- Academic Editor
The impact of previous embryo transfer failure on pregnancy outcomes following assisted reproductive technology (ART) treatments remains unclear. Thus, this study aimed to compare pregnancy outcomes between elective single blastocyst transfer (SBT) and double high-quality cleavage embryo transfer (DC-ET) after failure with SBT in the first embryo transfer cycle.
A total of 263 women who underwent a second frozen-thawed embryo transfer (FET) after failure with the SBT in the first embryo transfer cycle, from January 1, 2021 to December 31, 2023 at the Reproductive Medical Center of Peking University Shenzhen Hospital, were included. Patients were divided into the DC-ET and SBT groups based on the number and developmental stage of the embryos transferred. Clinical characteristics and pregnancy outcomes, including clinical pregnancy rate, live birth rate, embryo implantation rate, multiple pregnancy rate, and pregnancy loss rate, were retrospectively analyzed.
Baseline characteristics were similar between the DC-ET (n = 122) and SBT (n = 141) groups. However, the number of available blastocysts was significantly lower in the DC-ET group, as fewer embryos underwent blastocyst culture, whereas the implantation rate was significantly higher in the SBT group than in the DC-ET group (48.94% vs. 30.74%; p < 0.001, adjusted p = 0.002; odds ratio (OR): 2.023, 95% confidence interval (CI): 1.300–3.149). However, no differences were observed in clinical pregnancy rate, live birth rate, or pregnancy loss rate between the groups. The multiple pregnancy rate was significantly lower in the SBT group than in the DC-ET group (2.90% vs. 20.63%; adjusted p = 0.007; OR: 0.113, 95% CI: 0.023–0.549).
SBT results in similar pregnancy outcomes as DC-ET but carries a lower risk of multiple pregnancy after failure with SBT.
Various factors affecting the outcomes and safety of assisted reproductive technology (ART) have gained increasing attention with the widespread application of this technique. Previous unsuccessful ART treatments may influence the outcomes of subsequent embryo transfer cycles, as reported in several observational studies. For example, an unsuccessful ART cycle has been associated with decreased odds of ongoing implantation [1]. McLernon et al. [2] developed a predictive model estimating the chances of live birth over multiple complete cycles using population data from 113,873 women. They found that the chance of live birth was 21% lower after two unsuccessful in vitro fertilization (IVF) treatments compared with one, and 56% lower after six unsuccessful IVF treatments than one unsuccessful IVF treatment. External validation of this model using updated UK data between January 2010 and December 2016 reached similar conclusions [3]. In addition, the number of previous failed treatments has been identified as a prognostic factor for poor outcomes in models predicting embryo transfer success [4]. Repeated treatments also increase patients’ economic burden and psychological stress. Thus, efforts should be made to reduce the likelihood of repeated failures before or during the next embryo transfer after an initial failure. In good-prognosis patients without risk factors related to the endometrium, metabolic or endocrinologic disorders, or immunological disease, embryo-related factors become the primary consideration after the first failed frozen-thawed embryo transfer [5]. Regarding embryo selection, unlike repeated implantation failure cases where clinicians often recommend preimplantation genetic screening for aneuploidy, in the second transfer cycle following an initial failure, both doctors and patients tend to focus on embryo developmental stage and number. Increasing the number of embryos transferred may improve the chances of pregnancy in subsequent treatments. In clinical practice, infertile couples often choose double embryo transfer after failure with single embryo transfer, in consultation with their physicians.
Elective single blastocyst transfer (eSBT) has been recommended in ART to improve outcomes while minimizing the risk of multiple pregnancy [6]. Several international professional organizations, including American Society for Reproductive Medicine (ASRM) and European Society of Human Reproduction and Embryology (ESHRE), have issued related guidelines [7, 8, 9]. Meta-analyses of individual patient data from randomized trials have shown that elective single embryo transfer is associated with a higher chance of term singleton live birth compared with double embryo transfer [10]. As blastocyst culture has become more common as a selection method, the adoption of eSBT has increased. However, extended culture carries the risk of having no available embryos if none develop into blastocysts. Although this outcome indicates low developmental potential, it results in loss of time and financial resources for patients. To mitigate this, embryologists often recommend freezing two high-quality cleavage-stage embryos as a backup in case blastocyst culture fails. This raises the question of which type of embryo should be transferred after failure of an initial eSBT.
ART has significantly contributed to the global increase in multiple pregnancies [11, 12, 13]. Although the rate of multiple births has reduced in recent years owing to single embryo transfer and advancements in IVF techniques [14], the twin pregnancy rate remains high, due to nonadherence to the guideline recommending elective single embryo transfer (eSET) [15]. Many patients who insist on multiple embryo transfer are unaware of the risks associated with multiple pregnancy, including preterm birth, low birth weight, preeclampsia, gestational diabetes, placental abruption, intrauterine growth restriction, and perinatal mortality [16, 17]. Furthermore, the risk of ectopic pregnancy increases with the number of embryos transferred, up to 20-fold [18]. Singleton pregnancies following double embryo transfer (DET) are also associated with higher risks of neonatal death and low birthweight compared with singleton pregnancies after SET in frozen embryo transfer cycles [19]. Additionally, twin pregnancies conceived through ART have higher risks than naturally conceived twin pregnancies [17]. Thus, reducing the number of embryos transferred in ART is essential.
In clinical practice, when an eSET fails, patients and clinicians often choose to transfer two cryopreserved embryos in the next cycle to increase the likelihood of success. However, it remains uncertain whether transferring two high-quality cleavage-stage embryos improves pregnancy outcomes compared with eSBT, and whether this strategy increases the risk of multiple pregnancy. Therefore, this study aimed to evaluate the outcomes of frozen-thawed embryo transfer (FET) cycles in patients who failed to conceive after an initial eSBT cycle and subsequently underwent either double vitrified-warmed high-quality cleavage embryo transfer (DC-ET) or eSBT, with a focus on comparing clinical pregnancy and multiple pregnancy rates.
We included patients aged 20–39 years who had undergone their first IVF or intracytoplasmic sperm injection (ICSI) cycle with all embryos frozen at the Reproductive Centre of Peking University Shenzhen Hospital, Shenzhen, China, between January 1, 2021, and December 31, 2023. Their frozen embryos included at least two blastocysts as well as double cleavage-stage embryos. Patients who had a single high-quality blastocyst transferred in the first FET cycle but failed to achieve pregnancy were included. We excluded patients who had undergone preimplantation genetic testing (PGT) and those diagnosed with recurrent pregnancy loss, abnormal uterine structure, intrauterine adhesions, adenomyosis, hypothalamic amenorrhea, or other endocrine abnormalities, as well as those lost to follow-up. The inclusion and exclusion details of the analyzed cohort are shown in Fig. 1.
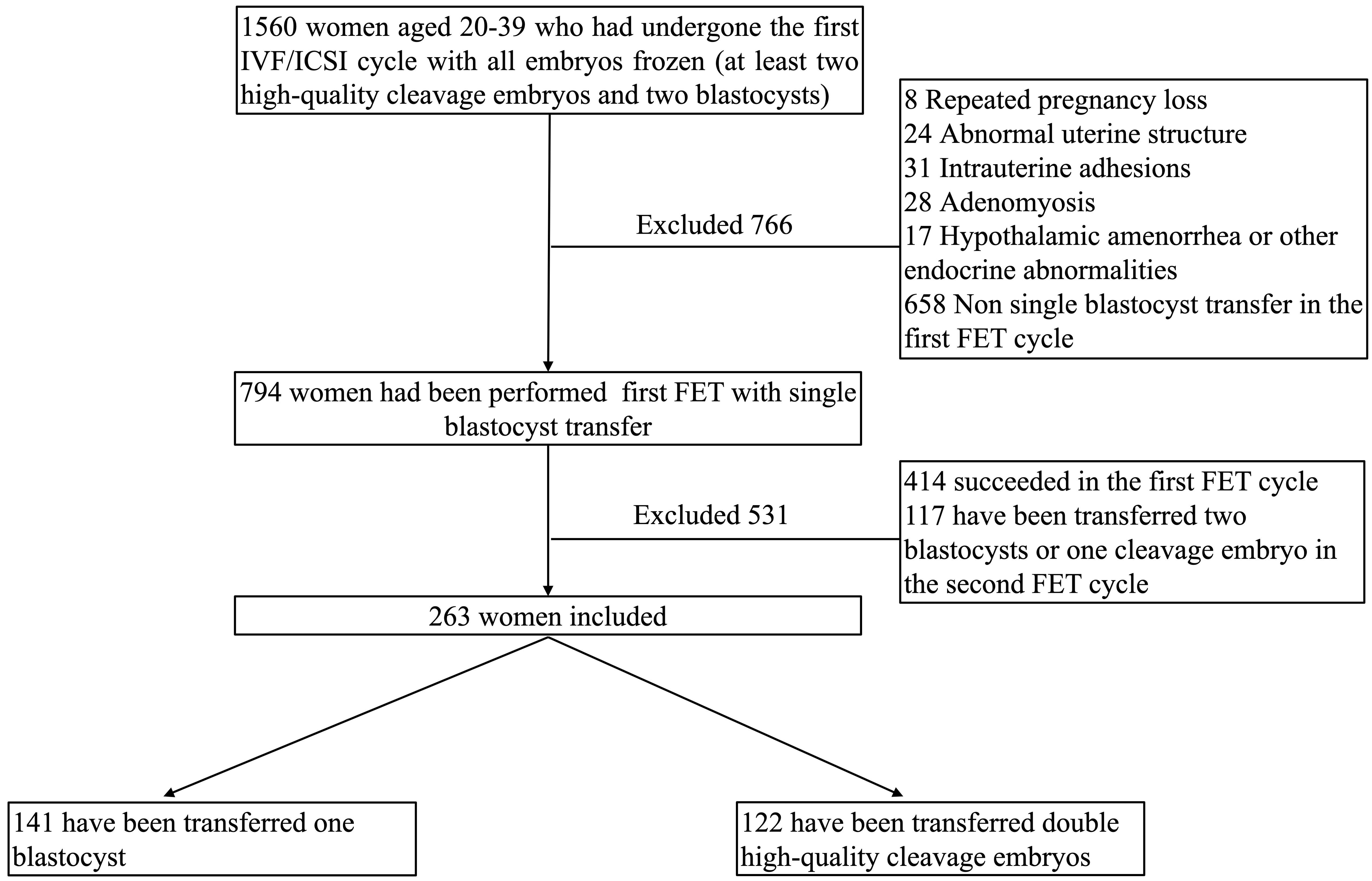 Fig. 1.
Fig. 1.
Flowchart of the cohort. IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; FET, frozen-thawed embryo transfer.
A total of 263 infertile women met the above criteria. Among them, 141 patients underwent transfer of one blastocyst, while 122 patients underwent transfer of two cleavage-stage embryos in the second FET cycle. This study was conducted in accordance with the principles of the Declaration of Helsinki. A waiver of informed consent was obtained for this study because it did not disclose any personal or identifying patient information and posed no risk to the participants. Approval was granted by the Research Ethics Committee of Peking University Shenzhen Hospital(approval number: 2024-133).
The ovarian stimulation protocols for fresh cycles, from which vitrified-warmed
embryos were obtained, were as follows. Gonadotropin dosing was individualized
and adjusted according to ovarian response. Human chorionic gonadotropin (hCG;
5000–10,000 IU; Livzon, Zhuhai, Guangdong, China) was administered to trigger final oocyte
maturation when at least two follicles reached 18 mm in diameter. Transvaginal
oocyte retrieval was scheduled 36 hours later. Fertilization was performed using
conventional IVF or ICSI, and all embryos were cryopreserved via vitrification.
Cleavage-stage embryo morphology was assessed on day 3 after oocyte retrieval.
Embryos were graded according to criteria described in our previous work [20]. A
good-quality cleavage embryo was defined as one consisting of seven to nine
symmetrical blastomeres with
Endometrial preparation before FET was performed using a natural cycle for
patients with regular menses or hormone replacement therapy (HRT) for those with
irregular cycles. In a natural cycle, transvaginal ultrasound was performed from
day 10 of the menstrual cycle to confirm follicular selection. When the dominant
follicle reached 14 mm, patients were instructed to monitor urinary luteinizing
hormone (LH) daily. Serum levels of LH, estrogen, and progesterone were measured
after urinary LH surge detection or when the dominant follicle reached 18 mm.
Transvaginal ultrasound was continued daily until ovulation. In HRT cycles, oral
estradiol (2 mg twice daily; Progynova, Bayer, Leverkusen, Saxony, Germany) was initiated on day 3 of
the cycle. When the endometrial thickness reached 8 mm, luteal support was
provided with either a 60 mg intramuscular injection of progesterone in oil or
vaginal progesterone gel (Crinone, Merck Serono, Geneva, Canton of Geneva, Switzerland), combined with oral
dydrogesterone (Duphaston, Abbott, Chicago, IL, USA) 10 mg twice daily. In some
patients with irregular cycles, an ovulation stimulation protocol was used. In
these cases, a low dose of gonadotropin or letrozole was administered to
stimulate follicular development. Transvaginal ultrasound was performed to
monitor follicular growth and ovulation. hCG was administered when the dominant
follicle reached
Cleavage-stage embryos were transferred on the fourth day after progesterone initiation or the third day after ovulation. Blastocysts were transferred on the sixth day after progesterone initiation or the fifth day after ovulation. All transfers were performed under transabdominal ultrasound guidance.
Serum
The primary outcome of this study was the clinical pregnancy rate. Secondary outcomes included implantation rate, live birth rate, pregnancy loss rate, and multiple pregnancy rate. Clinical pregnancy rate was defined as the proportion of cycles with a confirmed gestational sac and fetal heartbeat at 7 weeks among all FET cycles. Implantation rate was calculated as the number of gestational sacs divided by the total number of embryos transferred. Pregnancy loss rate was defined as the number of pregnancy losses divided by the number of cycles with confirmed clinical pregnancy. Live birth rate was defined as the proportion of cycles resulting in live birth among all FET cycles. Multiple pregnancy rate was defined as the proportion of cycles with more than one fetal heartbeat at 7 weeks among cycles with confirmed clinical pregnancy.
Continuous variables are expressed as mean
Baseline characteristics of oocyte retrieval cycle are shown in Table 1. There were no significant differences in mean maternal or paternal age, body mass index (BMI), duration or type of infertility, basal follicle-stimulating hormone (FSH), LH, estrogen (E2), antral follicle count, anti-Müllerian hormone (AMH), or parameters of controlled ovarian stimulation (COS) and fertilization protocols between the DC-ET and SBT groups. There were also no significant differences in the total number of oocytes, the number of mature oocytes available cleavage embryos, or endometrial thickness on the day of embryo transfer between the two groups. However, the number of available blastocysts was higher in the SBT group.
| Group | DC-ET | SBT | p-value | |
| N | 122 | 141 | ||
| Female age (years) | 31.58 |
32.49 |
0.056 | |
| Male age (years) | 33.62 |
34.01 |
0.477 | |
| BMI (kg/m2) | 21.37 |
21.40 |
0.958 | |
| Duration of infertility (years) | 3.57 |
3.87 |
0.322 | |
| Primary infertility, n (%) | 73 (59.84) | 75 (53.12) | 0.279 | |
| Antral follicle count | 16 (12, 20) | 16 (10, 20) | 0.640 | |
| Basal FSH (IU/L) | 7.80 |
7.58 |
0.235 | |
| Basal LH (IU/L) | 5.20 |
5.70 |
0.260 | |
| Basal E2 (ng/mL) | 35.61 |
36.03 |
0.825 | |
| AMH (ng/mL) | 4.37 |
4.87 |
0.208 | |
| COS protocols, n (%) | 0.128 | |||
| GnRH-agonist | 47 (38.52) | 42 (29.79) | ||
| GnRH-antagonists | 66 (54.10) | 93 (65.96) | ||
| Other protocols | 9 (7.38) | 6 (4.25) | ||
| Level of E2 on the day of hCG (pg/mL) | 3297.34 |
3420.27 |
0.592 | |
| ICSI fertilization, n (%) | 39 (32.97) | 43 (30.50) | 0.628 | |
| COS cycle | ||||
| Number of oocytes retrieved | 18 (13, 24) | 16 (12, 24) | 0.804 | |
| Number of matured oocytes | 17 (12, 20) | 14 (10, 21) | 0.830 | |
| Number of available cleavage embryos | 11 (9, 15) | 10 (7, 16) | 0.508 | |
| Number of available blastocysts | 3 (2, 6) | 5 (3, 8) | 0.001 | |
DC-ET, double cleavage embryo transfer; SBT, single blastocyst transfer; AMH, anti-Müllerian hormone; BMI, body mass index; FSH, follicle-stimulating hormone; LH, luteum hormone; E2, estrogen; hCG, human chorionic gonadotropin; ICSI, intracytoplasmic sperm injection; COS, controlled ovarian stimulation; GnRH, gonadotropin-releasing hormone; N, number.
As the baseline characteristics of FET between DC-ET group and SBT group, the female age at the time of embryo transfer were slightly higher in SBT group, while the embryo thawing survival rates, the endometrium preparation protocols, the endometrial thickness on the day of embryo transferred, were similar between the two groups (Table 2).
| Group | DC-ET | SBT | p-value | |
| n | 122 | 141 | ||
| Female age at the time of embryo transfer (years) | 31.99 |
32.99 |
0.017 | |
| Embryo thawing survival rates | 97.20% | 97.33% | 0.937 | |
| Endometrium preparation protocols, n (%) | 0.676 | |||
| Natural cycle | 25 (20.49) | 23 (16.31) | ||
| HRT | 46 (37.71) | 63 (44.68) | ||
| Ovulation stimulation cycle | 34 (27.87) | 37 (26.24) | ||
| GnRH-agonist-HRT | 17 (13.93) | 18 (12.77) | ||
| Endometrium thickness on the day of embryo transfer (mm) | 10.28 |
10.30 |
0.944 | |
HRT, hormone replacement treatment.
We then compared pregnancy outcomes between the DC-ET and SBT groups (Table 3).
The implantation rate was significantly higher in the SBT group than in the DC-ET
group (48.94% vs. 30.74%, p
| Group | DC-ET | SBT | p-value | Adjusted p-value | OR (95% CI) |
| n | 122 | 141 | |||
| Implantation rate, n (%) | 75 (30.74) | 69 (48.94) | 0.002 | 2.023 (1.300, 3.149) | |
| Clinical pregnancy, n (%) | 63 (51.64) | 69 (48.94) | 0.662 | 0.083 | 0.550 (0.280, 1.080) |
| Live birth, n (%) | 48 (39.34) | 55 (39.00) | 0.955 | 0.220 | 0.658 (0.337, 1.284) |
| Multiple pregnancy, n (%) | 13 (20.63) | 2 (2.90) | 0.001 | 0.007 | 0.113 (0.023, 0.549) |
| Pregnancy loss, n (%) | 13 (20.63) | 13 (18.84) | 0.796 | 0.892 | 1.076 (0.373, 3.106) |
OR, odds ratio; CI, confidence interval.
Binary logistic regression was used to calculate the adjusted p value and controlled for the female age at the time of oocyte retrieval, anti-Müllerian hormone, body mass index, the number of available blastocysts, and number of available cleavage embryos, female age at the time of embryo transfer, endometrium thickness on the day of embryo transfer.
This retrospective study showed no significant differences in pregnancy outcomes between eSBT and double high-quality cleavage embryo transfer after failure of an elective single vitrified-warmed blastocyst transfer cycle. However, the multiple pregnancy rate was much lower after eSBT compared with double cleavage embryo transfer. Therefore, eSBT appears to be the optimal choice in patients who have experienced failure in previous FET cycles.
Several studies have compared pregnancy outcomes across cycles with different numbers of embryos transferred. A meta-analysis of eighty-five studies (14 randomized controlled trials and 71 observational studies) concluded that in women younger than 40 years or in the presence of at least one high-quality embryo, single embryo transfer should be incorporated into clinical practice [21]. Another study evaluating single- and double- elective embryo transfers in oocyte donation cycles reported similar results [22]. However, it remains controversial how many embryos should be transferred after initial failure of eSBT.
Only one related study, by Monteleone et al. [23], evaluated pregnancy outcomes in patients who failed to conceive in fresh eSET cycles and subsequently underwent either elective double vitrified-warmed blastocyst transfer or elective single vitrified-warmed blastocyst transfer. They reported similar pregnancy rates between the two groups but a higher multiple pregnancy rate in the double transfer group (22.5% vs. 5.9%), consistent with our results. Accordingly, ESHRE guidelines recommend that the decision to perform double embryo transfer instead of eSET should not be based on the number of previous unsuccessful ART treatments [8], given the significantly higher risk of multiple pregnancies and associated obstetric and perinatal complications. However, Monteleone et al. [23] only included blastocyst transfers, and the clinical pregnancy rate in their fresh eSBT cycles was relatively low (24.8%), suggesting that the study population may not have had good prognoses, thus limiting the reliability of their findings. In contrast, our results indicate that in patients with good prognosis, eSBT is preferable to double high-quality cleavage embryo transfer, as it reduces multiple pregnancy risk without compromising pregnancy outcomes.
In our study, the implantation rate was significantly lower in double cleavage embryo transfer, which is expected because some cleavage embryos fail to develop into blastocysts. Extended embryo culture serves as a selection process, which explains why clinical pregnancy and ongoing pregnancy rates were similar between groups. Our findings align with previous studies comparing double cleavage embryo transfer and SBT. Long et al. [24] reported no statistical difference in clinical pregnancy rates between double cleavage embryo transfer and SBT, although the live birth rate was slightly lower in the SBT group (23.0% vs. 29.0%; aOR: 0.78; 95% CI: 0.72–0.85). However, the double cleavage embryo transfer group had higher risks of twin births, preterm births, low birth weights, and small-for-gestational-age infants. Similarly, Wei et al. [25] found that transferring two high-quality 8-cell cleavage embryos achieved comparable clinical pregnancy and live birth rates to low-quality blastocysts but resulted in higher multiple pregnancy rates. Several other studies reached similar conclusions [26, 27], although they did not account for prior FET failure. Given the significantly higher risk of multiple pregnancies after double embryo transfer, we recommend SBT in good-prognosis patients even after previous ART failures. Furthermore, our findings suggest that freezing two high-quality cleavage embryos may be unnecessary in such patients, and extended blastocyst culture of all cleavage embryos should be considered. Gingold et al. [28] reported that nonadherence to ASRM guidelines, which recommend eSET in patients with favorable prognoses, occurred in 42%–45% of cases. This nonadherence resulted in multiple live birth rates of around 40%, thereby increasing maternal risk and healthcare costs [15, 28]. Patient education, as well as improved adherence by reproductive endocrinology and infertility specialists, may help increase eSET uptake.
When selecting an embryo transfer strategy after initial failure, cost-effectiveness should also be considered. The primary concerns are the costs of extended embryo culture versus those associated with complications from multiple pregnancies. A systematic review comparing double embryo transfer with sequential SET [29] reported comparable cumulative live birth rates but significantly lower multiple pregnancy risks with sequential SET, albeit with longer treatment times. While sequential SET theoretically increases treatment costs by requiring more cycles, the reduced complications from multiple pregnancies may offset these expenses. In our study, the cost of extended embryo culture was lower than the potential costs associated with multiple pregnancy complications. Thus, extended embryo culture is preferable to the risks of multiple pregnancy. However, patients must also be informed of the risk that no embryos may remain available after extended culture, which could increase psychological stress.
This study has certain limitations. All data were derived from a single fertility center, and the retrospective design may have introduced confounding factors. Therefore, caution is required when generalizing these findings to broader populations with varied prognoses.
Single blastocyst transfer achieves similar pregnancy outcomes as double, high-quality cleavage embryo transfer but carries a lower risk of multiple pregnancies following failed single blastocyst transfer. Larger prospective randomized trials are needed to confirm these findings.
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
SL and CZL designed the present study. SL, ZXW and BLS collected raw data. SL and DGL checked and analyzed all data. SL were major contributors in writing the manuscript. CZL revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was carried out in accordance with the guidelines of the Declaration of Helsinki. A waiver of informed consent was obtained for this study because it did not disclose any personal or identifying patient information and posed no risk to the participants. Approval was granted by the Research Ethics Committee of the Peking University Shenzhen Hospital(approval number: 2024-133).
We thank all the staff in the center for reproductive medicine of Peking University Shenzhen hospital.
This work was supported by grants from Sanming Project of Medicine in Shenzhen (No. SZSM202211043), National Key Research and Development Program (No. 2024YFC2707503), Sanming Project of Cervical Disease Prevention and Control from Pingshan District Maternal and Child Health Hospital and Shenzhen Peking University Shenzhen Hospital (No. 2023-1).
The authors have no conflicts of interest to declare.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
