- Academic Editor
The study investigated the effectiveness of a modified enhanced recovery after surgery (mERAS) protocol in emergency cesarean deliveries (CDs), where its safety and applicability remain uncertain. Postoperative recovery was evaluated in pregnant women using the Thai version of the Quality of Recovery-35 (QoR-35) questionnaire and pain scores measured by the Visual Analogue Scale (VAS).
50 pregnant women were enrolled in a randomized controlled trial conducted at the Medical Education Center of Phayao Hospital. The primary outcomes were the 24-hour QoR-35 score and the 48-hour VAS pain score. Additional parameters, including postoperative hospital stay, opioid use, and the onset of gastrointestinal function, were also assessed. Postoperative complications, such as fever, wound dehiscence, and readmission, were also evaluated.
The mERAS group showed a significant reduction in 48-hour postoperative VAS scores (mean ± standard deviation [SD]: 4.0 ± 1.7 vs. 5.0 ± 1.3; mean difference: 1.0, 95% confidence interval [CI] 0.14, 1.86, p = 0.024). No significant differences were observed between the two groups in assessments conducted immediately postoperatively or at 24 hours across all parameters. The mERAS group experienced shorter hospital stays (p = 0.017), earlier onset of burping (p = 0.049), and earlier onset of flatulence (p = 0.011). Neither group required additional opioid administration or experienced postoperative complications, such as fever, wound dehiscence, or readmission.
Implementation of the mERAS protocol effectively reduced 48-hour postoperative VAS pain scores, shortened hospital stay, and improved patient outcomes without increasing morbidity or surgical complications in patients undergoing emergency CD.
The study has been registered on https://www.thaiclinicaltrials.org/ (registration number: TCTR20250627001; registration link: https://www.thaiclinicaltrials.org/export/pdf/TCTR20250627001).
Cesarean delivery (CD) is a life-saving procedure, and recent global data indicate its increasing prevalence, reflecting evolving trends in maternity care. In Thailand, the national CD rate has surpassed one-third (35%) over the past five years, showing a consistent upward trend [1]. This rising CD rate is associated with increased adverse outcomes, including surgical site pain, delayed recovery, and prolonged hospital stay, all contributing to higher healthcare costs [2, 3]. Traditionally, surgeons have employed a gradual postoperative feeding strategy, progressing from sips of water to liquids, then to a soft diet, and finally to a regular diet once bowel function returns—typically 8 to 20 hours after surgery. However, several studies suggest that early postoperative feeding, early mobilization, and effective pain control can shorten hospital stay and improve patient satisfaction [2, 4, 5, 6].
The enhanced recovery after surgery (ERAS) protocol was first introduced over three decades ago in colorectal surgery to promote faster recovery and reduce postoperative complications [7]. Since then, it has been adapted for various surgical procedures, including cesarean deliveries (CDs). The ERAS protocol incorporates preoperative, intraoperative, and postoperative elements, emphasizing preoperative counseling, minimal fasting, early postoperative feeding, early mobilization, and opioid-sparing analgesia. This approach facilitates faster recovery without increasing complication rates, demonstrates potential to enhance maternal recovery—as reflected by Obstetric Quality of Recovery (ObsQoR) scores—and may optimize neonatal outcomes, particularly in terms of nutritional status and breastfeeding success [2, 3, 4, 5, 6, 7, 8, 9].
Although numerous studies have demonstrated the benefits of the ERAS protocol in elective surgeries, its application in emergency procedures has been limited due to the need for preoperative and intraoperative preparations [2, 3, 4, 5, 6, 8, 9, 10]. These measures include the intake of clear fluids within two hours before surgery, administration of prophylactic antiemetics, and use of neuraxial anesthesia [8]. Nevertheless, one study in developing countries has shown that ERAS protocols may reduce the length of stay (LOS) and improve pain management even in emergency CDs [10]. Additionally, research on emergent colorectal surgeries suggests that a modified ERAS (mERAS) protocol—focused on postoperative elements such as early oral intake, multimodal analgesia, early mobilization, and timely removal of urinary catheters—can be safely adapted for emergency settings [11, 12].
This study aimed to evaluate the effectiveness of implementing the mERAS protocol in emergency CDs compared with the standard protocol. Postoperative recovery was assessed using pain scores measured by the Visual Analogue Scale (VAS) at 24 hours [2]. Additional variables included the Thai version of the Quality of Recovery-35 (QoR-35) questionnaire [2], duration of hospital stay, gastrointestinal function, and the incidence of postoperative complications [3].
This randomized controlled trial was conducted following approval from the Human Research Ethics Committee at Phayao Hospital (COA.210) and was registered in the Thai Clinical Trials Registry (https://www.thaiclinicaltrials.org/) (TCTR20250627001). The sample size was estimated using the two-independent, continuous-outcome formula, anticipating an alpha of 0.5 and 80% power. A total of 53 participants were assessed for the eligibility, of whom 50 consented to participate. The study flow of participants is presented in Fig. 1. 50 pregnant women with singleton pregnancies between 28 and 42 weeks of gestation, who were able to complete the questionnaire in Thai, were enrolled. All participants underwent CD at the Department of Obstetrics and Gynecology, a medical education center within Phayao Hospital, Thailand, between August and December 2023. Pregnant women were allocated in a 1:1 ratio to either the mERAS or standard protocol through simple randomization using opaque envelopes labeled with serial numbers. Outcome assessors were blinded throughout the study. Participants with high-risk obstetric comorbidities—such as placenta previa, placenta accreta spectrum, severe preeclampsia, and chorioamnionitis—were excluded. Additional exclusion criteria included intraoperative complications such as postpartum hemorrhage, bowel or bladder injury, sepsis, or known allergies to acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs).
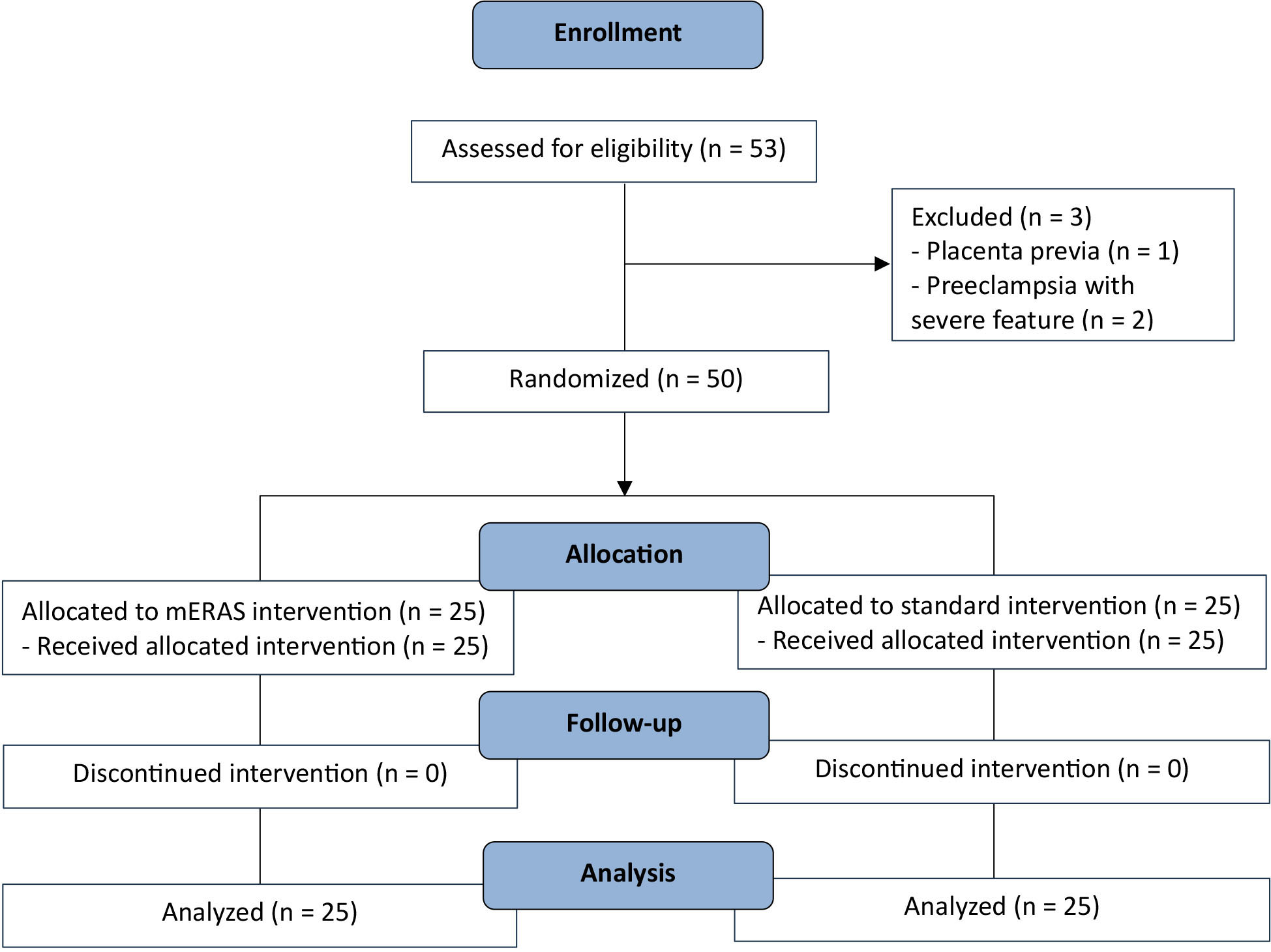 Fig. 1.
Fig. 1.
Study flow of the participants. mERAS, modified enhanced recovery after surgery.
Due to the absence of a standardized consensus on the complete ERAS protocol for emergency CD, this study focused on implementing its postoperative components, referred to as the mERAS protocol. Table 1 provides a detailed comparison of the mERAS and standard protocols. Clinical data were extracted from medical records and included baseline characteristics such as age at delivery, body mass index (BMI), comorbidities, gestational age at delivery, history of abdominal surgeries, and intraoperative findings (e.g., estimated fetal weight, operative time, and blood loss). The primary differences between the two protocols included the use of postoperative oral multimodal analgesia, shortened fasting duration, early mobilization, and timely removal of urinary catheters.
| mERAS protocol | Standard protocol | ||
| Preoperative | |||
| Patient counseling for the indications and complications of CD | Yes | Yes | |
| NPO time | At active phase or depending on CD indication | At active phase or depending on CD indication | |
| Bowel preparation | No | No | |
| Antiemetic drug | No | No | |
| Intraoperative | |||
| Anesthetic agent | Depending on CD indication | Depending on CD indication | |
| Prophylactic antibiotics | At operative room | At operative room | |
| Abdominal skin preparation | Yes | Yes | |
| Prevent hypothermia | Yes | Yes | |
| Postoperative | |||
| Early pain control | Acetaminophen and NSAIDs PO | Opioids IV | |
| Early feeding | Yes | No | |
| Early ambulation | Yes | No | |
| Early IV removal | Yes | No | |
| Early urethral catheter removal | Yes | No | |
mERAS, modified enhanced recovery after surgery; CD, cesarean delivery; NPO, nil per os; NSAIDs, non-steroidal anti-inflammatory drugs; PO, per oral; IV, intravenous.
Postoperative recovery was evaluated using the QoR-35 questionnaire at 24 hours or within a comparable timeframe. This tool assesses multiple dimensions of recovery, including physical comfort, emotional well-being, physical independence, need for assistance, and overall health, with a maximum score of 175. Pain was assessed using a 10-point VAS at 48 hours or a similar timeframe. Moreover, other measured parameters included the duration of postoperative hospitalization (from the start of surgery to discharge), the frequency of additional postoperative opioid administration, and the onset of gastrointestinal function, defined by the occurrence of burping or flatulence. Postoperative complications, such as fever, wound dehiscence, and readmission, were also recorded. Patients without complications were eligible for discharge at 48 hours, following a protocol similar to that used for vaginal deliveries. A surgical wound follow-up was conducted one week after delivery to monitor healing, and a six-week postpartum follow-up was performed to assess puerperium-related complications.
Descriptive statistics were applied as appropriate, including the mean (standard
deviation [SD]), median (interquartile range [IQR]), and proportions (%). A
per-protocol analysis was conducted. Differences between intervention groups were
assessed using the Student’s t-test for normally distributed continuous
variables, the Mann-Whitney U test for non-normally distributed continuous
variables, and the chi-square test for categorical variables. The baseline QoR-35
as reported by Pitimana-aree et al. [13] was 149.4
50 pregnant women who underwent emergency CD were randomly assigned to two
groups (25 per group), with all participants adhering to the study protocol. The
mean age of participants was 29 years, and the mean BMI was 28.2 kg/m2.
Underlying conditions included chronic hypertension, hyperthyroidism, and
pregestational diabetes mellitus. Antenatal complications included
diet-controlled gestational diabetes mellitus, preeclampsia without severe
features, isolated maternal fever, and acute gastroenteritis. The primary
indications for CD were cephalopelvic disproportion (34%), previous CD in labor
(28%), and non-reassuring fetal status (20%). Spinal anesthesia was the most
commonly used technique (80%). The average fetal weight was 3057
| Characteristics | mERAS protocol | Standard protocol | p-value | |
| n = 25 | n = 25 | |||
| Maternal age (years), mean |
30.2 |
28.4 |
0.285 | |
| BMI (kg/m2), mean |
28.4 |
27.9 |
0.660 | |
| Underlying disease, n (%) | 1.000 | |||
| None | 24 (96.0) | 24 (96.0) | ||
| Present | 1 (4.0) | 1 (4.0) | ||
| Antenatal complications, n (%) | 0.009 | |||
| None | 25 (100.0) | 19 (76.0) | ||
| Present | 0 (0.0) | 6 (24.0) | ||
| Gestational age of delivery, n (%) | 0.157 | |||
| Preterm | 4 (16.0) | 1 (4.0) | ||
| Term | 21 (84.0) | 24 (96.0) | ||
| Indication of CD, n (%) | 0.299 | |||
| Cephalopelvic disproportion | 8 (32.0) | 9 (36.0) | ||
| Previous cesarean section in labor | 9 (36.0) | 5 (20.0) | ||
| Fetal non- reassuring | 4 (16.0) | 6 (24.0) | ||
| Failed induction | 2 (8.0) | 5 (20.0) | ||
| Breech presentation | 2 (8.0) | 0 (0.0) | ||
| Anesthetic agent, n (%) | 0.157 | |||
| Spinal anesthesia | 18 (72.0) | 22 (88.0) | ||
| General anesthesia | 7 (28.0) | 3 (12.0) | ||
| Previous CD, n (%) | 0.344 | |||
| 0 | 16 (64.0) | 20 (80.0) | ||
| 1 | 8 (32.0) | 5 (20.0) | ||
| 1 (4.0) | 0 (0.0) | |||
| Skin incision, n (%) | 0.508 | |||
| Pfannenstiel incision | 18 (72.0) | 20 (80.0) | ||
| Low midline incision | 7 (28.0) | 5 (20.0) | ||
| Fetal presentation, n (%) | 0.149 | |||
| Vertex | 23 (92.0) | 25 (100.0) | ||
| Non-vertex | 2 (8.0) | 0 (0.0) | ||
| Fetal weight (grams), mean |
3026.4 |
3068.4 |
0.842 | |
| Operative time (minutes), mean |
41.88 |
37.48 |
0.307 | |
| Operative blood loss (mL), median (IQR) | 500 (400, 500) | 500 (400, 600) | 0.330 | |
SD, standard deviation; BMI, body mass index; IQR, interquartile range; n, number of samples.
Regarding the primary outcome, the mERAS group showed a significant reduction in
VAS scores at 48 hours or comparable timeframe (mean
| Outcomes | mERAS protocol | Standard protocol | p-value | |
| n = 25 | n = 25 | |||
| Outcomes evaluated at 24 hours and proximate timeframe | ||||
| Physical comfort, median (IQR) | 54.0 (50.0, 56.0) | 52.0 (49.0, 57.0) | 0.778 | |
| Emotional state, median (IQR) | 32.0 (30.0, 33.0) | 32.0 (28.0, 34.0) | 0.390 | |
| Physical independent, median (IQR) | 19.0 (17.0, 20.0) | 18.0 (14.0, 20.0) | 0.110 | |
| Psychological support, median (IQR) | 29.0 (27.0, 30.0) | 30.0 (27.0, 30.0) | 0.214 | |
| Pain, median (IQR) | 26.0 (24.0, 27.0) | 26.0 (23.0, 27.0) | 0.591 | |
| Outcomes evaluated at 48 hours and proximate timeframe | ||||
| VAS, mean |
4.0 |
5.0 |
0.024 | |
| Mild pain: 0–3, n (%) | 12.0 (48.0) | 1.0 (4.0) | 0.002 | |
| Moderate pain: 4–6 | 11.0 (44.0) | 22.0 (88.0) | ||
| Severe pain: |
2.0 (8.0) | 2.0 (8.0) | ||
| Other outcomes | ||||
| Length of postoperative hospital stay (hours), median (IQR) | 72.0 (66.0, 73.0) | 74.0 (72.0, 76.0) | 0.017 | |
| Duration until onset of burping symptoms (hours), median (IQR) | 17.0 (9.0, 31.0) | 24.0 (15.0, 48.0) | 0.049 | |
| Duration until onset of flatulence symptoms (hours), mean |
20.3 |
30.4 |
0.011 | |
VAS, Visual Analogue Scale.
Compared to the standard group, patients undergoing mERAS care have a significantly decreased risk of moderate pain relative to mild pain, with a relative risk ratio (RRR) of 0.04 (95% CI: 0.005, 0.36, p = 0.004). This mERAS group is also less likely to develop severe pain compared to mild pain, but the results are not statistically significant (RRR = 0.08 [95% CI: 0.005, 1.41], p = 0.080). In concordance with the pain severity outcome, individuals in the mERAS group have a lower VAS by approximately 24.5% compared to those in the standard-of-care group (beta-coefficient = –0.28 [95% CI: –0.49, –0.08], p = 0.008), as shown in Table 4.
| Outcomes | Effect size* (95% CI), p-value | |
| Primary outcomes§ | ||
| VAS (log scale) | –0.28 (–0.49, –0.08), 0.008 | |
| Mild pain (VAS 0–3) | Reference outcome | |
| Moderate pain (VAS 4–6) | 0.04 (0.005, 0.36), 0.004 | |
| Severe pain (VAS |
0.08 (0.005, 1.41), 0.080 | |
| Primary and secondary outcomes (log scale) | ||
| Physical comfort | 0.03 (–0.05, 0.12), 0.440 | |
| Emotional state | 0.08 (–0.02, 0.17), 0.100 | |
| Physical independent | 0.13 (0.02, 0.24), 0.020 | |
| Psychological support | –0.03 (–0.09, 0.04), 0.360 | |
| Pain | 0.05 (–0.05, 0.16), 0.280 | |
| Length of postoperative hospital stay (hours) | –0.06 (–0.12, –0.01), 0.020 | |
| Duration until onset of burping symptoms (hours) | –0.41 (–0.80, –0.01), 0.040 | |
| Duration until onset of flatulence symptoms (hours) | –0.48 (–0.82, –0.14), 0.007 | |
Note: Effect size in the mERAS group compared to the standard group. Embolden
figures represent statistically significant values. *Most of the effect sizes are
beta coefficients, except for mild, moderate, and severe pain, in which the
effect sizes are RRRs. §For primary outcomes, a
p-value of
Additionally, receiving mERAS care also significantly reduced the length of hospital stay, duration until onset of burping symptoms, and duration until onset of flatulence symptoms by 5.8% (–0.06 [95% CI: –0.12, –0.01], p = 0.020), 33.6% (–0.41 [95% CI: –0.80, –0.01], p = 0.040), and 38.1% (–0.48 [95% CI: –0.82, –0.14], p = 0.007), respectively, compared to the standard group. However, the mERAS group exhibited a slightly higher physical feeling score by 13.9% compared to the control group (0.13 [95% CI: 0.02, 0.24], p = 0.020).
This randomized controlled trial evaluated the efficacy of the mERAS protocol in emergency, uncomplicated CD. The sample size calculation was based on the assumptions of no participant dropout and the absence of postoperative complications. The study aims to investigate the impact of limited preoperative care, inherent in emergency CD, on intraoperative and postoperative outcomes. Previous research by Klangprapan [2] supports the QoR-35 questionnaire as a reliable instrument for evaluating postoperative recovery, especially given its availability of a validated Thai-translated version [13]. Additionally, the VAS was used to assess pain scores at 48 hours. The analysis also considered the duration of hospital stay, with findings from Tientong et al. [3] demonstrating a statistically significant reduction compared with the standard Thai protocol. Several studies have shown that partial implementation of ERAS protocols, including in emergency surgeries, can be beneficial [9, 10, 11, 12]. Comprehensive adherence throughout all perioperative phases may not necessary, and selected components can effectively manage postoperative pain [14].
Our pain management approach follows a multimodal analgesic strategy, consistent with previous studies using acetaminophen and NSAIDs to minimize opioid use, as recommended by the standard ERAS protocol [2, 3, 4, 5, 6]. However, the method of assessing postoperative pain scores differs in emergency settings. Pain scores were evaluated at both 24 and 48 hours postoperatively, with an additional assessment conducted within 1–6 hours to reduce patient discomfort. This broad timeframe may have influenced the reported pain scores. Notably, no additional opioid medications were administered for pain management in either group. Although the mERAS group tended to have higher number of CD, there was no significant difference in QoR scores, while VAS pain scores were significantly lower in this group. Moreover, although the standard group had a significantly higher number of antenatal complications—including diet-controlled gestational diabetes mellitus, preeclampsia without severe features, isolated maternal fever, and acute gastroenteritis—none progressed to more severe conditions, and all resolved after delivery.
Postoperative hospitalization was determined according to local Thai policy, requiring a minimum stay of 48 hours for vaginal deliveries and 72 hours for CD. However, early feeding and ambulation in the mERAS group facilitated a faster onset of burping and flatulence. Participants were allowed to choose their discharge time, provided they felt physically comfortable and ready after a minimum stay of 48 hours. This approach reduced the median postoperative hospital stay without increasing complications such as fever, wound dehiscence, postpartum hemorrhage, or readmission, consistent with findings from prior studies [3].
Strengths of this study include its randomized controlled trial design, with all
attending surgeons adhering to standardized, sealed protocols. Additionally,
questionnaires were administered by nurses blinded to treatment assignments,
minimizing the risk of bias. However, the study has several limitations. First,
it was conducted at a small, single-center hospital, which may introduce
selection bias. Second, variability in physician practices within the standard
care group—especially in the timing and progression of postoperative
feeding—may have influenced outcomes. Third, differences persist between the
mERAS protocol used in this study and the standard ERAS protocol, particularly in
term preoperative fasting, antiemetic drug use, and intraoperative neuraxial
anesthesia. Due to hospital protocols and patient safety concerns,
anesthesiologists recommended a minimum fasting period of 6 hours to reduce risk
of aspiration from a full stomach. As a result, all pregnant women remained nil
per os (NPO) after active labor (cervical dilation
In summary, the implementation of a mERAS protocol in emergency CD was associated with lower pain scores at 48 hours, shorter postoperative hospital stays, and improved bowel functions—without an accompanying increase in morbidity or surgical complications. Although no statistically significant differences were observed in recovery outcomes within the first 24 hours, the broader findings underscore the potential benefits of this approach. These findings support the integration of mERAS principles into clinical practice and highlight their potential to enhance recovery and optimize postoperative care in emergency obstetric settings.
Due to privacy and ethical concerns, details of the data and how to request access are available from the corresponding author.
PN designed the research study. PN performed the research. PS analyzed the data. Both authors contributed to editorial changes in the manuscript. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Human Research Ethics Committee at Phayao Hospital (COA.210) and was registered in the Thai Clinical Trials Registry (approval number: TCTR20250627001).
The authors would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
During the preparation of this work the authors used ChatGPT 4.0 in order to check and correct grammatical errors during the manuscript writing process. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
