- Academic Editor
Oxytocin, a peptide hormone, plays several physiological roles in the human body, particularly during childbirth and lactation. Inappropriate use of oxytocin may cause adverse events. Although acute coronary syndromes associated with oxytocin are rare, they should not be overlooked.
A 44-year-old female underwent hysteroscopic surgery for endometrial and cervical polyps, which were accompanied by increased menstrual flow. During the procedure, oozing from the wound was observed. To promote uterine contraction, oxytocin (10 U) was administered in 100 mL of 0.9% normal saline (NS) via slow intravenous infusion. Five minutes after the infusion, ST-segment elevation in lead II was noted. Oxytocin was immediately discontinued, and symptomatic treatment, including esmolol, was initiated. Under continuous cardiac monitoring, the dosage of medications was gradually adjusted, leading to the stabilization of vital signs, including blood pressure, heart rate, oxygen saturation, and respiration. The patient was discharged on the fifth postoperative day and has since been regularly followed up in the cardiology clinic. To date, no abnormalities have been observed.
This case underscores the importance of thoroughly informing patients about the potential uses and adverse effects of oxytocin prior to surgery. During the procedure, strict adherence to the indications for oxytocin use and close monitoring of vital signs are essential. This case serves as a reminder of the need for vigilance in managing potential complications associated with oxytocin administration.
Oxytocin is synthesized by the hypothalamus, but is stored in the posterior pituitary gland. The first physiological use of oxytocin was stimulating contractions in the uterus and stimulating lactation [1]. Oxytocin and its analogues are increasingly used clinically to induce labor, produce uterine contractions in childbirth and to prevent post-partum hemorrhage, as well as to treat complications during surgery of the uterus [2].
Numerous research studies show that the injection of oxytocin intracerebrally modifies sexual, maternal and social behavior in experimental animals; thus, oxytocin acts as a central regulator [3]. In addition, oxytocin’s influence may affect psychological and behavioral domains. Oxytocin can inhibit the release of stress hormones such as cortisol. Research indicates that it can help with stress and anxiety. Also, due to its ability to promote social interaction and emotional bonding, it has become a focus of research aimed at ameliorating social dysfunction in those affected by autism spectrum disorder. A study on the use of oxytocin in psychotherapy remain exploratory, although oxytocin’s potential in psychotherapy has received considerable attention [4]. The most important negative side effect seen following overdosing is excessive uterine contraction. Other rare side effects like acute pulmonary edema [5, 6] and acute schizophrenia [7] have also been associated with its use.
Acute coronary syndromes (ACS) refer to a wide spectrum of ischemic diseases of the myocardium due to the reduction or stopping of blood flow through the coronary arteries. Major clinical entities of ACS include unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI) [8]. The main physiological mechanisms behind ACS involve rupture of the atheroma/atherosclerotic plaque, development of coronary thrombosis caused by plaque rupture, vascular spasms, rapidly rising myocardial oxygen demand, as well as systemic factors including hypotension/shock, severe anemia, and critical arrhythmias. Coronary artery spasms have most often been triggered by prolonged smoking, acute emotional stress and cold exposure [9, 10]. Sympathomimetic drugs are a large group of drugs which can precipitate an ACS. Common examples include cocaine, amphetamines, ephedrine, and pseudoephedrine [11, 12]. At present, there have been few reported cases of oxytocin-induced ACS [13, 14].
This article describes a case of ACS brought about by the administration of oxytocin to enhance uterine contractions during hysteroscopic surgery. Reports like this one are rare, and it may prove beneficial for clinical practitioners when dealing with the rare side effects of oxytocin.
A 44-year-old female patient, gravida 2 para 1 was hospitalized with complaints of increased menstrual flow for the last three months. The patient stated that she had a spontaneous increase in menstrual flow, without any cause, three months ago. There was no change in her cycle or duration. There was no history of dysmenorrhea. Ever since the abnormal menstrual flow started, an ultrasound was completed each month, which showed a heterogeneous echo in the endometrium, multiple endometrial polyps and a cervical polyp. The patient refuted having a history of hypertension, diabetes mellitus, coronary heart disease, or family history. They reported no prior surgical history, trauma history, or drug allergies.
The patient’s age, symptoms, and ultrasound findings made the indications for hysteroscopic surgery clear. A gynecological examination was completed and pre-anaesthetic tests such as full blood count, coagulation function, biochemical profile, electrocardiogram (ECG) (Fig. 1) and Chest X-ray were done to rule out any contraindications for anaesthesia and surgery.
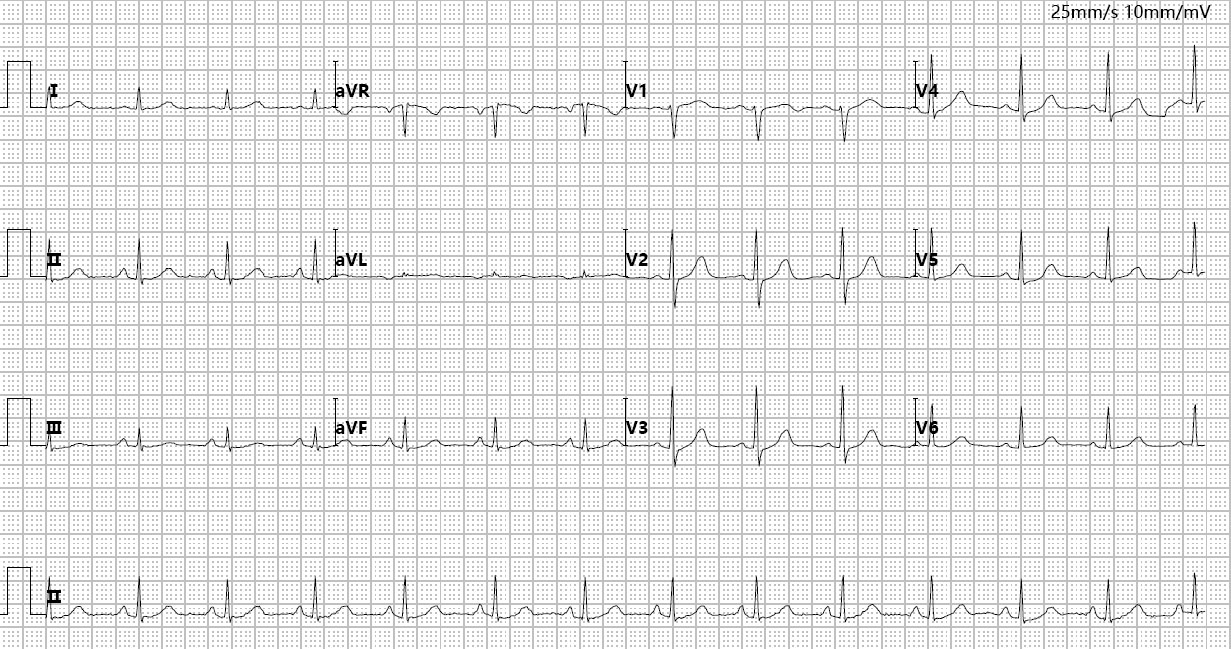 Fig. 1.
Fig. 1.
Preoperative routine ECG. aVR, augmented vector right; aVL, augmented vector left; aVF, augmented vector foot; ECG, electrocardiogram.
A hysteroscopic surgery was performed under general anesthesia. During the operation, polyps were found on the anterior and posterior walls of the uterine cavity with marked endometrial thickening and multiple cervical polyps. In the following course, hysteroscopic polypectomy, cervical polypectomy, and dilation and curettage was done. Due to the large uterine injury, risk of polyp recurrence, and expulsion of the intrauterine device (IUD), the patient also underwent insertion and fixation of a levonorgestrel-releasing intrauterine system (LNG-IUS) (Batch No.H20140237. Turku, Southwest Finland, Finland). Post-procedure, it was noted that the patient was oozing from the wound. To promote hemostasis and secure the LNG-IUS, 10 units of oxytocin (Batch No.H20233754. Chengdu, Sichuan, China) in 100 mL of 0.9% normal saline was given intravenously to stimulate uterine contraction. The surgery was completed once bleeding from the endometrium was ruled out and when the LNG-IUS was correctly positioned.
The patient developed abnormal symptoms after five minutes of slow infusion of oxytocin intravenously when the patient was under anesthesia. Sudden changes were noted in the heart monitoring with a change in the heart rate between 130–145/min, blood pressure about 200/110 mmHg, and oxygen saturation remained at 99%–100%. The ST-segment elevation on lead II was noted (monitor ECG in Fig. 2). The clinicians thought that there was an adverse event with the use of oxytocin and stopped the infusion. The elevation of the ST-segment in lead II remained for a duration of 10 seconds. As seen in Fig. 3, an emergency bedside ECG was urgently performed, together with arterial blood-gas analysis, troponin and myo Z tests and B-type natriuretic peptide (BNP). The myocardial zymography results and BNP levels can be found in Fig. 4. Furthermore, Esmolol (Batch No.H19991058. Jinan, Shandong, China) (20 mg) was injected intravenously for symptomatic relief within 30 seconds as per protocol. An urgent cardiology consultation was also requested. Considering the time lapse between oxytocin being administered and the beginning of ST-segment elevation, in addition to the stopping of the symptoms after the stop in the use of oxytocin and the preprocedural change or titration in either the type or dose of anesthetic, the cardiology team considered the ACS to be due to oxytocin-induced coronary spasm and not from any drug-drug interactions. The team suggested that oxytocin may trigger a coronary artery spasm in patients without previous coronary artery disease. It was wise to monitor the patient closely, use the nitroglycerin if required, and follow relevant indicators dynamically. Further investigation for coronary artery disease could also be completed as necessary.
 Fig. 2.
Fig. 2.
ST-segment elevation in ECG lead II for ST-segment elevation.
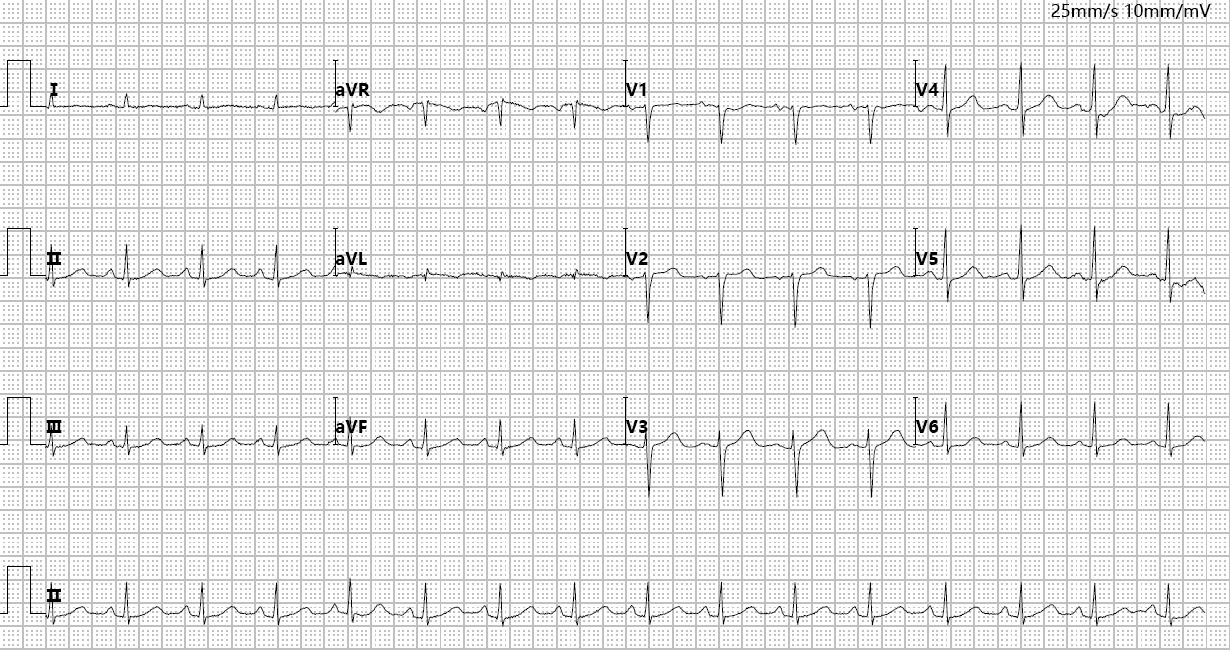 Fig. 3.
Fig. 3.
Emergency bedside ECG.
 Fig. 4.
Fig. 4.
Results of the blood test of indicators. (A) Aspartate aminotransferase. (B) Creatine kinase. (C) Creatine kinase-MB isoforms. (D) Lactate dehydrogenase. (E) High-sensitivity troponin I. (F) B-type natriuretic peptide.
Twenty minutes later, the patient’s blood pressure dropped. Subsequently, the blood pressure was increased with an intravenous bolus of 8 µg norepinephrine. Along with this, a continuous infusion of norepinephrine (Batch No.H42021301. Wuhan, Hubei, China) was given to keep the patient’s blood pressure at approximately 85/50 mmHg at a rate of 0.2 mg/h. Thirty-five minutes following the beginning of oxytocin infusion, the patient started to breathe. After the laryngeal mask was taken off, the patient was conscious and awake. The patient was well-oriented and mildly tired. The patient denied any chest-tightening. She also denied chest pain and pressure in the precordium. Upon further questioning, the patient also denied that she had ever reacted adversely to oxytocin. Under strict medical supervision, the blood pressure of the patient was slightly low; but, other vital signs were stable. Arterial computed tomography (CT) and echocardiography revealed no abnormalities. The dosage of norepinephrine was titrated under cardiac monitoring, ultimately resulting in stable vital signs. The blood pressure, pulse rate, oxygen saturation and respiration rate, showed improvement, with all parameters being stable. In this period, dynamic follow-up of myocardial zymography, BNP, and troponin I of the patient (Fig. 4). On the fifth postoperative day, the patient was discharged, and continued to be regularly followed up in the cardiology clinic. To date, no further abnormalities have been observed.
This article reviews the diagnostic and therapeutic process after admission, specifically reporting on the clinical manifestation of acute coronary syndrome due to the use of oxytocin in the intraoperative period. It reports on the medical team’s management measures and rationale for their clinical decision-making. It also describes phenomena that can happen to the patient. Cardiovascular events induced by the administration of oxytocin are rare. Adverse events that have been reported previously refer to pregnant women, fetuses and neonates. The clinical studies show that cardiovascular adverse events in pregnant women resulting from oxytocin mainly manifest as cardiac arrhythmias. Premature ventricular contractions have been found to be the most common among them.
The main use of oxytocin intrapartum and postpartum hemorrhage prevention and control. Also, some researchers have stated that oxytocin could control bleeding due to impaired uterine contraction during gynecologic procedures such as myomectomy, curettage and hysteroscopy [15, 16, 17, 18, 19]. According to the reports, the adverse reactions of oxytocin leading to ACS have all been reported only in obstetric practices related to cesarean section procedures with oxytocin. Crabbé et al. [13], a parturient with no cardiovascular risk factors, developed severe ACS followed by ventricular fibrillation (VF) after receiving 30 µg of carbetocin IV at the beginning of the cesarean section. Similarly, Jacquenod et al. [20] cited a case of a patient without cardiovascular risk factors to develop ACS following a single intramuscular injection of 100 µg carbetocin during emergency cesarean section. These two cases show that oxytocin analogues can cause ACS, regardless of whether an intramuscular or intravenous route of administration is used. Higher doses of medication or rapid administration rates may further augment the risk of ACS [21]. Furthermore, we must distinguish other specific diseases of the cardiovascular system that present with ACS-like symptoms. There was also a case of a multiparous woman who displayed significant ACS during an elective caesarean section, which was later diagnosed as Takotsubo cardiomyopathy, reported by Zdanowicz et al. [14]. Substantial short-term blood loss may also cause vasoconstriction [22, 23, 24]. It is necessary to distinguish whether the reaction to oxytocin, when given for massive uterine bleeding, is a drug or a blood disorder reaction.
The mechanism of oxytocin’s adverse effects, such as ACS, remains uncertain, although it could be connected to its mechanism of action. Signaling through the oxytocin receptor, expressed in the myometrium and mammary glands but also in endometrium, decidua, ovary, testis, epididymis, vas deferens, thymus, heart, kidneys, and brain, is required for the physiological effects of oxytocin [25]. The same receptors used in the myometrium can also produce vastly different side effects when oxytocin is given to the patient in clinical settings to induce contractions of the uterus. Moreover, this could be related to the considerable and temporary increase in the corrected QT (QTc) interval that occurs due to oxytocin, leading to prolonged cardiac repolarization [26, 27]. The electrical issues might cause blood flow changes, calling for more care from doctors.
Management of oxytocin-induced ACS is the same as typical ACS. The only
difference is the immediate stoppage of oxytocin. Strategies for treating ACS
caused by other drugs may act as a clinical reference. Prior applications have
emphasized the noteworthy clinical utility of the
This case carries significant clinical implications. To begin with, oxytocin is regularly used during delivery and post-abortion but it is not an essential drug for gynecological operations. Women at high risk of intraoperative or postoperative hemorrhage may require oxytocin to induce uterine contraction; therefore, the indication for its use and its possible adverse reactions should be thoroughly explained before the procedure. With this, the case highlights the importance of following oxytocin indication strictly, choosing standard dosages, and monitoring vital signs during the perioperative period. Studies show that early and accurate diagnosis, followed by timely and effective intervention, significantly improves the chances of a favorable outcome. As a result of this evidence, clinicians are much more aware of the possibility of a drug-related cardiovascular adverse event and this evidence should be useful in changing the drug use in the perioperative setting and in clinical decision-making.
However, this study has several limitations. Being a case report, it is inescapably influenced by individual differences and can, at most, only provide a limited degree of clinical evidence and reference information. Its main value lies in sharing the diagnostic and therapeutic experience of this unusual case. The exact molecular mechanisms of oxytocin-mediated constriction of coronary arteries are incompletely understood which calls for further in-depth mechanistic studies and clinical studies in this regard.
This is an unusual case of ACS following low-dose oxytocin during a gynecological surgery due to oxytocin-induced coronary artery spasm. The negative drug effect appeared suddenly, with ECG abnormalities in anesthetized patients followed rapidly by blood pressure instability. In this case, cessation of oxytocin was done immediately after the patient’s circulatory support was started. Through this case, we highlight that one must have clinical knowledge and consideration about the possible cardiovascular adverse effects of oxytocin. This ensures early recognition, timely intervention and regular postoperative follow-up in order to improve prognosis.
ACS, acute coronary syndromes; BNP, B-type natriuretic peptide; ECG, electrocardiogram; LNG-IUS, levonorgestrel-releasing intrauterine system; UA, unstable angina; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; IUD, intrauterine device; BNP, B-type natriuretic peptide; CT, computed tomography; aVR, augmented vector right; aVL, augmented vector left; aVF, augmented vector foot; QTc, corrected QT.
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the first author. Requests to access these datasets should be directed to Chuhan Wang, wang153000@sina.com.
Material preparation, data collection and analysis were performed by CW, HL, DM and YY. HY and YY contributed to the study as supervision. HY contributed to the conception, and design of the work. All authors contributed to the study conception and design. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was carried out in accordance with the guidelines of the Declaration of Helsinki. The studies involving human participants were reviewed and approved by this study was approved by the Ningbo NO.2 Hospital’s research ethics committee (Ethics Reference: PJ-NBEY-KY-2025-058-01). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. No potentially identifiable human images or data is presented in this study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG40626.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
