-
- Academic Editor
-
-
-
Endometriosis is a chronic gynecologic disorder characterized by systemic inflammation, with growing evidence implicating gut microbial dysbiosis. However, the relationship between inflammatory cytokines and gut microbiota across disease stages remains unclear.
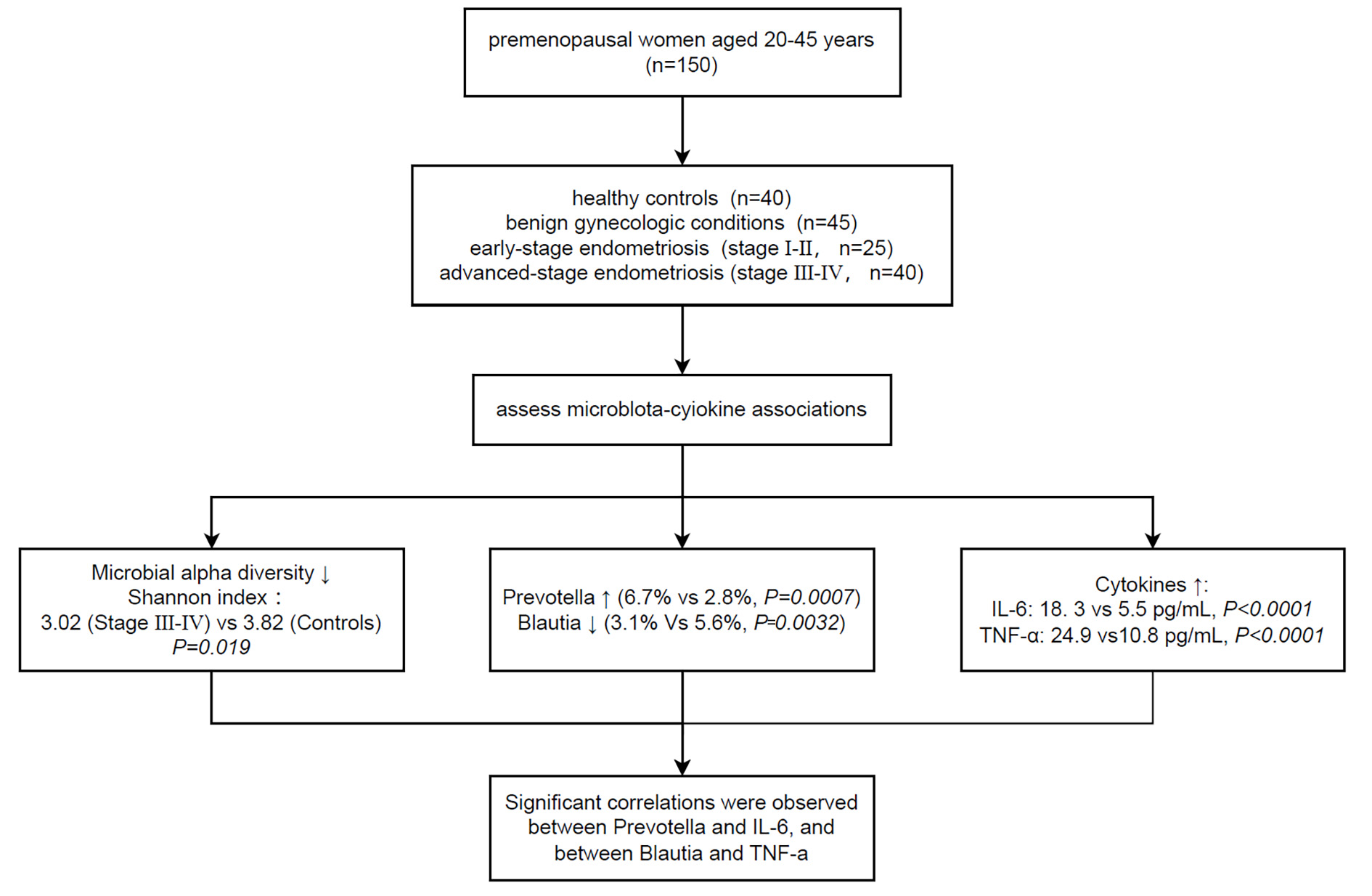
This retrospective cross-sectional study included 150 participants, divided into healthy controls (n = 40), benign gynecologic disease controls (n = 45), and patients with stage I–II (n = 25) or stage III–IV (n = 40) endometriosis. Levels of the serum cytokines interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) were evaluated by enzyme-linked immunosorbent assay (ELISA). Gut microbiota was profiled via 16S rRNA sequencing, followed by assessment of microbial alpha diversity, beta diversity (Bray-Curtis), and genus-level taxonomic composition.
Serum IL-6 and TNF-α levels increased progressively with disease severity. IL-6 levels differed significantly across groups (Kruskal-Wallis p < 0.0001), with the stage III–IV endometriosis group showing a median level that was 12.8 pg/mL higher compared to healthy controls 95% confidence interval (CI: 10.7 to 13.8). Shannon diversity decreased significantly across groups, and principal coordinate analysis (PCoA) demonstrated distinct clustering of microbial communities according to disease status. Spearman correlation analysis revealed that the genus Prevotella was positively correlated with IL-6 (ρ = 0.33, q = 0.018), whereas Blautia was negatively correlated with TNF-α (ρ = –0.32, q = 0.026), with both remaining significant after correcting for the false discovery rate (FDR).
These findings suggest that systemic inflammation and gut microbiota alterations progress alongside endometriosis severity. Specific genera, such as Prevotella and Blautia, may serve as potential microbial markers and modulators of inflammatory status in endometriosis.