diabetic mothers, indicating a possible link
between mitochondrial dysfunction and macrosomia, though the precise mechanisms
remain unclear. Additionally, fetal growth mainly depends on the availability of
sufficient nutrients and their transportation in the placenta, such as glucose.
Glucose has been proven to be a key component promoting cell proliferation [62].
Research has demonstrated that regulating maternal glucose levels is essential
for the growth and development of offspring. Furthermore, PGK1, a vital
enzyme in the glycolytic pathway, influences glucose utilization [63]. The
concentration of maternal glucose affects placental glucose transport, which
provides energy for normal fetal intrauterine development. Therefore, we infer
that PGK1 may regulate placental nutrient transport via glucose
metabolism, and its dysregulation may increase glucose transport, leading to
fetal overgrowth and macrosomia. Furthermore, fetal growth and development also
require an adequate and balanced supply of amino acids for physiological
processes such as protein synthesis. Methionine, an essential sulfur-containing
amino acid, is critical for fetal growth and development [64]. Research has shown
that methionine adenosyltransferase 2A (MAT2A) is involved in methionine metabolism and can catalyze the synthesis of
S-adenosylmethionine (SAM) (a precursor of cysteine) [65]. Rubini et al.
[66] confirmed that cysteine can affect fetal development. Furthermore, studies
have reported that overexpression of MAT2A can promote lipid accumulation and
significantly upregulate the expression levels of adipogenic marker genes such as
peroxisome proliferator-activated receptor γ (PPAR
This study constructed a differential transcriptome network based on systems biology to elucidate associations between differentially expressed RNAs and phenotypes (e.g., gestational weight gain), providing a basis for developing preventive strategies. However, there are several limitations. Firstly, its retrospective design inherently introduces selection bias. The inclusion criteria and patient characteristics may not reflect the broader population, and the availability and completeness of historical data may introduce additional confounding factors, thereby affecting the generalizability of the findings. Secondly, the single-center setting and relatively small sample size limit the robustness and generalizability of findings. Thirdly, there is a lack of comprehensive functional verification and a thorough assessment of clinical practicality. Although associations with exosome RNA were observed, the specific regulatory mechanisms (such as molecular pathways and interaction networks) remain unclear. Moreover, the clinical significance of exosomal RNA as a non-invasive screening biomarker, encompassing indicators such as sensitivity, specificity, and predictive value, requires further exploration. Future research should employ multi-center designs and expand sample sizes to verify result reliability. In vitro and in vivo functional experiments are needed to clarify the precise regulatory mechanisms of exosomal RNA. Additionally, well-designed prospective clinical trials should be conducted to systematically evaluate their diagnostic accuracy, prognostic value, and clinical applicability, thereby providing robust evidence for potential clinical application.
This study identified pre-pregnancy BMI, gestational weight gain, LDL levels, and ALP levels in the last trimester as risk factors for macrosomia in non-diabetic pregnancies. Through the investigation of umbilical cord blood plasma exosomes, TMEM175, PGK1, MAT2B, and HIF1A-AS2 were determined to be potential core regulators for macrosomia in infants born to non-diabetic mothers. In the future, additional research must be undertaken to investigate the specific molecular mechanisms of these potential core regulators, to elucidate their roles in metabolic disorders and how they affect the development of macrosomia. Secondly, more clinical indicators and molecular markers may be considered to develop a more comprehensive risk prediction model for macrosomia, thereby improving the ability for early prediction and intervention of macrosomia. Moreover, based on the risk factors identified in this study, targeted gestational intervention studies can be conducted. This could involve the development of appropriate gestational weight management plans and the regulation of blood lipid levels, to determine if the occurrence of macrosomia can be decreased, thereby offering more effective strategies for the clinical prevention of macrosomia.
ALP, alkaline phosphatase; AUC, area under the curve; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; GDM, gestational diabetes mellitus; GS, Gene Significance; HDL, high-density lipoprotein; KEGG, Kyoto Encyclopedia of Genes and Genomes; LDL, low-density lipoprotein; LGA, large for gestational age; MM, Module Membership; OGTT, oral glucose tolerance test; OR, odds ratio; SAM, S-adenosylmethionine; SBP, systolic blood pressure; SD, standard deviation; TG, triglyceride; WGCNA, weighted gene co-expression network analysis; TC, total cholesterol.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
JY and LS jointly designed the research study. JY, LS, JL, and KC were responsible for data acquisition. YM and DY performed the experimental procedures. JY and LS completed data analysis and interpretation. YL oversaw project supervision, funding acquisition, and provided guidance on research design. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was carried out in accordance with the guidelines of the Declaration of Helsinki. The study was approved by the Medical Ethics Committee of Guangxi Medical University (approval number: 2019-SB-067) and the written informed consent was obtained from the participants.
Not applicable.
This study was supported by the Guangxi Natural Science Foundation Program (2020GXNSFDA297024); the National Natural Science Foundation of China (81960282), the Self-Funded Scientific Research Project of Guangxi Health Commission (Z-A20220479 and Z-A20230789), the “Medical Excellence Award” funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University (201903), and the Guangxi Key Research and Development Program (Guike AB25069096).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG43904.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
