- Academic Editor
Microvascular-flow (MV-Flow) imaging (Samsung Medison Co., Ltd., Seoul, Korea) was utilized to assess placental perfusion in the first-trimester of pregnancy during the Omicron epidemic. The correlation between placental vascular index with microvascular flow (VIMV) and uterine artery (UtA) Doppler parameters, as well as pregnancy outcomes, was examined.
A total of 37 pregnant women who underwent early fetal screening ultrasound examinations at the First Affiliated Hospital of Zhejiang University School of Medicine from December 2022 to February 2023 were analyzed. Among them, 30 were infected with coronavirus disease 2019 (COVID-19) and 16 were delivered, while 7 were not infected. General information about the pregnant women, including age, gestational age, nuchal translucency (NT), maternal history, and placental location, was collected. MV-Flow technology was used to measure VIMV. Simultaneously, the systolic/diastolic (S/D) maximum blood flow velocity, resistance index (RI), and pulsatile index (PI) were measured. The differences in the ultrasound parameters mentioned above were compared among the various groups. The infection group was further categorized into good pregnancy outcome and adverse pregnancy outcome groups based on pregnancy results. The general data and ultrasound parameters of the adverse pregnancy outcome group were then compared and analyzed.
There were no statistically significant differences between the infection group and the normal group in terms of age, gestational age, parity, gestational number, placental position, placental thickness, and other factors (p > 0.05). The VIMV values obtained by elliptical, rectangular, and manual tracing methods at the same placental level in the MV-Flow mode showed no statistically significant differences (p > 0.05). Except for PI of right UtA, The VIMV values and the S/D, PI, and RI of UtA were not significantly different between the infection group and the normal group (p > 0.05). Within the infection group, no significant differences were observed in VIMV value, placental thickness, S/D ratio, PI, RI of UtA, or neonatal weight between the adverse pregnancy outcome good pregnancy outcome group (p > 0.05).
MV-Flow technology can quantitatively evaluate placental microvascular perfusion, and the VIMV is not influenced by the tracing method, making it a reliable indicator for monitoring the intrauterine status of the fetus. No significant differences in VIMV values were observed between the infection group and the normal group. The findings suggest that early infection with the Omicron variant does not impact placental blood perfusion. Infection with the Omicron variant during the first-trimester of pregnancy does not increase the risk of adverse pregnancy outcomes for the fetus. However, further studies are needed to reach a definitive conclusion on this topic.
Coronavirus disease 2019 (COVID-19) is a global respiratory infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The Omicron variant has become the dominant strain in the global pandemic, exhibiting significantly lower pathogenicity compared to earlier variants. Although the number of infections remains high, the illness caused by the Omicron variant is gradually transitioning into a common respiratory infectious disease. As the pandemic continues to spread, the potential risks to fetal growth and development, pregnancy outcomes, and vertical transmission of novel coronavirus during pregnancy have become major societal concerns. Although reports of poor maternal placental perfusion in the third-trimester of pregnancy have been documented since the onset of the COVID-19 pandemic, data on maternal placental perfusion and pregnancy outcomes during the first-trimester remain limited [1, 2].
Placenta is a crucial organ for the exchange of nutrients between the mother and the fetus, and its function directly affects fetal outcomes. During normal pregnancy, the uterine artery (UtA), a branch of the internal iliac artery, transforms from a high-resistance vessel to low-resistance vessel, forming spiral arteries. These arteries undergo trophoblast infiltration and remodelling, where vascular endothelial cells are replaced by trophoblast cells, and vascular smooth muscle is replaced by fibrin, widening the lumen to meet the demands of pregnancy and ensure adequate blood supply to the fetus [3]. Studies have reporter a significant increase in the levels of angiotensin-converting enzyme 2 (ACE2) receptors in the uterus and placenta during pregnancy following SARS-CoV-2 infection [4, 5]. The interaction between SARS-CoV-2 and endothelial cells can lead to microthrombosis, suggesting a potential link between infection and impaired placental function. In this study, Microvascular-flow (MV-Flow) technology was utilized to measure the placental vascular index, referred to as VIMV, in both the first-trimester infection group and normal pregnancy group. Additionally, Doppler ultrasound technology was used to measure the maximum systolic/diastolic (S/D) blood flow velocity, resistance index (RI), and pulsatile index (PI) of the bilateral UtA in pregnant women. The differences between the two groups of pregnant women and their fetuses were compared. Furthermore, the pregnancy outcome of the fetus in the infected group was observed in order to provide more accurate ultrasound quantitative indicators for clinical practice and to establish a theoretical basis for future diagnosis and treatment.
This single-center, retrospective study was conducted at the First Affiliated Hospital of Zhejiang University School of Medicine between December 2022 and February 2023. A total of 37 pregnant women who underwent early prenatal nuchal translucency (NT) ultrasound examination were included in the study, with 30 in the infection group and 7 in the normal group. The inclusion criteria for the normal group consisted of pregnant women in good physical health with a history of regular early pregnancy check-ups. Exclusion criteria for all participants included multiple pregnancies, fetal abnormalities, and the presence of other congenital or chronic conditions in pregnant women. Positive results from real-time polymerase chain reaction (RT-PCR) tests on nasopharyngeal swab samples confirmed SARS-CoV-2 infection during pregnancy [6]. All women infected with SARS-CoV-2 exhibited mild symptoms (such as fever, cough, sore throat, loss of smell and taste, diarrhea), and none required hospitalization.
During the examination, the pregnant woman should be positioned supine with her lower abdomen fully exposed, and both the woman and fetus should be in a calm state. The SAMSUNG W10 color ultrasound diagnostic instrument (Samsung Medison Co., Ltd., Seoul, Korea) was used for the examination, utilizing the CA2-9A convex array probe (Samsung Medison Co., Ltd., Seoul, Korea). The MV-Flow mode was activated to measure the placental thickness and VIMV value (ratio value on the image) at the center of the placenta. The region of interest (ROI) was automatically outlined using oval and rectangular shapes, with manual tracing employed if needed, ensuring that the ROI size remained within 2–3 cm2. To locate the external iliac arteries at the bilateral groin, the probe should be positioned at the midline, with the angle between the blood flow direction and the sound beam set at the intersection of the UtA and external iliac artery, approximately 1–2 cm away from the external iliac artery. At least 5 continuous spectra should be obtained, followed by automatic measurements to derive S/D, PI, and RI values.
The position of the ROI and the effect of different tracing methods on VIMV
values were observed. There were no significant differences in VIMV values
between the two groups regarding the UtA and placenta. The infection group was
further divided into two subgroups: the adverse pregnancy outcome group and the
good pregnancy outcome group, based on pregnancy outcomes. The inclusion criteria
for the outcome groups included preterm birth, stillbirth, neonatal asphyxia,
fetal growth restriction, low birth weight infants, an Apgar score of
Data analysis in this study was conducted using SPSS version 25.0 statistical
software (IBM Corp., Armonk, NY, USA). Count data were presented as rates and
analyzed using Fisher’s exact test. Normal distribution data were expressed as
mean
A total of 37 women with singleton pregnancies were included in this study, comprising 7 in the normal group and 30 in the infection group. No significant differences were observed in age, gestational weeks, number of pregnancies production times, placental position, or placental thickness between the two groups (Table 1).
| Characteristic | Normal group (n = 7) | Infection group (n = 30) | T | p | |
| Age (years) | 30.5 |
29.03 |
–1.318 | 0.175 | |
| Gestational weeks (weeks) | 12.55 |
12.60 |
0.23 | 0.814 | |
| Number of pregnancies (times) | 1 | 5 | 22 | 1.000 | |
| 2 | 8 | ||||
| Production times (times) | 0 | 6 | 23 | 1.000 | |
| 1 | 7 | ||||
| Site | Anterior | 4 | 13 | 0.680 | |
| Posterior | 3 | 17 | |||
| Placental thickness | 1.43 |
1.63 |
0.859 | 0.396 |
No significant differences in VIMV were observed between the two groups
when using ellipse, rectangular, and manual tracing methods to detect the center
of the placenta (Table 2 and Fig. 1). Compared to the ultrasound parameters of
the two groups, the VIMV value in the infection group were greater than that
in the control group. Except for PI of the right UtA, the S/D, PI, and RI of UtA
were not statistically significant between the two groups (p
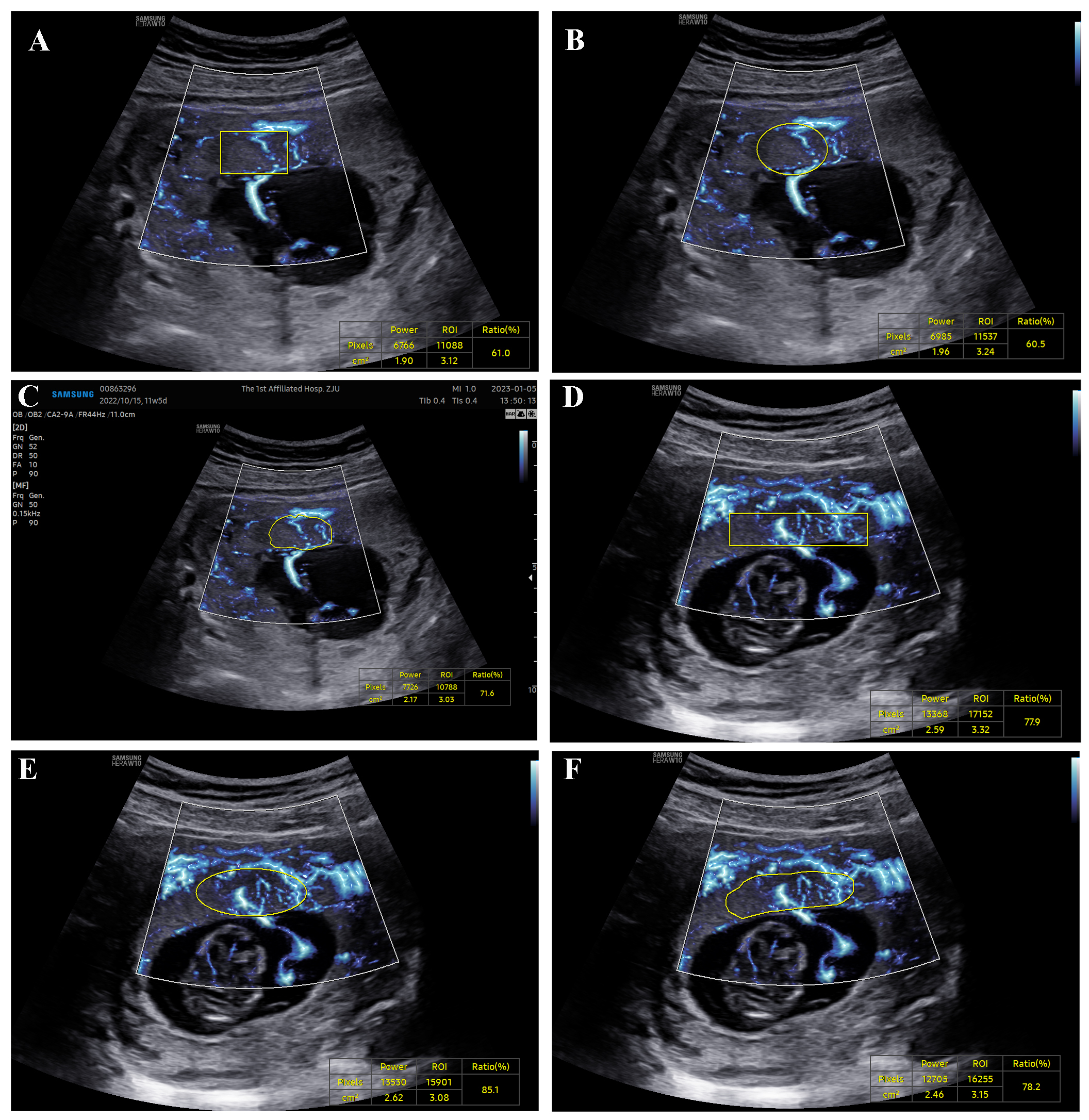 Fig. 1.
Fig. 1.
MV-Flow showing placental microvasculature. (A–C) Infection group: rectangle, ellipse, and manual trace ratios were 61.0%, 60.5%, and 71.6%, respectively. (D–F) Normal group: rectangle,ellipse,and manual trace ratios were 77.9%, 85.1%, and 78.2%. ROI, region of interest. MV-Flow, Microvascular-flow.
| Oval tracing percentage (%) | Rectangular tracing percentage (%) | Manual tracing percentage (%) | H | p | |
| Normal group (n = 7) | 47.9 (24.2, 73.5) | 45.3 (24.2, 70.5) | 48.9 (20.2, 70.5) | 0.091 | 0.955 |
| Infection group (n = 30) | 61.5 (45.12, 73.9) | 61.15 (42.18, 71.8) | 60.9 (44.9, 72.75) | 0.209 | 0.901 |
| Z | –1.125 | –1.163 | –1.128 | ||
| p | 0.261 | 0.245 | 0.201 |
ROI, region of interest; VIMV, vascular index with microvascular flow.
| Normal group (n = 7) | Infection group (n = 30) | Z | p | ||
| VIMV value | 47.367 (28.9, 67.517) | 60.683 (45.208, 76.117) | –1.241 | 0.215 | |
| Left UtA | S/D | 2.73 (2.66, 8.595) | 3.285 (2.67, 4.755) | –0.058 | 0.954 |
| RI | 0.63 (0.625, 0.88) | 0.695 (0.625, 0.79) | –0.029 | 0.977 | |
| PI | 1.17 (1.14, 2.92) | 1.34 (0.968, 1.848) | –0.549 | 0.583 | |
| Right UtA | S/D | 4.53 (2.954, 8.315) | 2.8 (1.983, 3.823) | –1.79 | 0.073 |
| RI | 0.78 (0.64, 0.88) | 0.645 (0.498, 0.73) | –1.736 | 0.083 | |
| PI | 1.82 (1.285, 2.715) | 1.165 (0.753, 1.523) | –2.079 | 0.038 |
VIMV, vascular index with microvascular flow; S/D, systolic/diastolic; RI, resistance index; PI, pulsatile index; UtA, uterine artery.
In the infection group, there were 12 cases with good pregnancy outcomes and 4 cases with adverse pregnancy outcomes. There were no statistically significant differences in VIMV values, placental thickness, S/D, PI, RI of the UtA, and neonatal values between the favorable pregnancy group and the adverse pregnancy group (Table 4).
| Good pregnancy outcome (n = 12) | Adverse pregnancy outcomes (n = 4) | Z | p | ||
| Age (years) | 28 (27, 28.5) | 27.5 (26.25, 32.5) | –0.123 | 0.902 | |
| VIMV value | 64.667 (48.3, 80.98) | 50.333 (42.317, 67.175) | –0.728 | 0.467 | |
| Placental thickness | 1.37 (1.19, 1.665) | 1.27 (1.133, 1.358) | –1.698 | 0.09 | |
| Left UtA | S/D | 3.18 (2.24, 5.18) | 3.71 (2.28, 4.83) | –0.154 | 0.877 |
| RI | 0.69 (0.55, 0.805) | 0.705 (0.56, 0.79) | 0.000 | 1.000 | |
| PI | 1.3 (0.865, 1.885) | 1.45 (0.87, 1.88) | –0.154 | 0.877 | |
| Right UtA | S/D | 2.66 (2.15, 3.385) | 3.58 (2.345, 5.183) | –0.926 | 0.355 |
| RI | 0.62 (0.5, 0.7) | 0.72 (0.548, 0.795) | –0.928 | 0.353 | |
| PI | 1.1 (0.72, 1.29) | 1.485 (0.913, 2.043) | –1.234 | 0.217 | |
| Neonatal weight (g) | 3190 (2885, 3565) | 3055 (2877.5, 3390) | –0.668 | 0.504 |
VIMV, Vascular Index with Microvascular Flow; S/D, systolic/diastolic; RI, resistance index; PI, pulsatile index; UtA, uterine artery.
Early pregnancy infection with COVID-19 during the Omicron pandemic did not significantly affect placental perfusion in pregnant women, nor were there significant differences in adverse pregnancy outcomes between women with early infection and those who were uninfected. These findings indicate that early Omicron infection does not significantly affect the growth and development of both placenta and fetus, nor does it increases the risk of adverse pregnancy outcomes.
During the first-trimester of pregnancy, the S/D value of the UtA, a branch of the internal iliac artery, is commonly used as an indicator of placental function on the maternal side. Adequate blood flow in the UtA is essential for maintaining normal placental function and ensuring fetal blood oxygenation in utero, which significantly impacts fetal intrauterine growth. Our study found no statistically significant differences in the RI and S/D values of the UtA between two groups, or between adverse and good pregnancy outcomes. These results suggest that early infection does not affect maternal UtA vascular remodeling.
MV-Flow technology can detect microvascular flow that is not visible with conventional Doppler imaging. It provides a detailed view of low-velocity blood flow associated with surrounding tissues, exhibiting high sensitivity and resolution. This technology has the potential to visualize microvessels with slow blood flow velocity. MV-Flow technology can visualize not only the placental villous shaft but also quantify the placental microvascular structure at the level of the villous lobe. Chen et al. [7] demonstrated that MV-Flow is the optimal method for assessing placental blood flow perfusion. Compared to other placental examination techniques, MV-Flow technology is easy to operate and fast, significantly improving examination efficiency.
Herein, we found that different tracing methods in the ROI had no significant effect on the results of measuring placental VIMV values using the MV-Flow technique, indicating high reproducibility of the technique. The VIMV value represents the percentage of the colored area within the placenta, indicating the density of microvessel per unit volume. Therefore, the placental VIMV value is directly proportional to the placental blood perfusion volume, with higher VIMV values indicating greater blood perfusion, and vice versa. There was no significant difference in placental VIMV values measured by MV-Flow technology between the adverse pregnancy outcome group and the normal pregnancy group in this study. The results suggest that early infection does not significantly impact maternal placental blood perfusion, as there was no significant difference in VIMV values between the positive and adverse outcome groups of early infection measured by MV-Flow technology. Additionally, SARS-CoV-2 infection in the first-trimester does not increase the risk of adverse fetal outcomes during pregnancy.
The primary limitation of this study is the potential for errors due to the small sample size, as only pregnant women with mild symptoms were included in the study. While severe SARS-CoV-2 infection is associated with a higher risk of vascular disease, our finding that early infection does not significantly impact placental perfusion, fetal growth, and development may be attributed to the inclusion of patients with mild symptoms. Nevertheless, it cannot be definitively ruled out that pregnant women with severe COVID-19 may be at an increased risk of fetal growth restriction. In addition, there are fewer control groups in this study, which leads to limited statistical research. In the later stage, the sample size will be increased on the basis of another study to carry out further exploratory research.
In conclusion, pregnancies affected by SARS-CoV-2-Omicron during the first-trimester do not have an increased risk of adverse fetal outcomes. Utilizing ultrafine blood flow imaging technology can effectively assess placental perfusion and provide valuable insights for clinical diagnosis and treatment. Further large-scale, prospective studies are needed to confirm these findings and to establish a definitive conclusion on this topic.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
SYY and PPZ designed the research study. ZHX and QL performed the research. XDZ and TAJ provided help and advice on the experiments. SYY analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (approval number: IIT20230328A).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This project was supported by the General Research Project of Department of Education in Zhejiang Province (No. Y202353427).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
