- Academic Editor
Obesity significantly influences female reproductive health; however, its specific impact on hormonal predictors of ovarian response remains uncertain. The follicle-stimulating hormone (FSH)/ anti-Mullerian hormone (AMH) ratio has recently gained attention as a potential marker of ovarian reserve and response to controlled ovarian stimulation. This study aimed to assess the association between the FSH/AMH ratio and oocyte count and to determine whether body mass index (BMI) modifies this relationship.
In this retrospective study, 185 women undergoing ovarian stimulation were reviewed, and 92 met predefined clinical and hormonal inclusion criteria. Baseline FSH, luteinizing hormone (LH), AMH, BMI, and oocyte counts were recorded, and the FSH/AMH ratio was calculated. Associations were assessed using Spearman correlation, Kruskal-Wallis tests, and linear regression analysis.
AMH levels showed a strong positive correlation with oocyte count, while the FSH/AMH ratio demonstrated a strong negative correlation. FSH exhibited a weak negative correlation, and no significant association was observed between BMI and hormonal markers. Neither oocyte count nor the FSH/AMH ratio differed significantly across BMI categories. Linear regression analysis confirmed that the FSH/AMH ratio was an independent predictor of oocyte yield (p < 0.001), whereas BMI and its interaction with the ratio were not statistically significant.
The FSH/AMH ratio is a reliable and BMI-independent predictor of ovarian response. These findings support its clinical utility in fertility assessment and treatment planning, particularly when standard markers are inconclusive.
Obesity represents a major global health issue and is increasingly prevalent among women of reproductive age. It is well-established that excess body weight negatively impacts female reproductive function, contributing to menstrual irregularities, anovulation, impaired oocyte quality, and reduced fertility rates [1, 2, 3, 4]. Epidemiological studies indicate that obese women are nearly three times more likely to experience infertility compared to women with a normal body mass index (BMI) [5]. Ovulatory dysfunction—manifesting as oligomenorrhea or anovulation—is more frequently observed in obese women and may occur independently or as part of polycystic ovary syndrome (PCOS), a condition closely linked with obesity [6]. Nevertheless, the precise mechanisms by which obesity impairs reproductive function remain incompletely elucidated.
In addition to reproductive disturbances, obesity is associated with systemic comorbidities such as insulin resistance, type 2 diabetes mellitus, hypertension, cardiovascular disease, and certain cancers, all of which may indirectly compromise reproductive health [7]. Notably, the pattern of fat distribution appears to influence reproductive outcomes. Central (visceral) adiposity, in particular, has been more strongly associated with ovulatory infertility than peripheral fat distribution [8, 9, 10]. Increasing evidence suggests that obesity is not solely a mechanical or metabolic burden but also an endocrine disorder, characterized by the secretion of bioactive molecules known as adipokines [11]. Among these, leptin plays a pivotal role in both energy homeostasis and reproductive regulation [12].
Leptin, primarily secreted by white adipose tissue, serves as a critical signal to the hypothalamic-pituitary-gonadal (HPG) axis and is essential for maintaining reproductive endocrine function. It promotes the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus, which subsequently stimulates the anterior pituitary to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [13]. The importance of leptin in reproductive physiology has been demonstrated in leptin-deficient animal models and in rare human cases of congenital leptin deficiency, both of which exhibit impaired reproductive function that improves with leptin supplementation [14, 15]. However, in the context of obesity, chronically elevated leptin levels may induce leptin resistance, impairing its regulatory effects and contributing to reproductive dysfunction [16]. Animal studies have further shown that obesity-induced hyperleptinemia can blunt the hypothalamic and ovarian responsiveness to leptin, thereby disrupting folliculogenesis and ovulation [17].
Beyond leptin, the hormones FSH and anti-Mullerian hormone (AMH) are widely
recognized as key indicators of ovarian reserve and reproductive capacity [18].
FSH, secreted by the anterior pituitary, stimulates the growth and maturation of
ovarian follicles. Elevated basal FSH levels—particularly during the early
follicular phase—may reflect diminished ovarian reserve due to decreased
negative feedback from estrogen and inhibin
Obesity has been shown to alter both AMH and FSH levels, although findings in the literature remain inconsistent [21]. Some studies have reported lower AMH levels in obese women, possibly due to impaired granulosa cell function or the inhibitory effect of leptin on AMH gene expression [22, 23]. Meanwhile, FSH levels in obese women may remain stable or slightly reduced, potentially due to increased peripheral estrogen synthesis via aromatase activity in adipose tissue, which suppresses FSH secretion through negative feedback mechanisms [24].
The hormonal dysregulation associated with obesity—including altered levels of leptin, insulin, estrogen, and androgens—may collectively interfere with normal folliculogenesis, ovulation, and fertility potential [2, 25]. To better understand this complex interplay, recent research has investigated composite hormonal indices, particularly the FSH/AMH ratio, as a more integrative marker of ovarian function [26, 27]. Since FSH and AMH exhibit opposing trends with declining ovarian reserve—FSH increasing and AMH decreasing—their ratio may more accurately reflect the dynamic hormonal milieu than either marker alone. Several studies have explored the utility of the FSH/AMH ratio in predicting ovarian response in assisted reproductive technology (ART) settings, with some reporting associations between higher FSH/AMH ratios and reduced oocyte yield or diminished ovarian function [28, 29].
Given the rising prevalence of obesity among reproductive-aged women and its multifaceted effects on ovarian physiology and endocrine function, further research is needed to clarify the relationships between obesity and reproductive hormone profiles. In the present study, we aimed to evaluate the associations between BMI and key reproductive markers—specifically FSH, AMH, and the FSH/AMH ratio—and to assess their predictive value for oocyte yield in women undergoing controlled ovarian stimulation. We further aimed to investigate whether the FSH/AMH ratio serves as a more sensitive indicator of ovarian reserve across different BMI categories, thereby enhancing fertility assessment and individualized treatment planning.
This retrospective cross-sectional study was conducted at the Assisted Reproductive Techniques Center of Acibadem Mehmet Ali Aydinlar University Atakent Hospital between 2015 and 2022. A total of 185 women who underwent controlled ovarian stimulation as part of infertility treatment were initially evaluated. The study received approval from the Institutional Review Board of Acibadem Mehmet Ali Aydinlar University School of Medicine (protocol code 2023-10/427) and was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Inclusion criteria were as follows: women aged 25 to 35 years, with baseline FSH
levels
All participants underwent controlled ovarian stimulation using a recombinant gonadotropin protocol. On the second day of the menstrual cycle, patients received recombinant FSH (Gonal-F®, Merck-Serono S.A., Darmstadt, Hesse, Germany) at a dose of 225–300 IU. A GnRH antagonist protocol was employed for all patients. Serum FSH and LH levels were measured on cycle days 2 or 3, and AMH levels were assessed prior to the initiation of stimulation. Hormonal assays were conducted using an electrochemiluminescence immunoassay (ECLIA) on the COBAS 8000 e801 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Baseline AMH values and post-treatment oocyte counts were recorded for all participants.
Additional parameters, including thyroid-stimulating hormone (TSH) levels and
BMI, were also documented. The FSH/AMH ratio was calculated for each patient by
dividing the serum FSH level by the AMH concentration. Oocyte retrieval was
performed following stimulation, and the total number of retrieved oocytes served
as the primary outcome measure. To assess the potential influence of body weight
on hormonal profiles and ovarian response, participants were stratified into five
BMI categories based on World Health Organization (WHO) classifications:
underweight (
Statistical analyses were performed using GraphPad Prism (version 9.5.1;
GraphPad Software Inc., San Diego, CA, USA). Continuous variables were presented
as mean
The normality of continuous variables was assessed using the Shapiro-Wilk test, and Levene’s test was used to evaluate the homogeneity of variances across BMI groups. For between-group comparisons, one-way analysis of variance (ANOVA) was applied to normally distributed variables (age, BMI, FSH), while the Kruskal-Wallis test was used for non-normally distributed variables (LH, TSH, AMH, FSH/AMH ratio, and oocyte count). Spearman’s rank correlation analysis was employed to examine associations between hormonal markers (FSH, AMH, FSH/AMH ratio), BMI, and oocyte yield.
To determine the independent contributions of the FSH/AMH ratio and BMI to
oocyte count, a multiple linear regression model was developed. An interaction
term (FSH/AMH ratio
In addition, a non-parametric bootstrap procedure was conducted to estimate the confidence intervals (CIs) of the mean oocyte count within each BMI category. This method involved 10,000 resampling iterations and was performed to assess the robustness and reliability of subgroup comparisons, particularly for BMI groups with limited sample sizes.
A total of 92 women met the predefined inclusion criteria: age between 25 and 35
years, FSH
Table 1 summarizes the baseline characteristics of the study population
stratified by BMI category. Across BMI groups, LH levels differed significantly
(p = 0.050), while no significant differences were observed for age,
FSH, AMH, TSH, or oocyte count (all p
| Underweight ( |
Normal weight (18.50–24.90 kg/m2) | Overweight (25.00–29.90 kg/m2) | Class I Obese (30.00–34.90 kg/m2) | Class II Obese (35.00–39.90 kg/m2) | p-value | |
| Age (years) | 33.33 |
33.50 [31.25–35.00] | 34.00 [32.00–35.00] | 34.00 [33.75–35.00] | 32.50 |
0.890 |
| Oocyte Counts | 11.33 |
7.00 [5.00–11.00] | 7.45 |
9.62 |
8.50 |
0.160 |
| BMI (kg/m2) | 18.00 [17.65–18.00] | 22.00 [20.86–23.41] | 26.98 |
31.98 |
36.07 |
|
| FSH (IU/mL) | 6.61 |
7.18 |
6.50 [5.44–8.75] | 6.94 |
7.05 |
0.780 |
| AMH (ng/mL) | 3.09 |
1.46 [0.90–2.43] | 1.56 |
1.76 |
1.89 |
0.080 |
| LH (IU/mL) | 6.50 |
5.43 |
5.12 |
3.69 |
5.64 |
0.050 |
| TSH (mIU/L) | 2.35 |
1.87 [1.31–2.52] | 1.87 |
1.80 |
2.48 |
0.740 |
Data are presented as mean
FSH, follicle-stimulating hormone; AMH, anti-Mullerian hormone; LH, luteinizing hormone; TSH, thyroid-stimulating hormone; BMI, body mass index.
Spearman’s correlation analysis revealed a strong positive association between
AMH and oocyte count (r = 0.63, p
Participants were stratified into five BMI categories based on WHO classifications: underweight (n = 3), normal weight (n = 50), overweight (n = 29), class I obese (n = 7), and class II obese (n = 3). The highest median oocyte count was observed in the underweight group (11), followed by class II obese (10), class I obese (9), overweight (8), and normal weight (7). Median FSH/AMH ratios were 2.24 (underweight), 4.77 (normal weight), 4.32 (overweight), 5.09 (class I obese), and 9.40 (class II obese), as illustrated in Figs. 1,2. However, the Kruskal-Wallis test revealed no statistically significant differences among BMI groups in either oocyte count (H = 5.35, p = 0.253) or FSH/AMH ratio (H = 5.58, p = 0.134). These findings suggest that BMI category was not significantly associated with ovarian response or hormonal ratio within this cohort, although observable trends warrant further exploration in larger populations. To further examine the robustness of oocyte count estimates across BMI subgroups, non-parametric bootstrap analyses (10,000 iterations) were performed. The 95% CIs for the mean oocyte counts were as follows: underweight, 6.67–13.67; normal weight, 6.45–8.31; overweight, 6.70–8.28; class I obese, 6.57–10.71; and class II obese, 5.50–10.50. Notably, the widest CIs were observed in the underweight and class II obese groups, consistent with their limited sample sizes (n = 3 for each). Despite numerical differences in mean oocyte yield across BMI categories, the overlapping CIs indicate that these variations are likely due to sampling variability rather than statistically robust subgroup effects.
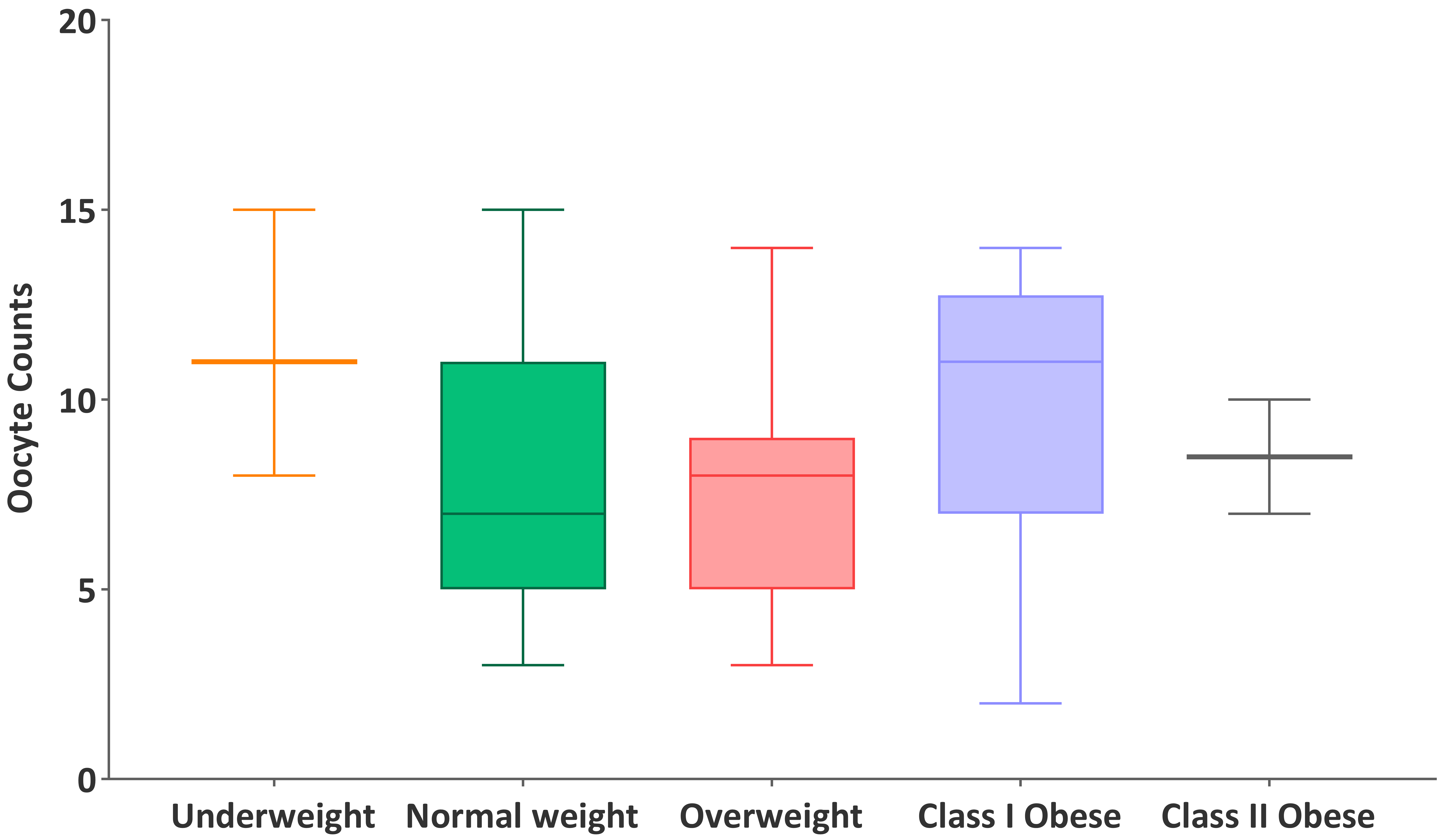 Fig. 1.
Fig. 1.
Distribution of oocyte counts across BMI categories.
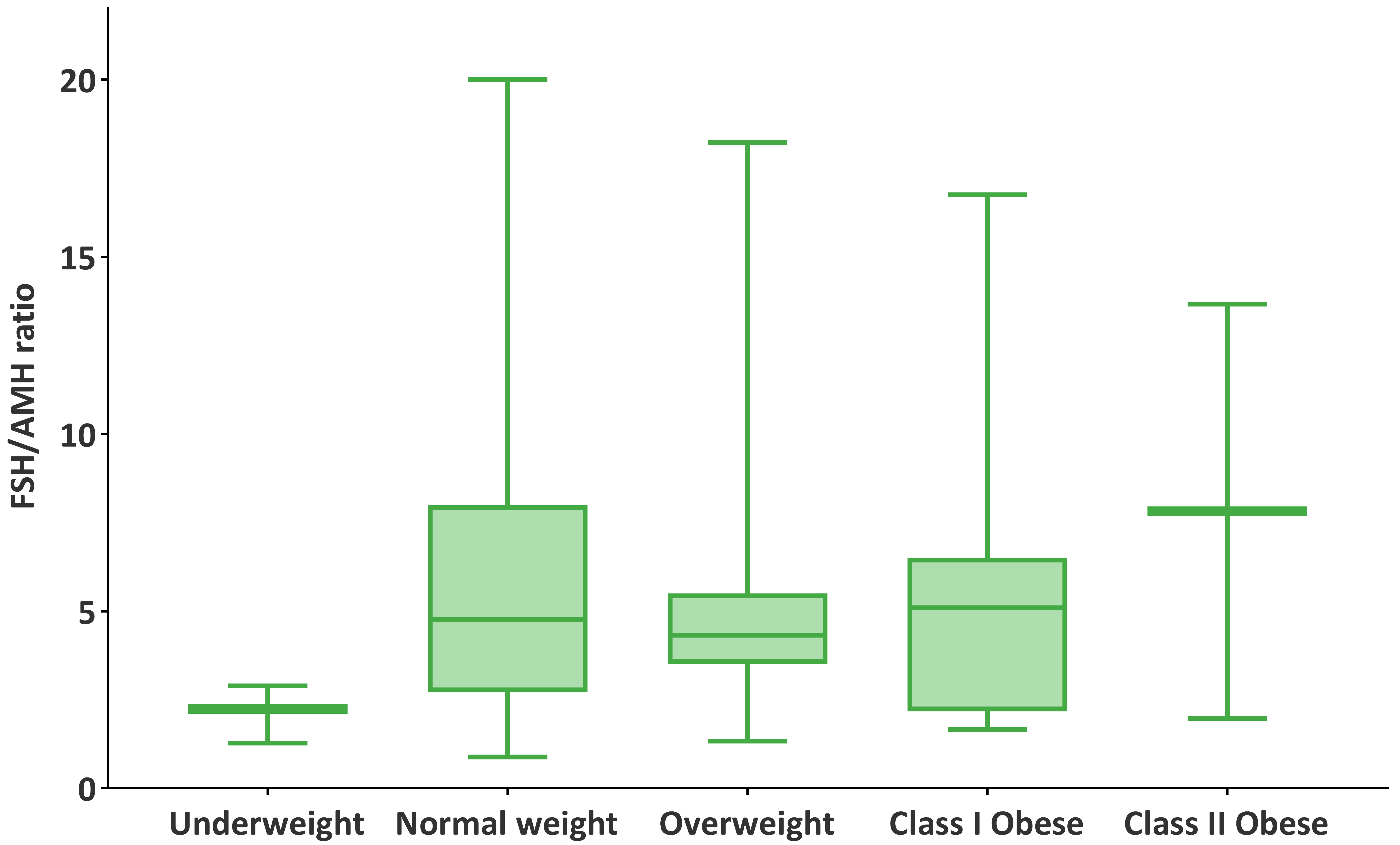 Fig. 2.
Fig. 2.
Distribution of FSH/AMH ratios across BMI categories.
To further assess predictors of ovarian response, linear regression analysis was
conducted (Fig. 3). In the initial model, which included both the FSH/AMH ratio
and BMI as continuous variables, the FSH/AMH ratio was significantly and
negatively associated with oocyte count (
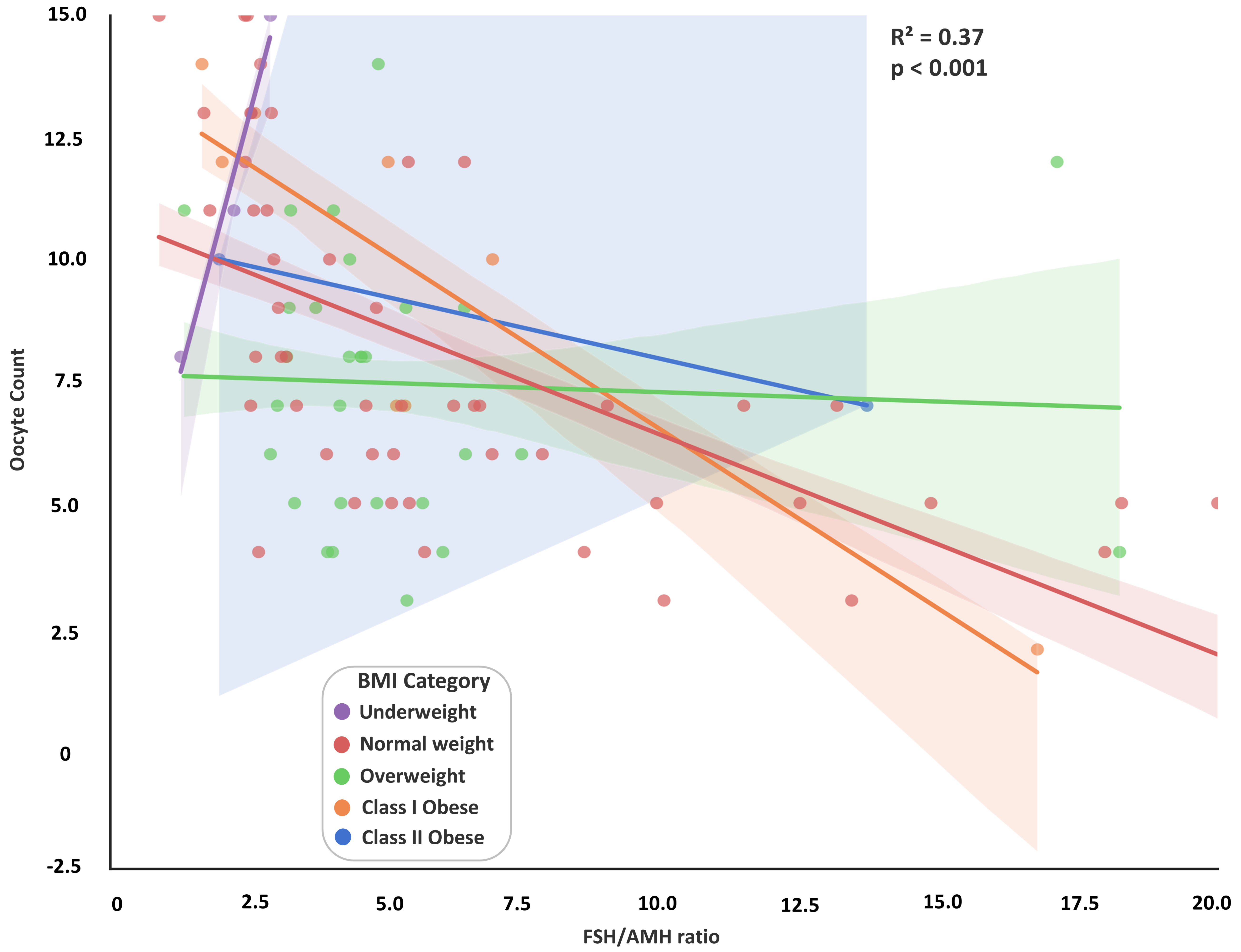 Fig. 3.
Fig. 3.
Relationship between FSH/AMH ratio and oocyte count across BMI
categories. A scatterplot illustrates the negative association between the
FSH/AMH ratio and the number of retrieved oocytes, stratified by BMI categories:
underweight (
This study evaluated the predictive value of the FSH/AMH ratio for ovarian response and assessed whether BMI modifies this relationship in infertile women undergoing controlled ovarian stimulation. Our findings demonstrate that the FSH/AMH ratio is a robust and independent predictor of oocyte yield, whereas BMI and its interaction with the ratio were not statistically significant. These results highlight the clinical utility of the FSH/AMH ratio as a composite endocrine marker that integrates both pituitary function and ovarian reserve, providing a more reliable indicator of ovarian responsiveness than either hormone alone, regardless of BMI.
The association between obesity and impaired fertility is well-established and multifaceted. Excess adipose tissue adversely affects ovulation, oocyte quality, and endometrial receptivity, contributing to suboptimal reproductive outcomes in women with elevated BMI [2, 30]. Emerging evidence suggests that obesity disrupts the hypothalamic-pituitary-ovarian axis through alterations in insulin signaling, steroidogenesis, and the secretion of bioactive adipokines, thereby impairing ovarian function and responsiveness to gonadotropins. In this study, we observed numerically higher FSH/AMH ratios and lower oocyte yields among women in higher BMI categories, aligning with previous reports of diminished ovarian responsiveness in obese patients [2].
Although BMI is often cited as a determinant of reproductive outcomes, our results suggest that, among women with a preserved ovarian reserve and within a moderate BMI range, BMI alone does not significantly influence AMH, FSH, or their ratio. This finding aligns with prior reports indicating that AMH remains a consistent predictor of ovarian response and is relatively unaffected by BMI [27]. In contrast, FSH tends to exhibit greater variability and is less reliable when considered in isolation [18]. The FSH/AMH ratio has emerged as a more integrative marker, combining pituitary activity (FSH) with ovarian follicular output (AMH). In this study, a higher FSH/AMH ratio was strongly correlated with reduced oocyte yield, confirming previous observations that elevated ratios are associated with diminished follicular development and an increased risk of cycle cancellation [28, 29].
The FSH/AMH ratio may be particularly valuable in clinical scenarios where individual hormone levels are borderline or contradictory. Prior research has demonstrated that this ratio exhibits higher sensitivity and specificity for predicting poor ovarian response compared to AMH or FSH alone [27, 28]. Evidence from animal models of diet-induced obesity supports these findings, showing that elevated FSH/AMH ratios are associated with reduced follicle counts and impaired ovarian function [29]. Similarly, in our cohort, higher ratios were negatively correlated with oocyte yield across all BMI groups, suggesting that this composite index effectively captures subtle disturbances in ovarian function that may not be evident from individual hormone levels.
Although AMH is widely regarded as a marker of ovarian reserve, it is influenced by dynamic intra-ovarian processes. AMH secretion begins during the transition from primordial to primary follicles and peaks at the antral stage. Its expression is regulated by intra-ovarian factors and may be modulated by FSH [31]. A high FSH/AMH ratio may therefore reflect both a diminished follicular pool and a compensatory increase in pituitary output, indicating accelerated ovarian reserve depletion [18]. Furthermore, obesity has been reported to reduce AMH production through impaired granulosa cell function and decreased FSH receptor expression [22]. In our study, patients with both higher BMI and elevated FSH/AMH ratios exhibited the lowest oocyte counts, suggesting that adiposity may exacerbate hormonal imbalance and compromise ovarian responsiveness.
The majority of evidence supports that women with elevated FSH and low AMH levels have reduced oocyte yield and possibly lower oocyte quality, highlighting the importance of the interplay between these hormones [18, 32, 33]. Consistent with this, we observed lower oocyte numbers in patients with higher FSH/AMH ratios, regardless of BMI. While no significant interaction between BMI and the ratio was identified, the combination of elevated BMI and high FSH/AMH ratio identified a subgroup at greater risk for poor ovarian response.
Our findings highlight the potential clinical utility of the FSH/AMH ratio as a
reliable predictor of ovarian response, independent of BMI. This marker may aid
clinicians in optimizing patient counseling and stimulation protocols, especially
in women with borderline ovarian reserve. Although BMI did not significantly
influence hormonal parameters in this study, its potential role in modulating
ovarian sensitivity warrants continued consideration during fertility assessment.
It is important to note that our cohort included women with BMI values ranging
from 17 to 39.9 kg/m2, and therefore, the results may not be generalizable
to individuals with class III obesity (BMI
Several limitations must be acknowledged. First, although this study focused on hormonal indicators of ovarian reserve, we recognize that metabolic factors—particularly insulin resistance and adipokine dysregulation—play a critical role in mediating obesity-related reproductive dysfunction. However, key metabolic parameters such as fasting insulin, leptin levels, and homeostasis model assessment of insulin resistance (HOMA-IR) scores were not available in our dataset. This absence restricted our ability to explore the mechanistic pathways underlying the observed associations. Second, the sample size was relatively small, and the representation of women at BMI extremes was uneven. In particular, both the underweight and class II obese groups included only three participants each, which may have contributed to statistical variability and limited our ability to draw definitive conclusions for these subgroups. This imbalance also affects the generalizability of our findings, as the associations observed in mid-range BMI categories may not fully reflect outcomes in populations at the extremes. Interestingly, class II obese women in our study demonstrated the highest median oocyte yield despite having the highest FSH/AMH ratio, a finding likely attributable to sampling variation within the small subgroup. This observation should be interpreted with caution. Lastly, while this study focused on oocyte yield as the primary outcome, we acknowledge that it represents only one component of ART success. Data on additional endpoints such as fertilization rates, embryo quality, and clinical pregnancy outcomes were not consistently available and therefore could not be analyzed.
Future research should include larger, multicenter prospective studies with more diverse populations to validate the predictive value of the FSH/AMH ratio across all BMI categories. Additionally, incorporating BMI-specific thresholds and including key metabolic markers—such as leptin, fasting insulin, and HOMA-IR—could improve the clinical applicability of this ratio. Given the increasing use of the FSH/AMH ratio in fertility decision-making, future studies should also investigate whether BMI-adjusted cutoffs could enhance its predictive performance and support individualized treatment planning. By accounting for both hormonal and metabolic factors, such composite models may provide a more comprehensive understanding of how adiposity affects reproductive outcomes and help guide more personalized, evidence-based fertility treatment strategies.
This study demonstrates that the FSH/AMH ratio is a valuable and BMI-independent predictor of oocyte yield in women undergoing controlled ovarian stimulation. While AMH and FSH are individually informative markers of ovarian reserve, their ratio provides a more integrated assessment of reproductive potential. Our findings confirm that higher FSH/AMH ratios are significantly associated with lower oocyte counts, supporting its clinical utility in identifying patients at risk for suboptimal ovarian response—particularly when standard markers yield inconclusive results.
Although BMI is a well-recognized factor influencing reproductive outcomes, it did not significantly impact hormonal profiles or the FSH/AMH ratio in this study. However, the combination of elevated BMI and a high FSH/AMH ratio was associated with a more pronounced reduction in oocyte yield, highlighting the importance of evaluating both endocrine and metabolic parameters during fertility assessment.
Given the rising prevalence of being overweight or obese among women of reproductive age, the FSH/AMH ratio may serve as a practical and reliable marker across BMI categories. Further prospective studies in larger, more diverse cohorts are warranted to confirm its clinical applicability and to explore whether BMI-adjusted thresholds could enhance its predictive performance.
AMH, Anti-Mullerian hormone; ART, Assisted Reproductive Technology; AFC, Antral Follicle Count; BMI, Body Mass Index; FSH, Follicle-Stimulating Hormone; GnRH, Gonadotropin-Releasing Hormone; HPG, Hypothalamic-Pituitary-Gonadal; LH, Luteinizing Hormone; PCOS, Polycystic Ovary Syndrome; TSH, Thyroid-Stimulating Hormone; WHO, World Health Organization.
The raw data supporting the conclusions of this article will be made available by the authors on request.
EK and EGA designed the research study, validated the data and results. EK developed the methodology, performed the software work, conducted the investigation, curated the data, and carried out the formal analysis and visualization. EGA provided resources. Both authors contributed to editorial changes in the manuscript. Both authors read and approved the final version of the manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Acibadem Mehmet Ali Aydinlar University School of Medicine (protocol code 2023-10/427). Written informed consent was obtained from all participants prior to inclusion in the study.
The authors gratefully acknowledge Seyma S. Celina for her valuable assistance in reviewing the English language, providing support with statistical analysis, and offering insightful feedback that greatly contributed to the quality and clarity of this work.
This research received no external funding.
The authors declare no conflict of interest.
During the preparation of this work the authors used ChatGpt-4.0 in order to check spell and grammar. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
