- Academic Editor
†These authors contributed equally.
Background:
Prenatal esophageal atresia (EA) diagnosis may improve outcomes by optimizing
prenatal and postnatal care. This study evaluated the feasibility of direct
antenatal visualization of normal longitudinal esophageal sections using
two-dimensional ultrasound between 21 and 23 gestational weeks. Methods:
A detailed prenatal anatomic survey was performed, followed by dedicated
esophageal imaging. The latter was performed as follows: coronal sections of the
thoracic aorta were obtained; the sound beam was then moved ventrally; and
strip-shaped high echoes with three or more hyperechoic layers were identified as
the esophagus. Images showing the longest esophageal segment were used to measure
the esophageal length and angle (between the thoracic aorta and esophagus).
Results: Longitudinal esophageal sections were visualized in 94.0% (n =
205/218) of patients. The visualization rates between fetuses in
different positions were statistically significant (p
Esophageal atresia (EA) and tracheoesophageal fistula (TEF) are major congenital malformations affecting 2.47/10,000 live births [1]. They are characterized by a disruption of esophageal continuity, with or without persistent communication with the trachea. Moreover, approximately 50% of patients with TEF/EA have associated congenital anomalies, including VACTERL (vertebral defects, anal atresia, cardiac defects, TEF, renal anomalies, and limb abnormalities) association or CHARGE (coloboma, heart defects, atresia choanae, growth retardation, genital abnormalities, and ear abnormalities) syndrome [2]. Prenatal diagnosis is helpful for counseling parents with affected fetuses, allowing them to plan for births in close proximity to a neonatal surgery unit to optimize postnatal transfer.
Ultrasonography is now a routine method for assessing pregnancy states, providing guidance for parents, and allowing medical practitioners to plan the care of newborns with congenital anomalies in advance. However, prenatal diagnosis of EA is challenging [3, 4]. Current ultrasound technology does not allow for definitive diagnosis of EA/TEF. EA is underdiagnosed antenatally. A recent systematic review and meta-analysis reported that prenatal ultrasound had a sensitivity of 31.7% and a high rate of false-positive diagnoses [5]. At present, a normal esophagus is not routinely examined during ultrasound screening. Prenatal diagnosis of EA/TEF relies on two nonspecific signs: polyhydramnios and an absent or small gastric bubble. However, these signs have very low sensitivities and positive predictive values (26–57% and 35–63%, respectively) [6]. Some investigators have used ultrasound to visualize the dilatation of the blind-ending upper esophageal segment in the neck and mediastinum during fetal swallowing [7]; nonetheless the upper pouch is most often identified during the third trimester, and its visualization may be challenging due to technical difficulties and time limitations.
These problems can be addressed by routine esophageal imaging, especially in all targeted high-risk scans, although the ideal situation would be to perform imaging in all fetuses during a screening anomaly scan. This study aimed to determine the consistency of prenatal ultrasound imaging of the longitudinal esophageal sections in fetuses between 21 and 23 gestational weeks.
This prospective observational study was conducted at an obstetrics and gynecology ultrasound center between June 2021 and June 2022. Only singleton pregnancies between 21 and 23 gestational weeks with no abnormalities were included in the study. Pregnancies referred for suspected fetal anomalies, polyhydramnios, and small or absent gastric bubbles were excluded from the study. Informed written consent was obtained for the study as well as for the use of data. We obtained approval for publishing this study from the ethics committee of our institution.
Imaging was conducted using Voluson E10 (GE Healthcare, Milwaukee, WI, USA) with a C2-9-D or RM7C probe (4–8 MHz) and Voluson E8 (GE Healthcare, Milwaukee, WI, USA) with RAB6-D or C1-5-D probe (2.5–5 MHz) were used for imaging. Fetal cardiac presets were used in this study to image fetal esophagus.
A detailed prenatal anatomic survey was first performed, followed by dedicated esophageal imaging. Esophageal imaging was performed as follows: coronal sections of the thoracic aorta were obtained first; the sound beam was then moved slightly to the ventral side of the fetus; strip-shaped high echoes with three or more hyperechoic layers were identified as the esophagus; and images showing the longest segment were stored. Esophageal length and angle (between the thoracic aorta and esophagus) were measured.
The collected data were computerized and analyzed using IBM SPSS Statistics
software version 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables were
expressed as mean
We examined 3525 consecutive women with singleton pregnancies who were referred
for detailed prenatal ultrasound imaging between June 2021 and June 2022 at the
Obstetrics and Gynecology Ultrasound Department of our institution. Of these
women, 218 were enrolled in the study. The mean age of the study population was
30.7
| Characteristics | Data |
|---|---|
| Age of the pregnant women, years | 30.7 |
| Gestational age of the fetus, weeks | 22.1 |
| BMI of the pregnant women | 22.9 |
BMI, body mass index.
With methodological guidance, which was detailed mentioned in the part of
Instruments and Methods, the time taken to visualize the longitudinal section of
the esophagus was 21.3
| Fetal position | n | Seen (%) | Not seen (%) |
|---|---|---|---|
| LOA | 74 | 74 (100) | 0 (0) |
| ROA | 55 | 55 (100) | 0 (0) |
| LOP | 6 | 4 (66.7) | 2 (33.3) |
| ROP | 3 | 2 (66.7) | 1 (33.3) |
| LSA | 32 | 32 (100) | 0 (0) |
| RSA | 13 | 13 (100) | 0 (0) |
| LSP | 14 | 9 (64.2) | 4 (35.8) |
| RSP | 5 | 3 (60.0) | 2 (40.0) |
| Transverse | 16 | 10 (62.5) | 4 (37.5) |
| Total | 218 | 205 (94.0) | 13 (6.0) |
LOA, left occiput anterior; ROA, right occiput anterior; LOP, left occipitoposterior; ROP, right occipitoposterior; LSA, left sacro anterior; RSA, right sacro anterior; LSP, left sacro posterior; RSP, right sacro posterior.
Two patterns of longitudinal esophageal sections in fetuses were observed when
collapsed on ultrasound: a tubular structure composed of three hyperechoic layers
(40.4%, 88/218) and a tube composed of multilayered hyperechoic structure
(59.6%, 130/218; Fig. 1). Swallowing actions were observed
in 28 cases (12.8%, 28/218; Fig. 2). The fetal esophagus was fluid-filled and
exhibited an appearance similar to a tram-track sign. The
mean esophageal length on longitudinal section was 25.4
 Fig. 1.
Fig. 1.Longitudinal sections of the fetal esophagus, seen as a
three-layered hyperechoic structure (A,B) and a multilayered hyperechoic
structure (C,D). (A) Longitudinal sections of fetal esophagus imaging at
22
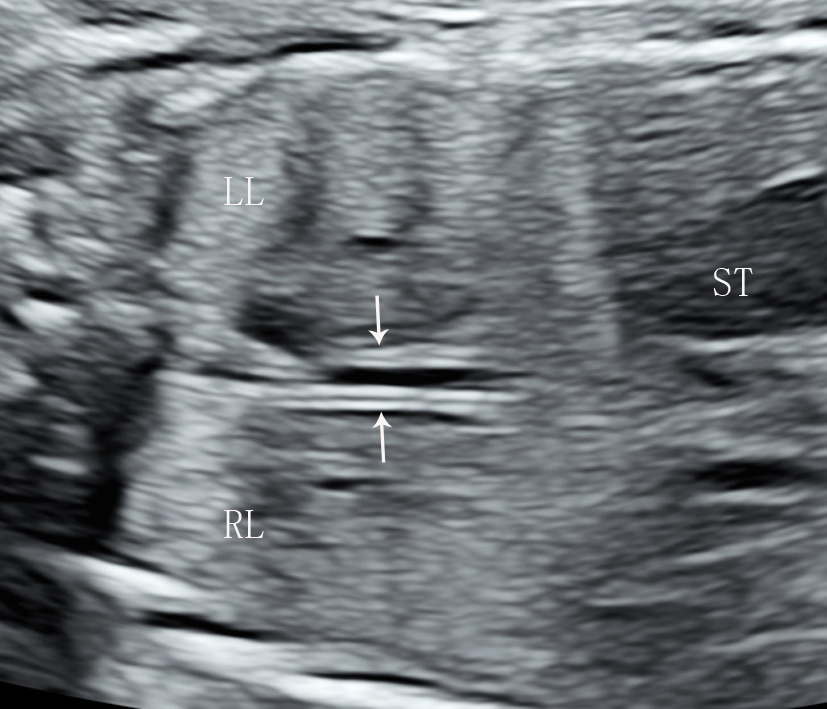 Fig. 2.
Fig. 2.Longitudinal section of a fluid-filled fetal esophagus,
appearing as a tram-track sign. Longitudinal section of
fetal esophagus imaging in a fetus in the LSA position at 22
 Fig. 3.
Fig. 3.Longitudinal sections of the fetal esophagus with color Doppler imaging. (A) Longitudinal sections of the fetal esophagus with color Doppler imaging in a LSA position fetus at 22 weeks of gestation, showed the positional relationship between the esophagus and aorta. (B) The angle between the thoracic aorta and the esophagus was measured at 14.4°. RL, right lung; LL, left lung; TA, thoracic aorta; AA, abdominal aorta; E, esophagus; ST, stomach.
Improving the diagnostic accuracy of prenatal EA assessments is challenging
owing to the limited visibility of the fetal esophagus. If the longitudinal
esophageal sections in normal fetuses can be antenatally visualized, we can
improve the EA detection rate and provide more information to the parents of
affected fetuses, as well as to surgeons. Therefore, we performed this study. The
primary types of congenital EA according to the Gross classification, are EA with
distal TEF (85%, Gross C), isolated EA without TEF (8%, Gross A), TEF without
atresia or H-type TEF (4%, Gross E), EA with proximal TEF (3%, Gross B), and EA
with proximal and distal TEF (
It is possible to visualize the normal fetal esophagus antenatally. Malinger et al. [11] showed that training could improve operators’ ability to directly visualize the esophagus. Quarello et al. [9] reported on seven fetuses with suspected EA who were assessed prospectively using targeted ultrasound imaging of cervical and thoracic structures. The defect length was assessed directly, with EA correctly diagnosed prenatally in six out of the seven cases. However, this article did not describe a method for displaying the longitudinal esophageal sections by prenatal ultrasound imaging. After visualizing the transverse section of the collapsed esophagus in the area behind the heart, Venkatesh [12] was able to identify the longitudinal esophageal section by rotating the probe 90°. Using this technique, the entire length of the esophagus could be traced in 92.3% of fetuses at 18–30 weeks and 88.23% of those at 11–14 weeks. However, it was performed by a single operator. In addition, the esophagus on the transverse section is very small, approximately 3 mm, and is very easy to lose during probe rotation. Our study aimed to consistently image the longitudinal esophageal sections.
As is widely accepted, esophageal echogenicity is difficult to demonstrate
without methodological guidance, even after extensive left-to-right and
ventral-to-dorsal thoracic scanning, especially with abundant amniotic fluid or
frequent fetal movement. Our study suggests that the position of the esophagus
can be inferred from its anatomic relations. The esophagus lies on the ventral
aspect of the thoracic aorta, and both are closely related structures within the
mediastinum. The upper part of the thoracic esophagus is located on the right
side of the thoracic aorta and the lower part is located in front of the thoracic
aorta. The mean angle between the thoracic aorta and esophagus was 10.5
At this stage, the longitudinal esophageal section is a tubular structure
composed of multilayer parallel echogenic lines obliquely coursing from the
stomach to the upper thorax. In our study, two patterns of the longitudinal
esophageal sections in normal fetuses were observed when collapsed on ultrasound:
(1) a tubular structure composed of three hyperechoic layers and (2) a tube
composed of a multilayered hyperechoic structure. The results of our study are
similar to the findings previously published by others [12, 13].
Swallowing actions could be observed in some fetuses whose
esophagus is fluid-filled, and these could appear as
tram-track signs. The esophagus and trachea can be
distinguished based on the location of the trachea (the middle and upper part of
the chest, on the right-hand side of the esophagus), its bifurcation, larger
diameter, and amniotic fluid anechoic area. Furthermore, the esophagus, the
length of which is 25.4
In our study, the fetal position affected the visualization of the longitudinal esophageal sections. The esophageal visualization rate of fetuses in occipitoposterior and sacro-posterior positions was low. In such cases, it is reasonable to wait until after fetuses change their position.
This study has some limitations. First, this is a single-center observational study that evaluated the feasibility of prenatal ultrasound imaging to assess for esophageal atresia in fetuses between 21 and 23 gestational weeks. Second, the number of cases was relatively small. In the future, we will increase the sample size and access fetuses in the first, second, and third trimester.
We suggest direct sonographic assessment of the esophagus to improve the specificity of diagnosis and prenatal evaluation. Prenatal evaluation of the esophagus in cases with high-risk scan findings (such as polyhydramnios, and small or absent gastric bubbles) should be helpful in counseling parents of affected fetuses.
All data generated or analyzed during this study are included in this published article.
XLC, YSZ and NZ designed the research study. XLC analyzed the data and wrote the manuscript. XLC, NZ, LM, MFX and SYZ performed the research. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
We obtained approval for publishing this study from The Ethics Committee of Shenzhen Hospital of Southern Medical University (No.: NYSZYYEC20210017). Informed written consent was received for the study as well as for the use of the data.
Not applicable.
This research project was supported by Fund of Shenzhen Science and Technology Innovation Commission (Ref No.: JCYJ20180306174225855).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
