- Academic Editor
Polycystic ovary syndrome (PCOS) is related to persistent low-grade inflammation and oxidative stress (OS). Resveratrol (RSV) exhibits potent anti-inflammatory and antioxidant effects, suggesting therapeutic potential for PCOS. However, its precise mechanisms of action, particularly concerning ferroptosis remain unclear. This study aimed to investigate whether RSV ameliorates PCOS by modulating inflammation, OS and ferroptosis features, thereby offering novel insights into its therapeutic role.
PCOS was induced in mice through once-daily subcutaneous dosing of dehydroepiandrosterone (DHEA: 0.6 mg/kg), alongside a 21-day continuous high-fat diet regimen. Following successful model induction, the mice were assigned to the PCOS group or the RSV group. RSV was orally gavaged at doses of 20 and 40 mg/kg/day for 30 days. The body weight, ovarian weight and estrous cycle were monitored. Serum is used for the detection of hormones [follicle stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T) and inflammatory markers [interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α)]. Then the ovarian tissue samples were utilized for the assessment of OS indicators [malondialdehyde (MDA), glutathione (GSH)], glutathione peroxidase 4 (GPX4), and transcriptomic profiling.
RSV was shown to reduce body weight and ovarian weight. RSV also ameliorated symptoms of PCOS by decreasing chronic inflammation, OS and ferroptosis features in the ovaries. All the detected inflammatory factors in the PCOS group were significantly higher than those in the control group (IL-1β, p < 0.0001; IL-6, p < 0.001; TNF-α, p < 0.01) and were significantly decreased with the supplementation of RSV. Ovarian tissue MDA levels were markedly elevated in PCOS mice compared to controls (p < 0.0001), with RSV treatment significantly attenuating this increase. While GSH levels were significantly depleted in PCOS ovaries compared to controls (p < 0.0001), with RSV treatment effectively restoring concentrations. Western blot analysis revealed that GPX4 expression in ovaries was significantly diminished in the PCOS group compared with the control group (p < 0.01), but this reduction was effectively reversed by RSV treatment.
RSV demonstrated therapeutic efficacy in PCOS mice by normalizing hormonal profiles and mitigating chronic inflammatory responses and oxidative damage. Furthermore, our investigation also revealed that RSV ameliorates PCOS pathology by modulating GPX4 pathways, which is consistent with ferroptosis features.
Polycystic ovary syndrome (PCOS) represents a complex endocrinopathy affecting 15–20% of reproductive-age women [1]. The condition is marked by oligo-anovulation, polycystic ovarian morphology, hyperandrogenism, insulin resistance, hirsutism, and elevated luteinizing hormone (LH) levels [2, 3]. PCOS pathogenesis involves an intricate interplay of genetic predisposition, environmental triggers, oxidative damage, persistent low-grade inflammation, and lifestyle determinants [4, 5, 6]. Clinical evidence demonstrates marked increases in proinflammatory mediators in women with PCOS. Furthermore, oxidative stress (OS) plays a pivotal role in PCOS pathogenesis, and individuals with PCOS have an imbalance in antioxidant status [7]. Despite advances in understanding the disease, targeted therapeutic strategies remain limited. Therefore, elucidating the molecular mechanisms underlying PCOS is essential to improve diagnostic accuracy, optimize treatment regimens, and enhance long-term reproductive and metabolic outcomes.
Ferroptosis is a programmed form of cell death driven by iron-catalyzed lipid peroxidation, and it has emerged as a pathogenic mechanism in cancer, cardiovascular, neurodegenerative, metabolic and renal diseases [8, 9, 10, 11, 12, 13, 14]. Ferroptosis is mechanistically characterized by iron-mediated lipid peroxide deposition and reactive oxygen species (ROS) production [15]. A key regulator of ferroptosis is glutathione peroxidase 4 (GPX4), which serves a critical role in suppressing ferroptotic cell death [16, 17]. Biomarkers such as elevated malondialdehyde (MDA) and depleted glutathione (GSH) levels have been identified as hallmarks of ferroptosis, reflecting its progression and severity. Emerging evidence suggests a potential link between ferroptosis and PCOS [17]. In line with these observations, Li et al. [18] showed that baicalein ameliorates PCOS manifestations through attenuation of oxidative injury and blockade of ferroptotic demise. These findings highlight ferroptosis as a promising therapeutic target in PCOS management.
Resveratrol (RSV) is widely distributed in various plant species. Mounting studies have established that RSV displays a versatile bioactivity profile, encompassing antitumor, anti-inflammatory, and powerful antioxidant capacities [19, 20, 21]. Emerging evidence positions RSV as a key modulator of ovarian physiology, governing folliculogenesis, ovulation, steroidogenesis, and embryo development, while exerting therapeutic influence over ovarian disorders and assisted reproductive outcomes [22, 23]. Meanwhile, RSV can reduce the development of chronic inflammation during follicular development [22]. RSV administration significantly ameliorates follicular abnormalities in PCOS rat models by reducing the population of small antral and atretic follicles while promoting follicular maturation, thereby underscoring the influence of RSV on the preservation of follicular development [24]. Furthermore, recent investigations have identified the capacity of RSV to scavenge reactive ROS, inhibit ferroptotic pathways, and ameliorate intestinal barrier dysfunction [25]. While these findings collectively suggest the therapeutic potential of RSV in PCOS management [26], the specific mechanisms through which RSV may ameliorate PCOS symptoms (particularly via modulation of OS and ferroptosis pathways) remain to be fully elucidated.
Emerging evidence indicates that PCOS development involves a complex interplay among three key pathological mechanisms: chronic low-grade inflammation, redox imbalance, and dysregulated GPX4, yet the central regulatory factors and signaling cascades remain incompletely defined. Given the well-documented anti-inflammatory and antioxidant actions of RSV, our work was designed to delineate how ferroptosis intersects with inflammation and OS in PCOS development and whether RSV exerts its therapeutic benefits, at least in part, by modulating ferroptosis.
All experimental procedures were approved by the Experimental Animal Welfare
Committee of Lanzhou University Second Hospital (approval number: D-2023-071). All animal procedures were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and adhered to the “3Rs” principles (Replacement, Reduction, Refinement). The number of animals used was minimized through appropriate statistical study design.
Three-week-old female C57BL/6J mice (body weight 12.5
Mice were randomly allocated into two experimental cohorts: a control group (n = 10) and a dehydroepiandrosterone (DHEA) (D106380, Aladdin Reagent Co., Ltd., Shanghai, China) group (n = 30). The PCOS model was induced by daily subcutaneous injections of DHEA (0.6 mg/kg body weight in 0.1 mL vehicle) for 21 consecutive days. The vehicle consisted of a 9:1 (v/v) mixture of sesame oil and 95% ethanol. Control mice received equivalent volumes of vehicle alone administered identically (0.1 mL/mouse daily for 21 days). PCOS was defined by the presence of irregular ovarian cyclicity. Following successful PCOS induction, DHEA-treated mice were further randomized into three subgroups: PCOS model group, RSV (20 mg/kg) treatment group, and RSV (40 mg/kg) treatment group. During the RSV (R8350, Solarbio, Beijing, China) intervention period, control and PCOS model mice received daily intragastric administration of normal saline, whereas RSV-treated mice were administered RSV at doses of 20 mg/kg and 40 mg/kg body weight, respectively. Following a 30-day RSV intervention, mice were humanely euthanized with an overdose of pentobarbital sodium (200 mg/kg) for the collection of serum samples and ovarian tissues. During ovarian tissue dissection, the ovarian tissue was rapidly transferred to ice-cold phosphate-buffered saline (PBS) to maintain tissue integrity. One ovary per mouse was fixed for histopathology; the contralateral ovary was snap-frozen at –80 °C for downstream molecular analyses.
In this study, the estrous cycle was monitored daily from day 15 post-modeling through day 14 of RSV post-treatment (14 consecutive days). 3 mice per group underwent estrous cycle analysis. Briefly, vaginal secretions were obtained using a sterile Pasteur pipette containing 0.9% normal saline, gently rinsed, and transferred onto clean glass slides. The samples were air-dried, fixed in methanol, and stained with hematoxylin and eosin (H&E). Estrous cycle stages were determined by light microscopic examination of vaginal cytology, including the presence and proportion of nucleated epithelial cells, cornified epithelial cells, and leukocytes.
Fresh ovaries were fixed in 4% paraformaldehyde (24 h), washed, then dehydrated through ascending ethanol (70%, 80%, 90%, 95% and 100%), paraffin-embedded, and sectioned at 5 µm; slides were stored at 4 °C. After xylene deparaffinization and graded-ethanol rehydration, 5 µm sections were stained with H&E (Solarbio G1120, Beijing, China) per standard protocol.
Total RNA was isolated from mouse ovaries with TRIzol reagent (9108; Takara,
Beijing, China). RNA-seq libraries were constructed and sequenced on the Illumina
sequencing platform (Novogene Co., Ltd., Beijing, China), with two biological
replicates prepared for each experimental group. Quality-filtered reads were
mapped to the mouse reference genome using Spliced Transcripts Alignment to a
Reference (STAR) with default parameters. Gene Ontology (GO) term enrichment
among Differentially expressed genes (DEGs) was assessed with the clusterProfiler
R package. GO terms were deemed significantly enriched at a
Benjamini-Hochberg-adjusted p-value
The concentrations of follicle stimulating hormone (FSH), LH, testosterone (T),
interleukin-6 (IL-6), interleukin-1
Total protein extracts from mouse ovaries were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene fluoride membranes (MSBVN1B50, Sigma-Aldrich, St. Louis, MO, USA). The membranes were blocked with 5% skimmed milk (D8340, Servicebio, Wuhan, Hubei, China) and probed overnight at 4 °C with primary antibodies as listed below: GPX4 (Rabbit polyclonal, A1933, ABclonal, Wuhan, Hubei, China; 1:2000 dilution). Following three washes, membranes were probed with horseradish peroxidase (HRP)-linked secondary antibodies at ambient temperature for 2 h. Protein bands were imaged on a Tanon system (V2.0 software) and quantified with Gel Pro Analyzer 4. GPX4 abundance was quantified by integrated optical density (IOD) of the corresponding bands.
Statistical analyses were performed in GraphPad Prism 8.0 (GraphPad Software,
San Diego, CA, USA). Continuous data are expressed as mean
Vaginal cytology was employed to monitor the influence of RSV on estrous cyclicity. Fig. 1 illustrates the four stages of the murine estrous cycle. As described previously [27], the dominant cell type in the proestrus phase consists of nucleated epithelial cells, whereas cornified epithelial cells dominate during the estrus phase (Fig. 1A,B). The metestrus phase is distinguished by the presence of leukocytes and cornified epithelial cells (Fig. 1C). While the diestrus phase is predominantly composed of leukocytes (Fig. 1D). As anticipated in the PCOS model, DHEA-treated mice demonstrated complete estrous cycle arrest, persistently remaining in the diestrus phase. In contrast, RSV administration significantly restored cyclic progression through all estrous stages, indicating normalization of reproductive cyclicity in the PCOS mice (Fig. 2).
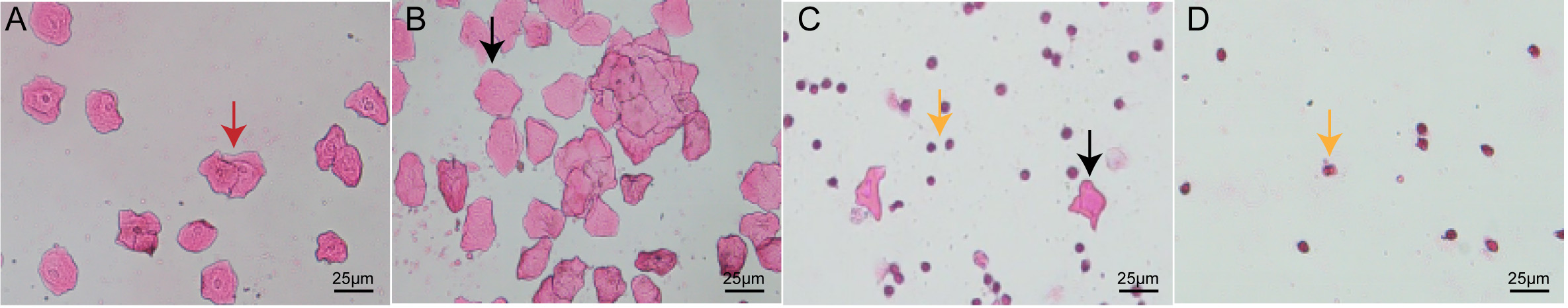 Fig. 1.
Fig. 1.
The four phases of the control group mouse estrous cycle. Scale bar: 25 µm. (A) Proestrus: nuclear epithelial cells are the predominant cell type in the proestrus stage (red arrowhead). (B) Estrus: cornified epithelial cells (black arrowhead) are the predominant cell type in theestrus stage (black arrowhead). (C) Metestrus: leukocytes (yellow arrowhead) and cornified epithelial cells (black arrowhead) are present in the metestrusstage, dominant cells in the metestrus are leukocytes. (D) Diestrus: fewer cell numbers, leukocytes (yellow arrowhead) are the predominant cell type in the diestrus stage.
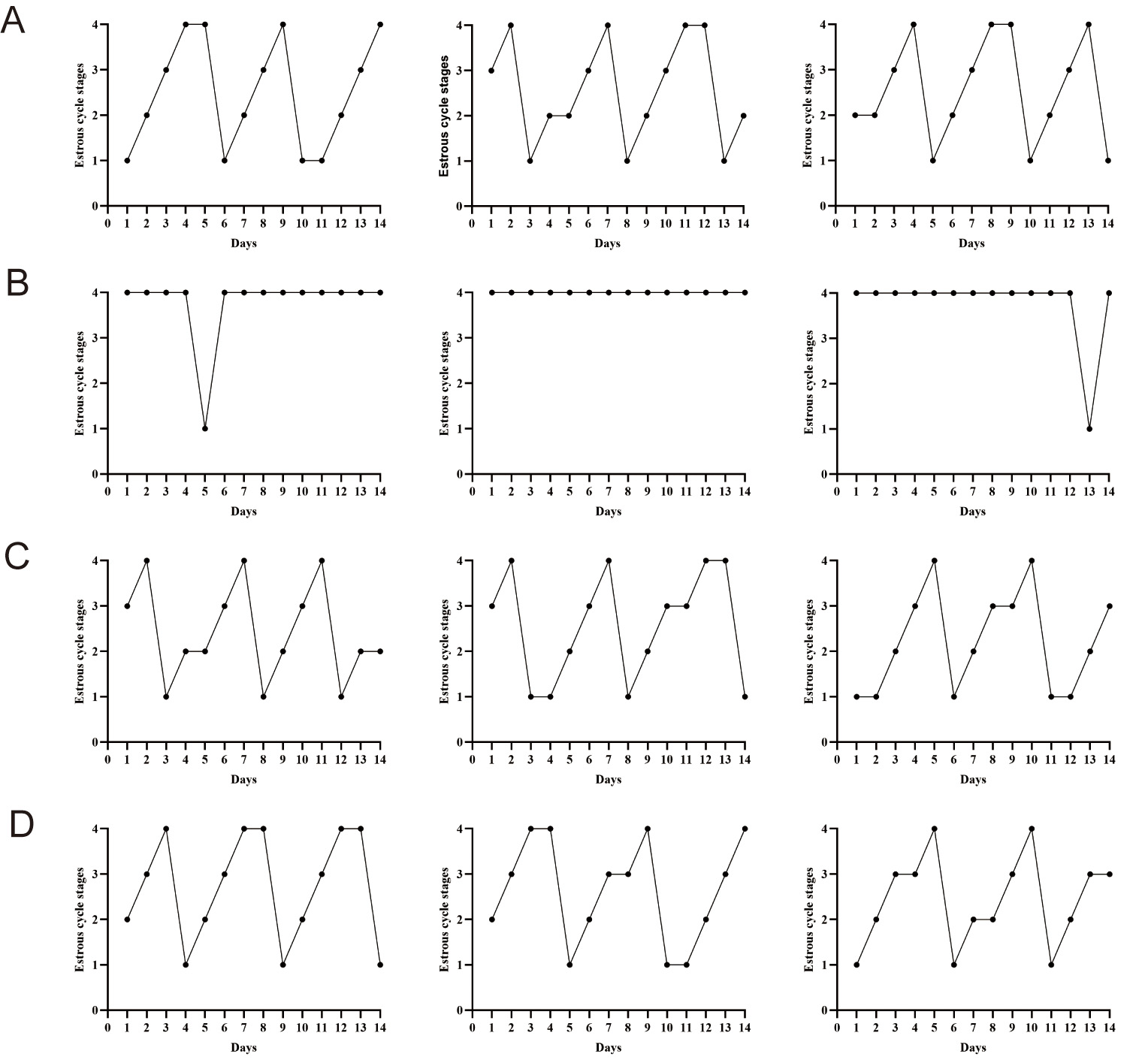 Fig. 2.
Fig. 2.
RSV improves ovarian function in PCOS mice. (A) CON group: representative estrous profiles from three normal-group mice. (B) PCOS group: representative estrous profiles from three PCOS groups. (C) 20 mg/kg RSV-treated group: representative estrous profiles from three RSV (20 mg/kg) groups. (D) Representative estrous profiles from three RSV (40 mg/kg) groups. CON, control; RSV, resveratrol; PCOS, polycystic ovary syndrome.
Ovarian architecture was assessed histologically by H&E staining (Fig. 3). In the control group, ovarian sections exhibited follicles of all levels including primordial follicles, early antral follicle, antral follicle, and luteum (Fig. 3A). Follicular and luteal architecture remained intact, with clearly discernible cellular morphology (Fig. 3A). In contrast, ovaries from the PCOS model group displayed multiple cystic dilatations with attenuated granulosa cell layers and a significant reduction in corpora lutea (Fig. 3B). Following treatment with RSV (20 mg/kg and 40 mg/kg), H&E staining revealed a marked decrease in cystic follicles and a restoration of granulosa cell layers (Fig. 3C,D). These present observations suggest that RSV exerts a beneficial influence on ovarian dysfunction in PCOS mice by improving follicular development and luteal formation.
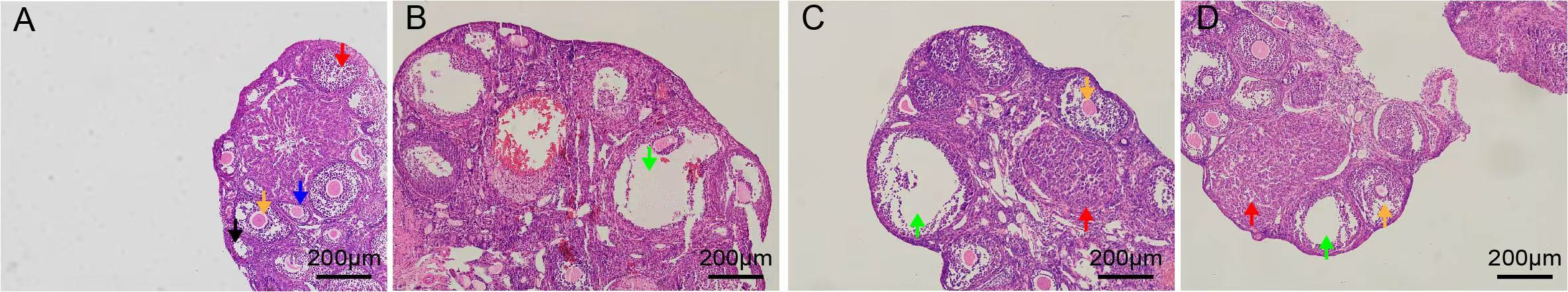 Fig. 3.
Fig. 3.
Pathological images of ovarian tissues in mice. Scale bar: 200 µm. (A) CON group. (B) PCOS group. (C) 20 mg/kg RSV-treated group. (D) 40 mg/kg RSV-treated group. Primordial follicle marked with a black arrowhead, the early antral follicle marked with a blue arrowhead, the antral follicle marked with a yellow arrowhead, the luteum marked with a red arrowhead, large cystic follicles marked with a green arrowhead.
To gauge the impact of RSV on PCOS, we tracked body weight, ovarian weight, and ovarian index (Fig. 4). When compared to the control group, the PCOS group exhibited pronounced increases in body weight, ovarian weight, and ovarian index. Following treatment with RSV at 40 mg/kg, body weight, ovarian weight and ovarian index significantly reduced. However, relative to the PCOS group, the 20 mg/kg RSV cohort showed no appreciable change. Collectively, RSV partially normalized body weight, ovarian weight, and ovarian index in PCOS mice.
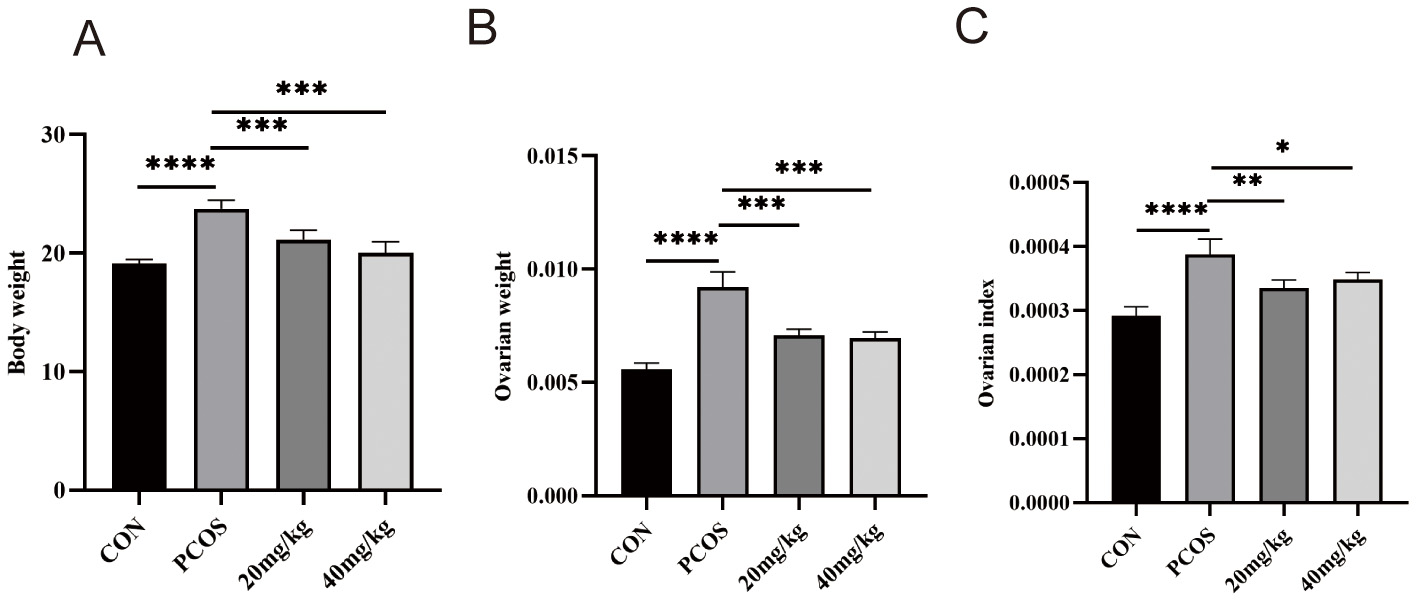 Fig. 4.
Fig. 4.
Effect of RSV on body weight, ovarian weight, and ovarian index
in mice. (A) Body weights. (B) Ovarian weight. (C) Ovarian index. ns (not significant) p
To elucidate the molecular basis of RSV’s therapeutic action, we conducted a
comprehensive transcriptomic analysis of ovarian tissues. Comparative analysis
revealed significant alterations in gene expression profiles (Fig. 5). Relative
to controls, PCOS ovaries exhibited 1120 up-regulated and 1915 down-regulated
genes (p
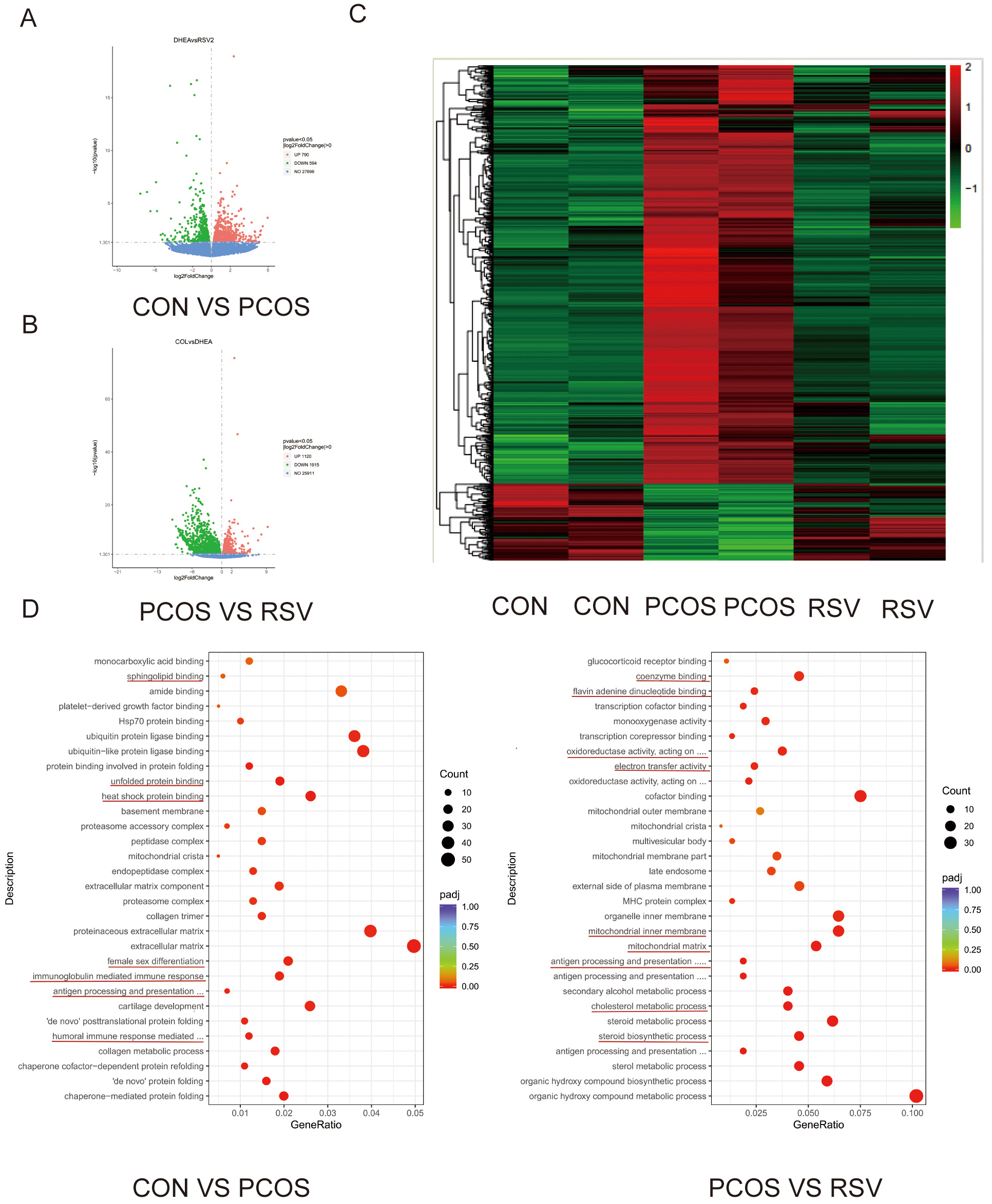 Fig. 5.
Fig. 5.
Effects of RSV on the ovarian transcriptome in mice. (A) Volcano plots illustrate gene expression differences in CON versus PCOS. (B) Volcano plots illustrate gene expression differences in PCOS and RSV groups. (C) A heatmap in the CON, PCOS, and RSV groups. (D) Enrichment Analysis of GO Terms. GO, Gene Ontology.
We measured hormones closely related to ovarian function including FSH, LH, and T (Fig. 6). Levels of FSH, LH, and T in the serum of mice in each group were detected using ELISA. Serum concentrations of LH and T were significantly higher in all the PCOS groups compared to the control group (Fig. 6B,C), while the FSH content was not significantly altered (Fig. 6A). Compared with controls, PCOS mice exhibited a markedly elevated LH/FSH ratio. Both 20 and 40 mg/kg RSV treatments significantly lowered serum LH and T levels, restoring the LH/FSH ratio to near-normal values (Fig. 6B–D).
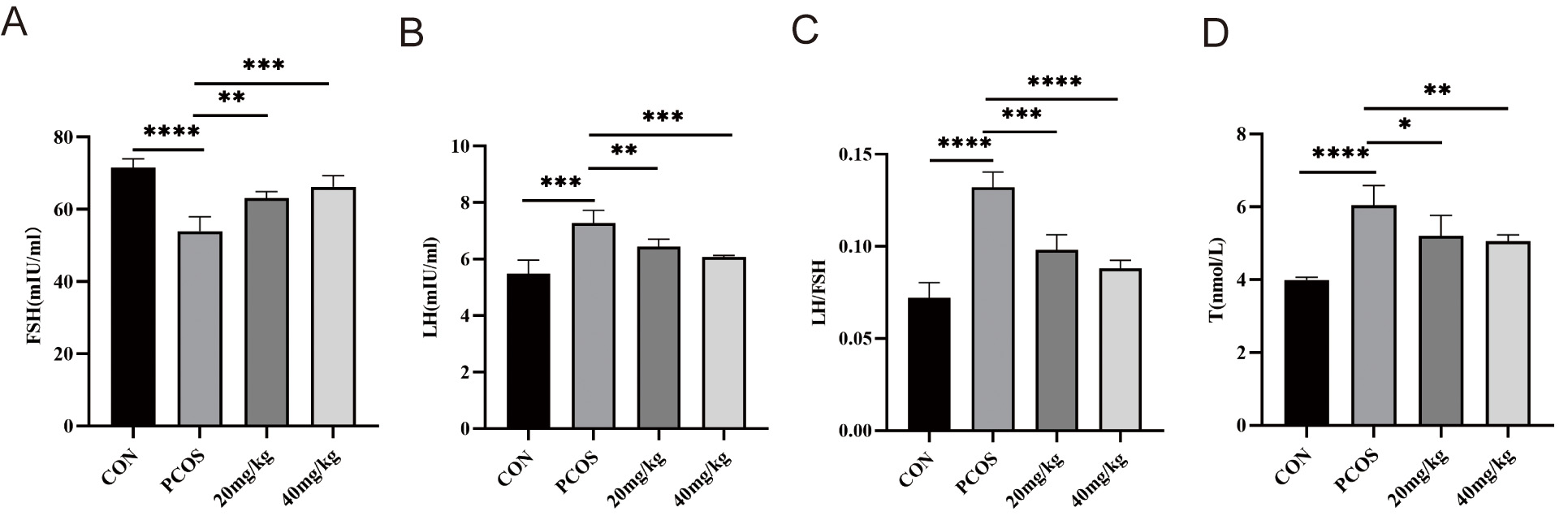 Fig. 6.
Fig. 6.
Effects of RSV on serum LH, FSH, LH/FSH ratio, and T levels in
mice. (A) Serum FSH levels in mice. (B) Serum LH levels in mice. (C) Ratio of
serum LH/FSH in mice. (D) Serum T levels in mice. * p
To assess the anti-inflammatory activity of RSV, serum IL-1
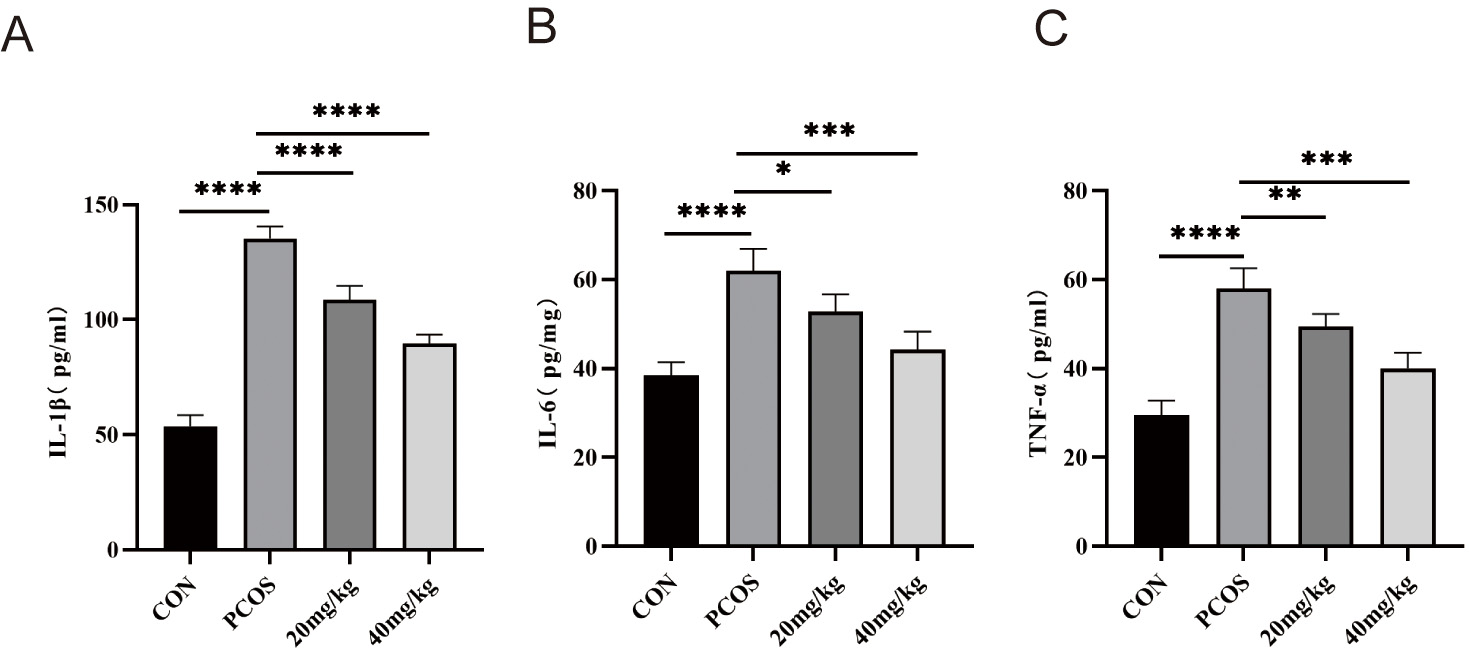 Fig. 7.
Fig. 7.
Effects of RSV on serum IL-1
As a marker of OS, MDA levels in PCOS ovaries were significantly higher than those in control ovaries (Fig. 8A), indicating the occurrence of OS, and MDA in the 20 mg/kg and 40 mg/kg RSV groups showed obvious down-regulation compared with the PCOS group. In addition, the GSH levels in the ovarian tissue was sharply reduced in PCOS mice, and then showed a dose-dependent recovery following treatment with RSV (20 mg/kg and 40 mg/kg) (Fig. 8B).
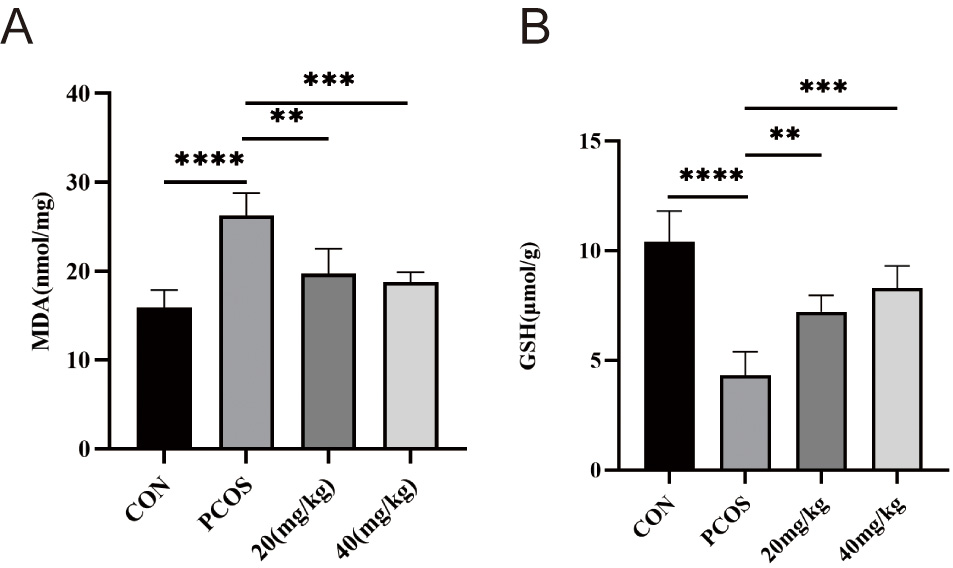 Fig. 8.
Fig. 8.
Effects of RSV on MDA and GSH levels in mice ovaries. (A) The
ovarian MDA levels in mice. (B) The ovarian GSH levels in mice. ** p
GPX4 is localized in the cytoplasm, mitochondria, and nucleus of mammalian cells [28]. This protein serves as a central mediator of ferroptosis induction while simultaneously functioning as a master regulator of its suppression [29]. Western blot analysis revealed significantly decreased GPX4 expression in PCOS ovarian tissue compared to controls, which was restored by RSV treatment (Fig. 9). The decline in GPX4 expression is consistent with the hallmarks of ferroptosis.
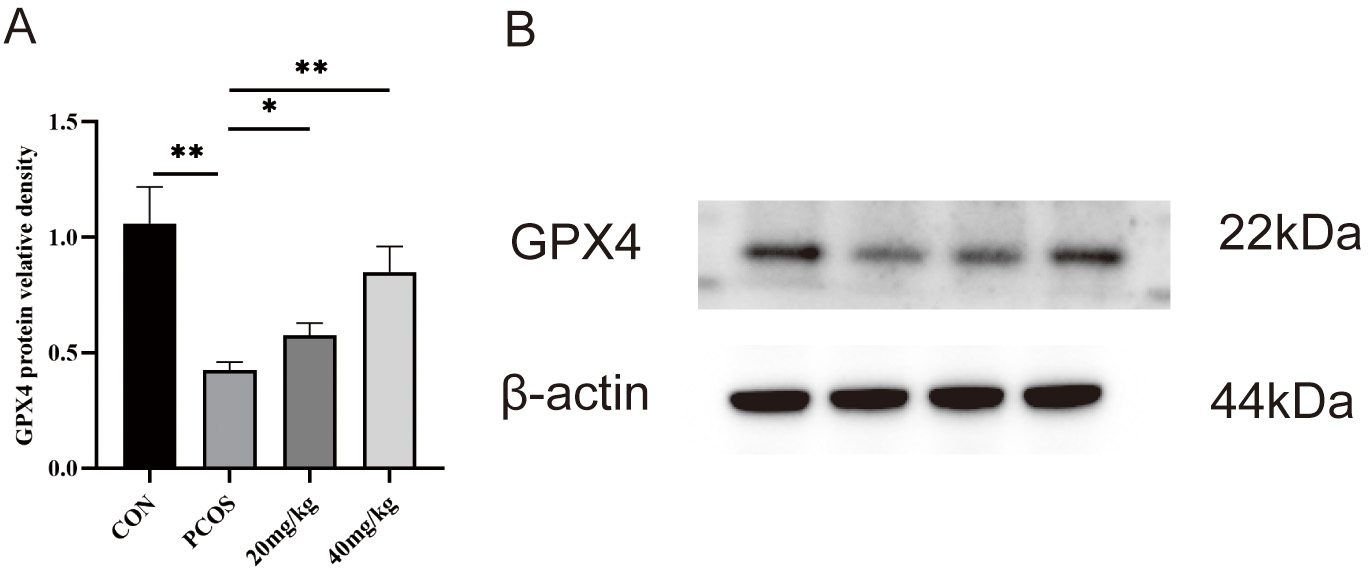 Fig. 9.
Fig. 9.
The protein levels of glutathione peroxidase 4 (GPX4) in the
ovaries were determined via western blot assays. (A) Protein relative identity
of GPX4. (B) Western blot of GPX4. * p
In this study, a DHEA-induced PCOS mouse model was successfully established. Our findings demonstrate two key observations: First, PCOS was associated with elevated chronic inflammation, OS, and ferroptosis features. Second, RSV treatment significantly ameliorated DHEA-induced ovarian dysfunction and suppressed chronic inflammatory, OS, and ferroptosis features in PCOS mice.
Among the available pre-clinical models, DHEA induction is rapid (2–3 weeks), reproducible, and yields both PCOS and metabolic dysfunction similar to human PCOS. Compared with letrozole (aromatase inhibition) or testosterone propionate administration, DHEA increases circulating androgens without markedly altering oestrogen levels, thereby better mimicking the mild hyperandrogenism characteristic of the adolescent-onset PCOS phenotype. Nevertheless, DHEA does not induce the full neuro-endocrine disruption seen in prenatal androgenization models, and it lacks the obesity phenotype evident in high-fat-diet combined models. We acknowledge that daily DHEA administration can transiently raise serum DHEA-S and androstenedione. To minimize carry-over interference, blood was collected 24 h after the last injection for ELISA.
PCOS is a common endocrine and metabolic dysfunction affecting women of reproductive age. The condition is primarily driven by insulin resistance and hyperandrogenism, accompanied by chronic low-grade inflammation and elevated OS levels [30]. These pathological mechanisms collectively contribute to ovulatory dysfunction and impaired ovarian physiological activity. Currently, the etiology and pathophysiological mechanisms underpinning PCOS are not entirely elucidated, thus, the primary therapeutic approach focuses on symptomatic management [18]. This study successfully induced a PCOS mouse model by subcutaneous injection of DHEA. Following RSV treatment, the PCOS group showed marked decreases in body weight, ovarian weight, and the ovarian index. Notably, the 20 mg/kg RSV group displayed no significant change in body weight, ovarian weight, and ovarian index. This may be because the 20 mg/kg dose may be insufficient in fully counteracting the hyperandrogenism or insulin resistance. Higher doses could more robustly activate the Sirtuin 1 (SIRT1)/AMP-activated protein kinase (AMPK) axis, enhancing insulin sensitivity and attenuating ovarian lipid accumulation. Furthermore, PCOS mice exhibited significantly elevated serum levels of LH, T, and LH/FSH ratio compared with controls. These findings align with previous reports by Peng et al. [31]. However, the results of FSH contrast with the findings of Luo et al. [32], who reported decreased FSH levels in their PCOS mouse model. This disparity in FSH levels between studies may reflect methodological variations, including: strain selection, modeling protocol, and assay sensitivity, which highlights the need for standardization in PCOS research models. These findings demonstrate that RSV treatment can restore normal gonadotropin secretion patterns and ameliorate hyperandrogenemia in PCOS mice, indicating its promise for correcting PCOS-related endocrine dysfunction. We speculate that the effects of RSV on serum T and LH levels appears to relate to the modulation of aromatase activity within the ovarian environment. Furthermore, RSV treatment exhibited a notable positive correlation with the restoration of ovarian function, as reflected by marked improvements in body weight dynamics.
Chronic low-grade inflammation is another pathognomonic trait of PCOS, with
elevated pro-inflammatory cytokines aggravating metabolic and reproductive
dysfunction [33]. Our model replicated this inflammatory milieu, consistent with
clinical studies linking inflammation to insulin resistance and hyperandrogenemia
[34]. Notably, anti-inflammatory agents like curcumin ameliorate PCOS by
suppressing cytokine production [24], indicating that targeting inflammation may
be a feasible treatment approach. RSV has demonstrated anti-inflammatory activity
in models of inflammatory disease, and RSV administration is expected to
attenuate the release of pro-inflammatory mediators [35]. In our study, RSV
supplementation markedly lowered circulating IL-1
Growing evidence implicates OS as a central player in PCOS pathogenesis. Our findings align with clinical observations demonstrating elevated oxidative markers (e.g., MDA) and diminished antioxidant defences (e.g., GSH, GPX4 activity) in PCOS patients [36, 37, 38]. OS exacerbates insulin resistance and hyperandrogenism, creating a vicious cycle that perpetuates PCOS phenotypes [39, 40]. Emerging evidence indicates that ferroptosis—an iron-driven, lipid-peroxidation-mediated mode of regulated cell death—may also compromise ovarian function in PCOS [41]. GPX4 plays a central role in ferroptosis regulation across in vitro and in vivo systems [42]. A key characteristic of ferroptosis is the accumulation of lipid peroxidation, which can be measured by detecting MDA. In addition, during the process of ferroptosis, the expression of GSH decreased. RSV demonstrates both anti-inflammatory and antioxidant activities. For assessment of the impact of RSV on OS, we examined the ovarian tissue levels of OS markers and antioxidant substances. In PCOS ovaries, we observed down-regulated GPX4 and GSH alongside elevated MDA levels—hallmarks of oxidative damage and consistent with ferroptosis features. In addition, the results suggest that the therapeutic benefits of RSV in PCOS may be mediated, in part, through its ability to restore redox homeostasis in ovarian tissue. These findings support the hypothesis that ferroptosis features exacerbate follicular atresia and ovarian fibrosis in PCOS, and the precise triggers (e.g., iron overload, GSH depletion) warrant further study [42, 43]. Although our study revealed ferroptosis-associated signatures in PCOS ovaries—namely decreased GPX4 and GSH alongside elevated MDA—the current dataset does not provide definitive evidence of ongoing ferroptosis. Key indicators of iron metabolism and canonical execution markers remain unexamined. Comprehensive profiling of iron homeostasis and downstream ferroptosis effectors is therefore required to substantiate active ferroptotic cell death in this context. This study specifically characterized the effects of RSV on OS and ferroptosis features. Future studies should establish causal relationships among RSV, OS, and ferroptosis. Moreover, our study lacked a standalone RSV-only group, leaving the compound’s independent effects on murine reproductive structure and function undetermined, and this question warrants dedicated investigation.
In summary, our study underscores the roles of inflammation, OS and ferroptosis (particularly through GPX4 regulation) in PCOS pathogenesis and demonstrates that RSV ameliorates these disturbances by restoring redox homeostasis and potentially upregulating GPX4 activity, thereby improving ovarian function. RSV shows promise as an adjunct therapy for PCOS, and confirmation of its GPX4-targeted mechanism and refinement for clinical use remain to be established.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
JC designed the research study. JC, FW, JY, YW and JW performed the research. JY, YW and JW provided help and advice on the HE, ELISA and WB experiments. JC analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All animal experiments were conducted in accordance with protocols approved by the Experimental Animal Welfare Committee of Lanzhou University Second Hospital (approval number: D-2023-071). All animal procedures were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and adhered to the “3Rs” principles (Replacement, Reduction, Refinement).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This project was supported by the Youth Science and Technology Fund of Gansu, China (Grant No.: 23JRRA1639).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
