- Academic Editor
This study is a prospective cohort study that aimed to explore the mechanism involved in myocardial energy metabolism disturbance in breast cancer patients with stages I to II. Notably, myocardial energy metabolism disturbance is induced by epirubicin, cyclophosphamide, and paclitaxel taxane (EC-T) sequential chemotherapy after breast-conserving surgery.
Stage I and stage II breast cancer patients treated in the breast or oncology departments of the hospital from February 2021 to May 2023 were invited to participate in the study. The patients were treated with EC-T sequential chemotherapy after breast-conserving surgery. The patients were divided into a control group (no cardiotoxic event, n = 155) and an observation group (cardiotoxic event, n = 45) based on clinical evaluations and laboratory tests. All patients signed informed consent for the protocol during study inclusion. Two groups were compared for clinically relevant differences. Blood biochemical analyses were performed to detect the levels of myocardial injury and myocardial oxidative stress. Enzyme linked immunosorbent assay (ELISA) was employed to detect serum mitochondrial respiratory enzyme activity. Cardiac magnetic resonance spectroscopy (MRS) was used to detect myocardial energy metabolism. The blood flow and metabolic status of the heart were assessed by positron emission tomography (PET).
The general data were identical in the two groups (p > 0.05). The observation group had significantly higher levels of troponin, creatine kinase, creatine kinase-MB (CK-MB) isoenzyme, and lactate dehydrogenase compared with the control group; meanwhile, the activities of complex I, complex III, and complex V were significantly lower (p < 0.05). In the observation group, the levels of adenosine triphosphate and creatine phosphate were lower than those in the control group, while the levels of reactive oxygen species (ROS) and malondialdehyde (MDA) were higher (p < 0.05). The levels of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in the observation group were lower than those in the control group, while the levels of pyruvate and β-hydroxybutyric acid were higher (p < 0.05). Moreover, cardiac blood flow, myocardial glucose uptake, myocardial fatty acid uptake, and myocardial metabolic efficiency in the observation group were lower compared with the control group (p < 0.05).
In breast cancer patients with stages I to II who were receiving an EC-T sequential chemotherapy regimen, myocardial energy metabolism disorders are associated with increased markers of myocardial injury. This phenomenon is associated with decreasing mitochondrial respiratory enzyme activity, changes in energy metabolic pathways, and reduced cardiac blood flow and metabolic efficiency. These results suggest that EC-T chemotherapy may cause direct damage to cardiomyocytes and affect the normal metabolic function of the heart.
Breast cancer is among the most prevalent types of malignant tumors in women worldwide. Consequently, the development of treatment strategies for breast cancer has been a primary focus of medical research [1]. Patients suffering from breast cancer with stages I to II are usually cured by breast-conserving surgery followed by chemotherapy. The aim is to minimize the risk of cancer recurrence through systematic treatment while preserving breast tissue. In this treatment mode, the sequential chemotherapy regimen epirubicin, cyclophosphamide, and paclitaxel taxane (EC-T) is widely used due to its good efficacy. However, with the further study of the EC-T chemotherapy regimen, its potential cardiotoxicity has gradually attracted clinical attention. Cardiotoxicity, especially disturbance of myocardial energy metabolism, is an important side effect of EC-T chemotherapy [2, 3, 4, 5]. Myocardial energy metabolism disorders involve abnormalities in the production and use of energy by cardiomyocytes, which are essential for the normal function of the heart. As an organ with high energy requirements, the heart has strict requirements for the efficiency and stability of energy metabolism [6, 7]. Cardiomyocytes are responsible for the production of a significant quantity of adenosine triphosphate (ATP), primarily through the process of mitochondrial oxidative phosphorylation. This process is essential for maintaining sustained cardiac contraction [8, 9]. Kuang Z et al. [10] reviewed the current molecular pathways leading to cardiac toxicity in cancer treatment and systematically discussed prevention and treatment strategies for cardiac toxicity, providing a new perspective on the management of cardiac toxicity. Giffoni de Mello Morais Mata D et al. [11] reviewed randomized controlled trials conducted before March 11, 2024, to investigate the effects of docetaxel and cyclophosphamide on cardiac toxicity. The results showed that compared with anthracycline taxanes, docetaxel and cyclophosphamide had cardiotoxicity. In a retrospective review of patients with stages I–III cancer, Tsai JH et al. [12] compared the effectiveness of combination therapy involving epirubicin and PEGylated liposomal doxorubicin in treating cancer. The results indicated that the cardiac toxicity of patients under the proposed treatment plan was lower than that of EC-T.
In the EC-T chemotherapy regimen, epirubicin and paclitaxel are considered to be the main sources of cardiotoxicity. Epirubicin blocks the replication of cancer cells by cross-linking with DNA, but may also cause damage to heart muscle cells. Paclitaxel affect cell division by blocking microtubule dynamics, and their effects on cardiomyocytes may involve interference with the microtubule network and subsequent metabolic effects [13, 14]. Myocardial cell damage may lead to mitochondrial dysfunction, affecting the balance of ATP synthesis and energy metabolism. The mechanism of myocardial energy metabolism disorder may include many aspects, such as direct cytotoxic effects, oxidative stress, impaired mitochondrial function, and changes in energy metabolic pathways [15, 16, 17]. Oxidative stress refers to the inequality of intracellular reactive oxygen species (ROS) and antioxidant mechanisms, which can lead to damage to myocardial cell membranes, changes in protein function, and DNA damage. Mitochondria are energy factories in cardiomyocytes, and their dysfunction directly affects the energy supply of the myocardium [18, 19]. In addition, cardiomyocytes may adapt to chemotherapy-induced stress by altering energy metabolic pathways, such as increasing glycolysis as an energy source. At present, the treatment strategy for breast cancer is constantly being optimized, and it is urgent to comprehensively understand the cardiotoxicity caused by EC-T chemotherapy. A deep understanding can help develop effective prevention strategies, thereby improving treatment safety. This study aims to investigate how EC-T sequential chemotherapy regimens induce myocardial energy metabolism disorders and the potential mechanisms involved in this process.
This study adopted a prospective cohort study design. The subjects were 200 patients with stage
I-II breast cancer who were cured in the Department of Breast and the Department of Oncology of
Baoding No.2 Central Hospital from February 2021 to May 2023. Among them, some patients were
initially diagnosed or consulted by general surgery and then transferred to breast or oncology
departments for specialized treatment. The patients were
treated with an EC-T sequential chemotherapy regimen after breast-conserving
surgery. The EC-T sequential chemotherapy regimen consists of 90
mg/m2 Cefpirome Sulfate for Injection (Pfizer (Wuxi) Co., Ltd., Wuxi, Jiangsu, China),
600 mg/m2 Cyclophosphamide for Injection (Baxter Oncology GmbH, Halle,
Germany), and 100 mg/m2 paclitaxel (Luye Pharma Group Ltd., Hong Kong,
China), once a day for 21 d/cycle. These patients were aged 42~70,
with an average age of 56.34
Patients aged between 18 and 70, diagnosed with stages I to II breast cancer. They have good living ability, and their performance status scores are 0 to 1 point in Eastern Cooperative Oncology Group (ECOG). Baseline hematology, liver, and kidney function indexes are within the normal range. The patient has not been diagnosed with any other serious heart diseases or systemic conditions, including but not limited to unstable angina, recent myocardial infarction, uncontrolled heart failure, severe arrhythmia, severe liver dysfunction, severe kidney dysfunction, uncontrolled hypertension, or diabetes. They sign the informed consent.
Patients suffer from unstable angina pectoris, recent myocardial infarction, and uncontrolled arrhythmia. Patients have had a severe cardiotoxic reaction to a drug or a similar drug in the EC-T sequential chemotherapy regimen. Patients suffer from serious liver or kidney dysfunction or other uncontrolled major diseases. Patients cannot understand the study nature or comply with the study requirements.
The levels of troponin, creatine kinase, creatine kinase-MB (CK-MB) isoenzyme, and lactate dehydrogenase, which are markers of myocardial injury, were tested by blood biochemical analysis. The level changes of myocardial injury markers could reflect the development process of cardiac toxicity. Before chemotherapy, baseline testing of biomarkers such as troponin and CK-MB was performed to determine the patient’s initial state. Biomarker testing was required before each chemotherapy cycle, as well as 24, 48, and 72 hours after chemotherapy, once a week to capture dynamic changes in biomarkers and detect abnormalities promptly. After chemotherapy, it was necessary to continue monitoring the levels of biomarkers. If the biomarkers’ levels continue to rise or slowly recover, it might indicate more severe potential cardiac toxicity. 5–10 mL of blood was intravenously drawn and centrifuged (3000 rpm, 10 minutes) to separate the blood to obtain serum or plasma. Enzyme linked immunosorbent assay (ELISA, Fuzhou Yilisha Biotechnology Development Co., Ltd., YLS-P6931, Fuzhou, Jiangxi, China) was commonly used for the detection of cardiac troponin. The signal strength of a specific antibody binding to troponin was measured and subsequently converted into a concentration (ng/mL). Enzyme activity assay was used to evaluate the activity of creatine kinase and CK-MB isoenzymes. Creatine kinase substrate was creatine; CK activity was quantified by measuring changes in absorbance at specific wavelengths (340 nm). The CK-MB level was monitored by electrophoresis. The lactate dehydrogenase activity was evaluated by catalyzed lactate-to-pyruvate reactions. The activity of mitochondrial respiratory enzyme in serum was detected by ELISA. ELISA mainly measured the protein concentration rather than directly measuring enzyme activity, and it might be limited by the characteristics of serum samples. Therefore, even if mitochondrial respiratory enzymes were present in the serum, ELISA could not provide direct information about the activity of these enzymes. The use of tissue assay was necessary for directly measuring mitochondrial function in myocardial cells, as it could provide a more accurate and direct assessment of mitochondrial function. The reaction was performed using specific antibodies and substrates, and the absorbance values were read by a spectrophotometer to calculate the protein concentrations of complexes I, III, and V.
Cardiac MRS was used to determine myocardial energy metabolism. After the patient lay flat and entered the magnetic resonance imaging (MRI) scanner, standard cardiac imaging sequences were employed to locate the heart. Time echo (TE, about 30 ms) and time repetition (TR, about 3000 ms) of parameters could be scanned to get adequate signals. According to the target metabolite resolution, it was required to adjust the sampling points, bandwidth, and scanning layers while ensuring the uniformity of the B0 field. The line width, signal-to-noise ratio, baseline stability, and the presence of artifacts were required to be checked. During the above process, patients must remain still. The metabolite peaks in the MRS Spectra, adenosine triphosphate at 8.5 ppm, creatine phosphate at 3.0 ppm, and pyruvate and beta-hydroxybutyric acid at their specific chemical shifts, were analyzed and quantified by professional software. The concentrations of these metabolites (usually expressed as mmol/L) and their ratios were calculated to assess the energy metabolic status of the heart muscle, such as phosphocreatine (PCr) or ATP.
Myocardial oxidative stress is tested by blood biochemical analysis. It mainly includes the determination of ROS, malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx). ROS is the core product of oxidative stress. An increase in ROS levels directly indicates that cells are in an oxidative stress state. MDA is the main product of lipid peroxidation. An increase in MDA levels indicates an exacerbation of lipid peroxidation reactions. SOD is an important antioxidant enzyme, while GPx is a non-enzymatic antioxidant substance. The increased activity of SOD and GPx indicates an enhanced antioxidant capacity of the body. In summary, ROS, MDA, SOD, and GPx play critical roles in oxidative stress response, and their detection methods are mature and reliable. Therefore, these four biomarkers are selected as markers for myocardial oxidative stress in the study. Venous blood is centrifuged to obtain serum. ROS is determined by the chemiluminescence method. As an indicator of lipid peroxidation, MDA is evaluated by the thiobarbituric acid reactants assay. The activities of SOD and GPx are determined by the spectroscopic method.
Cardiac blood flow and metabolic status were assessed by PET. Cardiac blood flow was measured using oxygen-15 labeled water as a tracer, with an intravenous injection dose of 600 MBq. Imaging typically began immediately after injection and lasted for approximately 10 minutes. The pharmacokinetic analysis of cardiac blood flow was performed using a dual tissue compartment model, which was calibrated using arterial input functions for calculation. The myocardial glucose uptake was evaluated using 18F fluorodeoxyglucose and quantitatively analyzed using standardized uptake values. The intravenous injection dose was 3.7 MBq/kg, and a waiting time of 60 minutes was required after injection to allow the tracer to be taken up in the myocardium. The imaging time was usually 30 minutes. The myocardial fatty acid uptake was measured using carbon-11 palmitic acid as a tracer, with an intravenous injection dose of 380 MBq and a typical imaging time of 15–20 minutes. The analysis of myocardial fatty acid uptake used a single tissue compartment model to calculate inflow and outflow rates. The metabolic correction was applied to the input function to eliminate the interference of tracer metabolites in the blood. Myocardial metabolic efficiency was determined by calculating the ratio of the heart’s work output to energy expenditure.
The mean and standard deviation of continuous variables were calculated. The
categorical variables were calculated for frequency and percentage. The
comparison of continuous variables between two groups was conducted using a
t-test, and the comparison of categorical variables between two groups
was conducted using the chi-square test. Statistically, the whole analyses were
carried out by means of SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). If there
was missing or dropout data, relevant records should be deleted when analyzing
variables involving missing data, and complete data for other variables should be
retained. The research data were obtained through normality testing using the
Shapiro-Wilk method. The p
| Variable | Group | Shapiro-Wilk | p | Distribution state |
| Age (years) | Control | 0.927 | 0.089 | Normal distribution |
| Observation | 0.948 | 0.254 | ||
| BMI (kg/m2) | Control | 0.903 | 0.052 | |
| Observation | 0.924 | 0.112 | ||
| Troponin (ng/mL) | Control | 0.943 | 0.198 | |
| Observation | 0.935 | 0.151 | ||
| Creatine kinase (U/L) | Control | 0.873 | 0.062 | |
| Observation | 0.902 | 0.074 | ||
| CK-MB isoenzyme (U/L) | Control | 0.896 | 0.072 | |
| Observation | 0.911 | 0.062 | ||
| Lactate dehydrogenase (U/L) | Control | 0.955 | 0.294 | |
| Observation | 0.959 | 0.380 | ||
| Complex I (IU/L) | Control | 0.948 | 0.142 | |
| Observation | 0.963 | 0.358 | ||
| Complex III (IU/L) | Control | 0.912 | 0.068 | |
| Observation | 0.934 | 0.117 | ||
| Complex V (IU/L) | Control | 0.909 | 0.055 | |
| Observation | 0.885 | 0.031 | ||
| ATP (mmol/L) | Control | 0.865 | 0.045 | |
| Observation | 0.849 | 0.025 | ||
| Creatine phosphate (mmol/L) | Control | 0.895 | 0.063 | |
| Observation | 0.915 | 0.078 | ||
| Pyruvate (µmol/L) | Control | 0.887 | 0.054 | |
| Observation | 0.918 | 0.114 | ||
| Control | 0.934 | 0.116 | ||
| Observation | 0.942 | 0.145 | ||
| ROS (U/mL) | Control | 0.921 | 0.084 | |
| Observation | 0.914 | 0.092 | ||
| SOD (U/mg protein) | Control | 0.887 | 0.056 | |
| Observation | 0.919 | 0.131 | ||
| GPx (U/mg protein) | Control | 0.900 | 0.066 | |
| Observation | 0.926 | 0.075 | ||
| MDA (µmol/L) | Control | 0.943 | 0.094 | |
| Observation | 0.956 | 0.201 | ||
| Cardiac blood flow (mL/min/g) | Control | 0.935 | 0.118 | |
| Observation | 0.948 | 0.195 | ||
| Myocardial glucose uptake (µmol/min/g) | Control | 0.927 | 0.085 | |
| Observation | 0.917 | 0.107 | ||
| Myocardial fatty acid uptake (µmol/min/g) | Control | 0.941 | 0.133 | |
| Observation | 0.949 | 0.191 | ||
| Myocardial metabolic efficiency (%) | Control | 0.928 | 0.175 | |
| Observation | 0.920 | 0.171 |
Control group (n = 155), Observation group (n = 45). BMI, body mass index; CK-MB, creatine kinase-MB; ATP, adenosine triphosphate; ROS, reactive oxygen species; SOD, superoxide dismutase; GPx, glutathione peroxidase; MDA, malondialdehyde.
First, statistical analysis was performed on the general clinical data of two
groups of patients. In the control group, the average age was 56.17
| Items | Control group (n = 155) | Observation group (n = 45) | t/ |
p |
| Age (years) | 56.17 |
57.25 |
1.386 | 0.167 |
| BMI (kg/m2) | 23.68 |
23.47 |
0.681 | 0.497 |
| Stage of breast cancer (I:II) | 70:85 | 19:26 | 0.122 | 0.727 |
| High blood pressure (%) | 26 (16.77%) | 8 (17.77%) | 0.025 | 0.875 |
| Diabetes mellitus (%) | 17 (10.96%) | 6 (13.33%) | 0.192 | 0.661 |
| Smoking (%) | 15 (9.67%) | 5 (11.11%) | 0.000 | 1.000 |
| Alcohol consumption (%) | 22 (14.19%) | 7 (15.55%) | 0.052 | 0.819 |
Indicators of myocardial injury markers were detected by blood biochemical
analysis, and the levels of troponin, creatine kinase, CK-MB isoenzyme, and
lactate dehydrogenase in the observation group were significantly higher than
those in the control group (p
| Items | Troponin (ng/mL) | Creatine kinase (U/L) | CK-MB isoenzyme (U/L) | Lactate dehydrogenase (U/L) |
| Control group (n = 155) | 1.03 |
115.63 |
28.64 |
162.66 |
| Observation group (n = 45) | 2.86 |
275.49 |
81.43 |
315.42 |
| t | 259.578 | 64.306 | 32.309 | 32.904 |
| p | ||||
| Cohen’s d | 31.17 | 10.46 | 4.65 | 4.76 |
| 95% CI | [30.56, 31.78] | [9.58, 11.34] | [4.03, 5.27] | [4.15, 5.37] |
CI, confidence interval.
Serum mitochondrial respiratory enzyme activity is tested by ELISA. Fig. 1 shows
that the activities of complex I, complex III, and complex V in the observation
group are significantly lower than those in the control group (p
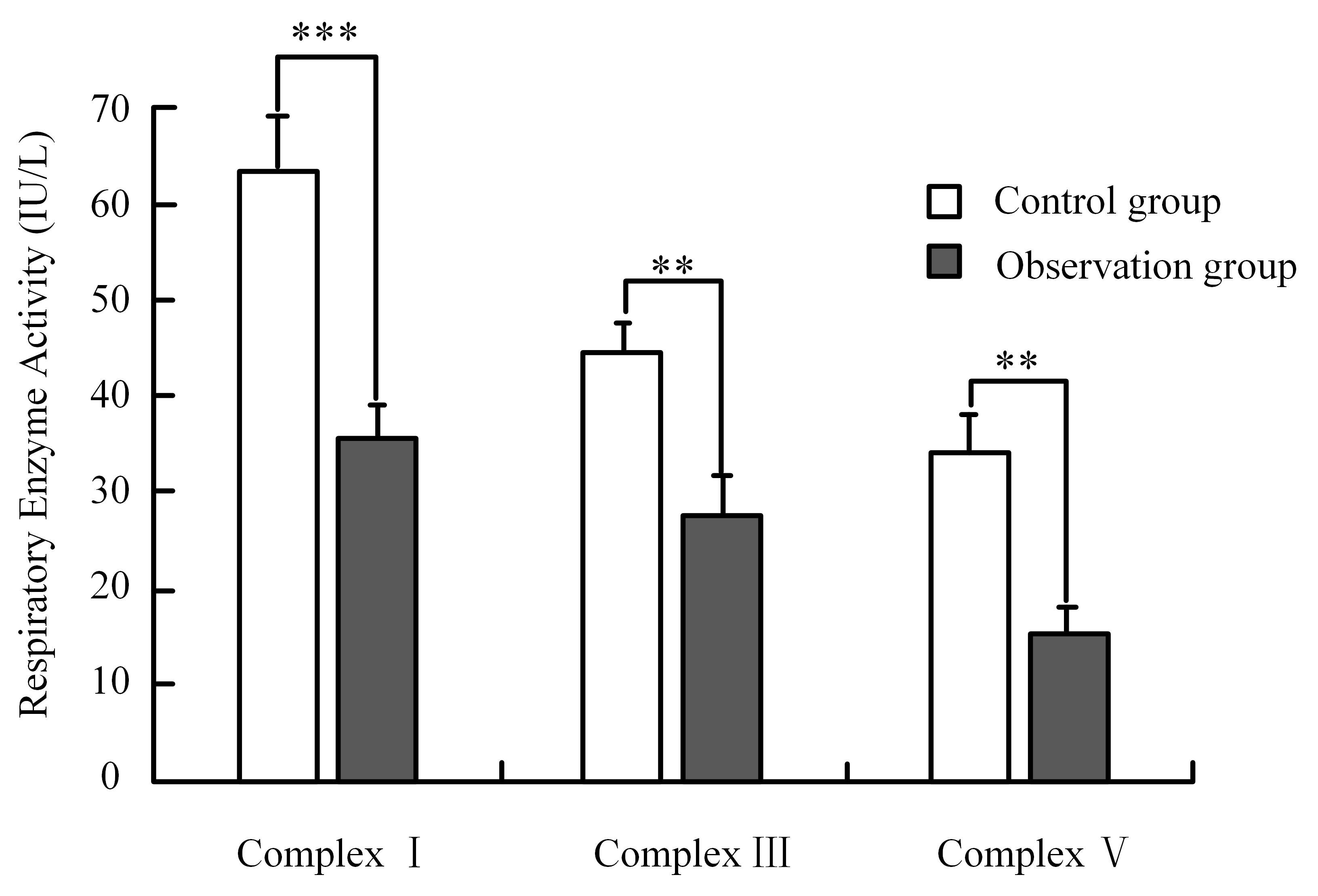 Fig. 1.
Fig. 1.
Respiratory enzyme activity. Note: *** means p
| Items | Complex I (IU/L) | Complex III (IU/L) | Complex V (IU/L) |
| Control group (n = 155) | 63.42 |
44.37 |
34.18 |
| Observation group (n = 45) | 35.37 |
27.65 |
15.44 |
| t | 32.882 | 29.045 | 33.017 |
| p | |||
| Cohen’s d | 5.52 | 4.79 | 5.38 |
| 95% CI | [5.03, 6.01] | [4.37, 5.21] | [4.92, 5.84] |
MRS is used to test the relevant indices of myocardial energy metabolism. The
levels of adenosine triphosphate and phosphocreatine in the observation group are
significantly lower than those in the control group (p
| Items | Adenosine triphosphate (mmol/L) | Creatine phosphate (mmol/L) | Pyruvate (µmol/L) | |
| Control group (n = 155) | 5.26 |
11.64 |
142.16 |
53.68 |
| Observation group (n = 45) | 3.14 |
5.84 |
215.37 |
84.23 |
| t | 36.969 | 47.850 | 30.887 | 40.446 |
| p | ||||
| Cohen’s d | 6.55 | 7.89 | 5.13 | 6.50 |
| 95% CI | [6.05, 7.05] | [7.31, 8.47] | [4.59, 5.67] | [5.97, 7.03] |
MRS, magnetic resonance spectroscopy.
Blood biochemical analysis is employed to detect myocardial oxidative stress.
ROS and MDA in the observation group are higher than those in the control group
(p
| Items | ROS (U/mL) | SOD (U/mg protein) | GPx (U/mg protein) | MDA (µmol/L) |
| Control group (n = 155) | 0.23 |
54.68 |
65.27 |
2.15 |
| Observation group (n = 45) | 0.51 |
35.72 |
35.58 |
4.38 |
| t | 46.666 | 18.084 | 36.800 | 26.635 |
| p | ||||
| Cohen’s d | 7.00 | 3.16 | 6.09 | 4.35 |
| 95% CI | [6.51, 7.49] | [2.66, 3.66] | [5.56, 6.62] | [3.92, 4.78] |
PET is employed to evaluate the cardiac blood flow and metabolic status. From
Fig. 2, the images of the observation group show an uneven distribution of
myocardial blood flow, while the myocardial blood flow distribution of the
control group is relatively uniform. The cardiac blood flow, myocardial glucose
uptake, myocardial fatty acid uptake, and myocardial metabolic efficiency of the
observation group are significantly lower than those of the control group
(p
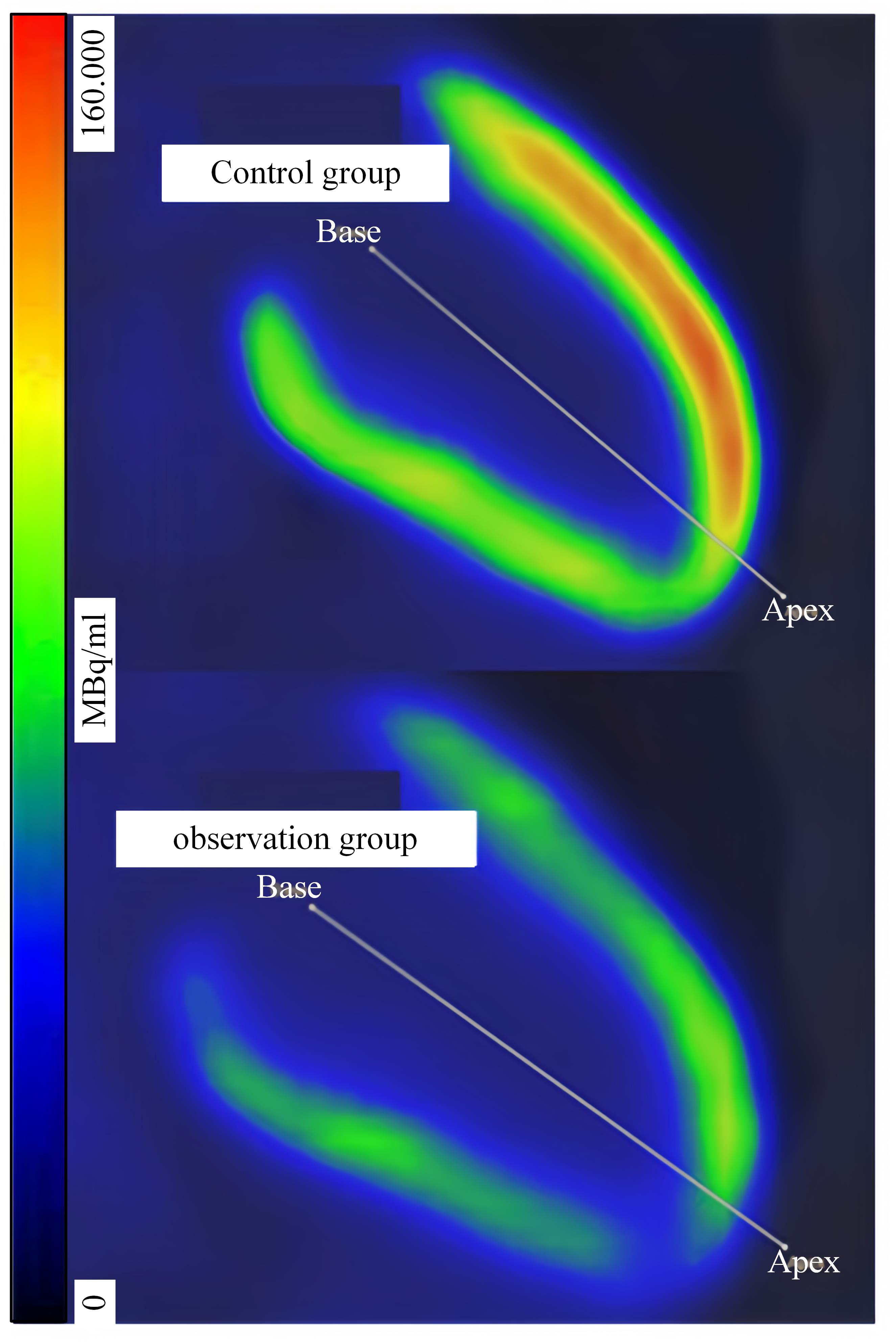 Fig. 2.
Fig. 2.
Results of myocardial glucose uptake.
| Items | Cardiac blood flow (mL/min/g) | Myocardial glucose uptake (µmol/min/g) | Myocardial fatty acid uptake (µmol/min/g) | Myocardial metabolic efficiency (%) |
| Control group (n = 155) | 1.15 |
0.55 |
0.44 |
74.35 |
| Observation group (n = 45) | 0.74 |
0.32 |
0.27 |
58.67 |
| t | 17.975 | 12.593 | 13.496 | 20.430 |
| p | ||||
| Cohen’s d | 2.93 | 2.30 | 2.43 | 3.25 |
| 95% CI | [2.58, 3.28] | [1.94, 2.66] | [2.08, 2.78] | [2.79, 3.71] |
PET, positron emission tomography.
In early breast cancer (stages I to II), breast-conserving surgery and adjuvant treatment are considered good approaches [20, 21]. With the development of medicine, the treatment plan for breast cancer is increasingly personalized, focusing on the balance of comprehensive treatment effect and patient quality of life. Breast cancer with stages I to II refers to the early stage of breast cancer in which the tumor is confined to the breast and may have slight metastases to local lymph nodes. At this stage, breast-conserving surgery (only the tumors and a small number of normal tissue are taken out to leave most of the breast tissue intact) is a common treatment option because it can maximize the appearance and function of the breast while removing the cancer. After surgery, adjuvant chemotherapy is often recommended to reduce the risk of cancer recurrence. EC-T sequential chemotherapy regimen is a common therapy. This regimen is first cured by an “EC” combination and then switched to “T” drug therapy. Epirubicin is an anthracycline antibiotic with anti-tumor activity, while cyclophosphamide is an alkylating agent that can interfere with DNA replication and repair [22]. Paclitaxel stop cancer cells from dividing by inhibiting the normal function of microtubules. This sequential chemotherapy regimen is designed to maximize the killing of cancer cells while minimizing damage to normal cells through a drug combination with different mechanisms. Although EC-T has shown good results in treating early-stage breast cancer, it can also be associated with side effects, including cardiotoxicity, hair loss, nausea, and vomiting. There is a close correlation between cardiotoxicity and myocardial energy metabolism. Cardiotoxicity refers to the negative effects of certain drugs or treatments on cardiac function, which may be manifested as myocardial damage, arrhythmia, heart failure, etc. [23, 24]. Myocardial energy metabolism disorders refer to the decline in the ability of cardiomyocytes to produce and use energy (primarily adenosine triphosphate). This study finds that the EC-T sequential chemotherapy regimen may impair cardiac function, especially in terms of myocardial energy metabolism.
By biochemical analysis, it is found that indicators of troponin, creatine kinase, CK-MB isoenzyme, and lactate dehydrogenase in the observation group are greatly higher than those in the control group, which has important clinical significance. Troponin is a specific marker of myocardial injury and occupies a vital place in the early diagnosis of acute myocardial infarction [25]. It is quickly released into the bloodstream after heart muscle cell damage. Therefore, the increase in levels usually indicates damage or necrosis of heart muscle cells. Similarly, creatine kinase and its MB isoenzyme are another sensitive indicator of myocardial injury. CK-MB is in high amounts in cardiac muscle tissue and is released into the blood when the heart muscle is damaged. An increase in CK-MB levels is frequently associated with myocardial damage [26]. In addition, as an enzyme that is widely present in various tissues of the body, an increase in lactate dehydrogenase may also reflect the overall situation of myocardial cell injury [27]. The elevation of these biochemical markers indicates that EC-T chemotherapy may cause some degree of toxic effects on the heart. Epirubicin and paclitaxel are known to have direct or indirect toxic effects on the heart, which can lead to damage and even necrosis of heart muscle cells. Specifically, epirubicin, an anthracycline chemotherapy agent, has gained significant recognition for its cardiotoxic effects, which may result in damage to cardiomyocytes by increasing oxidative stress, leading to mitochondrial dysfunction, and ultimately, apoptosis. The activity of complex I, complex III, and complex V in the observation group is significantly reduced compared to the control group, indicating that the EC-T chemotherapy regimen may have a negative impact on cardiac mitochondrial function. As an important place for energy metabolism in cardiomyocytes, the reduction of mitochondrial function may have a significant impact on the normal physiological activities of the myocardium [28]. Complex I, complex III, and complex V are key enzymes in the respiratory chain within the mitochondria, and are responsible for electron transport during oxidative phosphorylation and adenosine triphosphate synthesis [29]. The main function of complexes I and III is to transfer electrons in the electron transport chain, while complex V uses the proton gradient produced by electron transport to synthesize adenosine triphosphate. The observed reduction in the activity of the complexes indicates that mitochondria are engaged in the process of electron transfer and energy conversion efficiency. Chemotherapy drugs, especially anthracyclines such as epirubicin, may inhibit the activity of these complexes by causing mitochondrial DNA damage, increasing oxidative stress, or directly affecting the structure and function of mitochondria [30]. In addition, paclitaxel may have further indirect effects on mitochondrial function by affecting microtubule dynamics. The impairment of mitochondrial function has been demonstrated to result in a reduction of energy output by cardiomyocytes. In addition, this impairment may lead to a process of apoptosis and necrosis of cardiomyocytes, which can adversely affect the contractile function and overall performance of the heart. The changes in metabolic pathways are directly related to changes in mitochondrial function and energy production. Mitochondria are the main organelles responsible for energy conversion and supply within cells. In myocardial energy metabolism, changes in mitochondrial function directly affect the energy supply and cardiac function of the heart. In the state of heart failure, there may be significant changes in energy metabolism in the myocardium, leading to a decrease in mitochondrial adenosine triphosphate production capacity. In addition, myocardial metabolic disorders may lead to substrate utilization changes, mitochondrial structural alterations and dysfunction, adenosine triphosphate synthesis and transport disorders, etc., further exacerbating cardiac structure and dysfunction.
Cardiac MRS showed that the indices of adenosine triphosphate and phosphocreatine in
the observation group were not greatly higher than those in the control group,
while the indices of pyruvate and
However, there are still issues with the small sample size and reliance on a single medical center in this study, which may affect the generalizability and external validity of the results. Therefore, future research should further conduct multicenter studies, including more patients to improve statistical efficacy and enhance the generalizability of results. And conduct long-term follow-up to evaluate the long-term effects of cardiac toxicity.
In summary, the results showed that in breast cancer patients with stages I to II who received an EC-T sequential chemotherapy regimen, myocardial energy metabolism disorders were associated with several factors. These factors included the increasing myocardial damage markers, the decreasing mitochondrial respiratory enzyme activity, the changes in energy metabolic pathways, and the reduced cardiac blood flow and metabolic efficiency. These results explained that EC-T chemotherapy might cause direct damage to cardiomyocytes and affect the normal metabolic function of the heart. Therefore, attention should be paid to monitoring cardiac function and preventing cardiotoxicity during therapy. This study is helpful for medical professionals to better understand the cardiac toxicity risk that breast cancer patients may face during chemotherapy. By monitoring myocardial energy metabolism markers, cardiac toxicity can be identified early, allowing for timely adjustment of treatment plans, reduction of serious cardiac events, and provision of valuable information for future research and clinical practice. However, the study only included 200 patients from a single hospital, with a relatively small sample size, which may lead to insufficient statistical power of the results and limit their generalizability. Consequently, subsequent research endeavors should prioritize the augmentation of the sample size and the incorporation of multicenter data to enhance the generalizability and reliability of the findings.
The dataset analyzed during the current study are not publicly available due to the involvement of sensitive patient information but are available from the corresponding author on reasonable request.
CL designed the research study. ML and WX conducted experiments. CW recorded data. HZ analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Baoding No.2 Central Hospital (Approval Number: 23). All participants signed informed consent forms before being included in the study, and the informed consent forms were archived by the Ethics Committee of Baoding No.2 Central Hospital, ensuring the confidentiality and security of the documents.
Not applicable.
The research is supported by: the assignment of Baoding Science and Technology Plan Project, clinical research on heart injury caused by EC-T sequential chemotherapy after breast conserving surgery for patients with stage I–II breast cancer (No. 2241ZF019).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG26415.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
