- Academic Editor
†These authors contributed equally.
Background: To investigate the diagnostic value of SMARCE1,
cysteine-rich secreted protein 3 (CRISP3) combined with tumor markers in the
diagnosis of cervical cancer. Methods: A total of 80 patients with
cervical lesions who were diagnosed and treated in our hospital from January 2020
to March 2022 were selected and divided into control group (chronic cervicitis, n
= 30) and observation group (cervical cancer, n = 50). Immunohistochemistry was
used to detect the expression levels of SMARCE1 and CRISP3 in cervical
tissue of the two groups of subjects, and the relationship between the expression
of SMARCE1 and CRISP3 in cervical cancer tissue and the
clinicopathological data of the patients was analyzed. In addition, the serum
tumor marker levels of the two groups of subjects were detected, and the
diagnostic value of SMARCE1 and CRISP3 combined with tumor markers in
cervical cancer was analyzed. The female sexual function index (FSFI) and the
functional assessment of cancer therapy-general (FACT-G) score were used to
evaluate female sexual function and quality of life. Results: The
positive expression rates of SMARCE1 and CRISP3 in the observation group
were significantly higher than those in the control group (p
Cervical cancer is a common gynecological malignant tumor in clinical practice. As the second most common cancer among female in the world, cervical cancer has become the second leading cause of death of malignant tumors in the female genital system and posing a serious threat to the safety and health of female in China [1]. Cervical cancer is a long-term process and it takes a long time (5 to 10 years) to develop from cervical of precancerous lesions to cervical intraepithelial neoplasia (CIN). Therefore, early diagnosis and treatment of cervical cancer patients is of great significance to improve the prognosis of patients [2]. Patients with early cervical cancer have no conscious symptoms, and their cervical tissues are also lack of special changes with naked-eye, leading to missed diagnosis or misdiagnosis in clinical examination that affects early treatment of patients [3]. Therefore, it is very important to select reasonable and effective detection methods to improve the early diagnosis rate of cervical cancer. The studies found that the expression of SMARCE1 in cancer tissues of patients with gastric cancer, ovarian carcinoma and liver cancer are closely related to prognosis, and SMARCE1 is a critical gene to promote the invasion and metastasis of breast carcinoma cells [4, 5, 6]. cysteine-rich secretory protein 3 (CRISP3) is the third member of the cysteine-rich secretory protein family that has been confirmed to be low expressed in carcinoma of prostate, breast carcinoma and ovarian carcinoma, and the low expression of CRISP3 is related to the sur the stimulation reaction of malignant tumor cells or the body by tumors, the levels survival rate of breast carcinoma patients [7]. In addition, tumor markers refer to the special biochemical substances that exist in the body fluid, urine or blood of tumor patients and are generated byre higher than those of normal people, the changes can reflect the occurrence and development of tumors and play a role in early screening of cancer [8]. The study is to explore the diagnostic value of SMARCE1 and CRISP3 combined with tumor markers in cervical cancer, so as to provide reference for clinical diagnosis and treatment.
80 patients with cervical diseases were diagnosed and treated in Tianjin Fifth
Central Hospital from January 2020 to March 2022, and were divided into the
control group (with chronic cervicitis, n = 30) and the observation group (with
cervical cancer, n = 50) according to the pathological examination results. The
observation group was 35~58 years old, with an average age of
(47.63
The following inclusion criteria were established for cervical cancer: (1) Meet the diagnostic criteria for cervical cancer in the Expert Consensus On Immunoprophylaxis of Human Papillomavirus-Related Diseases [9]. (2) Patients with available 3-year follow-up data (for non-recurring patients; patients who recurred were included even if they did not complete the three-year follow-up period). (3) It be longed to the early stage of cervical cancer. (4) The clinical data were complete and all patients with cervical cancer were operated on in Tianjin Fifth Central Hospital. (5) Good cognitive function. (6) All subjects signed informed consent and was approved by the ethics committee of Tianjin Fifth Central Hospital.
Exclusion criteria were the following: (1) Age
Rabbit Anti-Human SMARCE1 and CRISP3 monoclonal antibodies were purchased from Abcam Corporation, Trading Co., Ltd. (batch number: EPR8848, Shanghai, China) and the immunohistochemistry kits were purchased from ZSGB-BIO Co., Ltd. (batch number: ab105951, Beijing, China).
SP (streptavidin-perosidase) immunohistochemical method was used to detect the
immunoreactivity of SMARCE1 and CRISP3 proteins. Fix the cervical
tissues sample with formalin solution (10%) (batch number: RY0380, ZSGB-BIO Co., Ltd., Beijing, China), embed them with paraffin (batch number: 8002-74-2, ZSGB-BIO Co., Ltd., Beijing, China), and cut
the samples into 5
Five visual fields were randomly selected from each section under high power
microscope (CX22LED Olympus (China) Co., Ltd., Beijin, China) for observation, and the percentage of positive cells and the staining
intensity of cells were judged. The positive signals of SMARCE1 and
CRISP3 proteins were located in the cytoplasm, and the positive cells were brown
yellow or brown granules. (I) According to the staining intensity of positive
cells, it is judged that: colorless was 0 score, light yellow (weak positive) was
1 score, brown yellow (medium intensity) was 2 score, brown (strong positive) was
3 score. (II) According to the percentage of positive cells, positive cells
accounted for 0% was 0 score, positive cells
Took 5 mL of fasting peripheral venous blood from all subjects in the morning, centrifuged for 10 minutes at a rate of 3500 r/min (BY-600A Beijing Baiyang Co., Ltd., Beijing, China), and placed in a refrigerator at –80 °C temperature for testing. The level of serum CEA (batch number: 11731629322), CA125 (batch number: CA125 11776223322) and CA153 (batch number: 03045838122) were measured by electrochemiluminescence immunoassay. The detection instrument was the automatic electrochemiluminescence immunoanalyzer of Roche (Roche cobas 8000, Roche Diagnostics GmbH, Mannheim, Germany). The Kits were purchased from Beijing Lidman Biochemical Co., Ltd., China and operated strictly according to the instructions.
The statistical analyses were performed using the Statistical Package for the
Social Sciences version 20.0 (SPSS Inc., Chicago, IL, USA), and the counting data
were chi-square test or rank sum test for comparison. The measurement data were
expressed by mean
The positive expression rates of SMARCE1 and CRISP3 in the observation
group were significantly higher than the control group (p
| Group | Case | SMARCE1 | CRISP3 | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Control group | 30 | 14 (46.67) | 16 (53.33) | 11 (36.67) | 19 (63.33) |
| Observation group | 50 | 38 (76.00) | 12 (24.00) | 31 (62.00) | 19 (38.00) |
| 7.092 | 4.825 | ||||
| p | 0.008 | 0.028 | |||
CRISP3, cysteine-rich secreted protein 3.
There was no significant difference in the positive expression of
SMARCE1 and CRISP3 among the age, lymph node metastasis and tumor node
metastasis (TNM) stage of cervical cancer patients (p
| Group | Case | SMARCE1 | CRISP3 | |||||
|---|---|---|---|---|---|---|---|---|
| Positive | p | Positive | p | |||||
| Age | ||||||||
| 35 | 27 | 0.005 | 0.943 | 21 | 0.198 | 0.656 | ||
| 15 | 11 | 10 | ||||||
| Lymph node metastasis | ||||||||
| Yes | 18 | 16 | 1.576 | 0.209 | 14 | 2.972 | 0.085 | |
| No | 32 | 22 | 17 | |||||
| Degree of tumor differentiation | ||||||||
| Highly differentiated | 20 | 11 | 6.255 | 0.012 | 9 | 4.089 | 0.043 | |
| Medium and low differentiation | 30 | 27 | 22 | |||||
| TNM staging | ||||||||
| Stage I | 36 | 25 | 1.882 | 0.170 | 23 | 2.266 | 0.132 | |
| Stage II and III | 14 | 13 | 8 | |||||
CRISP3, cysteine-rich secreted protein 3; TNM, tumor node metastasis.
The level of the serum CEA, CA125 and CA153 in the control group were
significantly higher than observation group (p
| Group | Case | CEA (ng/mL) | CA153 (U/mL) | CA125 (U/mL) |
|---|---|---|---|---|
| Control group | 30 | 6.24 |
15.46 |
66.28 |
| Observation group | 50 | 2.85 |
6.84 |
41.34 |
| t | 8.673 | 12.312 | 13.285 | |
| p | 0.000 | 0.000 | 0.000 |
CEA, carcinoembryonic antigen; CA, carbohydrate antigen.
The ROC curve results shown that the AUC of SMARCE1, SMARCE1 + tumor marker, CRISP3, CRISP3 + tumor marker, SMARCE1, CRISP3 combined with tumor marker for diagnosis of cervical cancer were 0.760, 0.851, 0.739, 0.810 and 0.944 respectively. As shown in Table 4 and Fig. 1.
| Screening method | 95% CI | AUC | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|
| CRISP3 | 0.655–0.812 | 0.739 | 64.62 | 75.38 |
| SMARCE1 | 0.678–0.831 | 0.760 | 72.31 | 70.77 |
| CRISP3 + tumor markers | 0.732–0.873 | 0.810 | 75.38 | 70.77 |
| SMARCE1 + tumor markers | 0.778–0.908 | 0.851 | 89.23 | 72.31 |
| SMARCE1, CRISP3 Combined tumor markers | 0.889–0.977 | 0.944 | 95.38 | 83.08 |
ROC, receiver operating characteristic; CRISP3, cysteine-rich secreted protein 3; CI, confidence interval; AUC, area under curve.
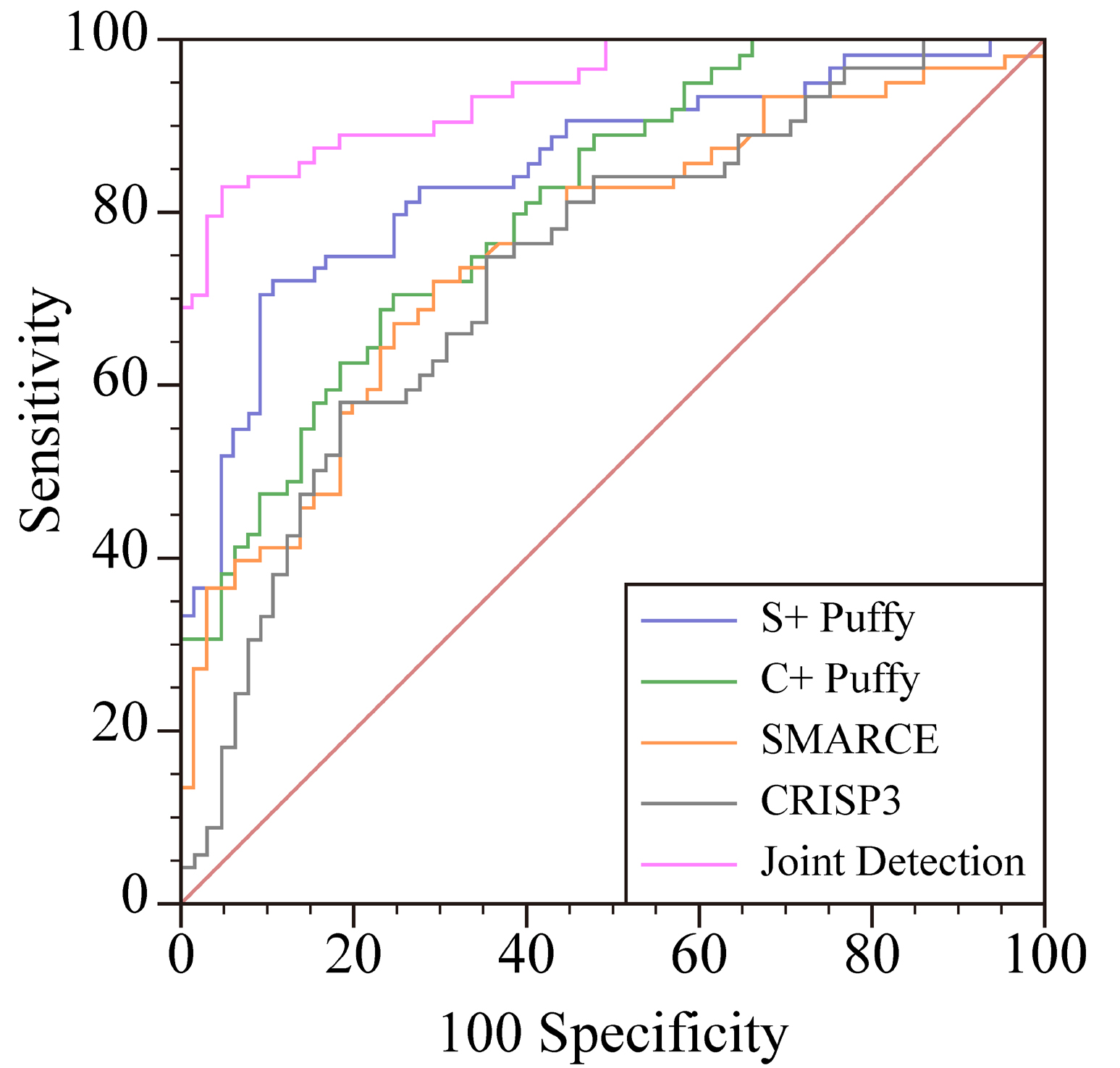 Fig. 1.
Fig. 1.ROC curve of the clinical value of SMARCE1, CRISP3 combined with tumor markers in the diagnosis of cervical cancer. CRISP3, cysteine-rich secreted protein 3.
The FSFI and FACT-G scores of cervical cancer group were lower than control
group (p
| Groups | n | FSFI (Score) | FACT-G (Score) |
|---|---|---|---|
| Control group | 30 | 35.69 |
89.68 |
| Cervical cancer group | 30 | 26.35 |
64.21 |
| t | 9.197 | 14.240 | |
| p | 0.001 | 0.001 |
FSFI, female sexual function index; FACT-G, functional assessment of cancer therapy-general.
The expression of SMARCE1 CRISP3 were positively correlated
with FSFI and FACT-G score (p
| FSFI | FACT-G | |
|---|---|---|
| SMARCE1 | r = 0.529 | r = 0.507 |
| p = 0.001 | p = 0.001 | |
| CRISP3 | r = 0.532 | r = 0.557 |
| p |
p |
FSFI, female sexual function index; CRISP3, cysteine-rich secreted protein 3; FACT-G, functional assessment of cancer therapy-general.
In recent years, with the change of people’s life and eating habits, the incidence of cervical cancer has been increasing year by year [11]. Cervical cancer is also known as Invasive Carcinoma of Cervix. Cervical intraepithelial neoplasia is the early stage of cervical cancer, also known as Precancerous Lesion Phase [12]. According to clinical studies, patients with cervical cancer have a long Precancerous Lesion State, and it takes about 5–10 years to develop from cervical intraepithelial neoplasia to cervical cancer [13]. Therefore, early detection and diagnosis of cervical intraepithelial neoplasia and cervical cancer, and active treatment of precancerous lesions can effectively reduce the incidence and mortality of cervical cancer and improve the quality of life of patients with cervical lesions. In clinical screening and diagnosis of cervical cancer with vinegar white test combined with iodine test, colposcopy, human papillo avirus (HPV) screening, cervical smear cytology, cervical and cervical tube biopsy, cervical conization screening [14]. The emergence of various screening technologies has improved the detection rate of clinical cervical cancer, but the screening costs of various screening methods are different. For cervical cancer, no effective screening methods exist. There is an urgent need to identify novel strategies to detect all gynecologic tumors as early as possible, thus reducing mortality and improving the quality of care [15].
It was found that human SWI/SNF chromatin-remodeling complex consists of 9~12 subunits, and SMARCE1 was one of the subunits of human SWI/SNF chromatin remodeling complex [16]. The human SWI/SNF chromatin-remodeling complex contains one of the ATPases of the SMARCA4 or SMARCA4 and three major core subunits and other complex specific variant subunits. The subunits together played biological roles in regulating cell cycle progress, differentiation, DNA repair, activation, genomic instability, and programmed cell death [17]. The results of the study shown that the positive expression rates of SMARCE1 and CRISP3 in the observation group were significantly higher than the control group. It was indicated that SMARCEI was expressed in cervical cancer patients and the abnormal expression of SMARCEI maid participate in the occurrence and development of cervical cancer. The results of the study also found that the positive expression rate of SMARCE1 was statistically significant in different tumor differentiation degrees of cervical cancer patients, and the lower the tumor differentiation degree, the higher the positive expression rate of SMARCE1 and CRISP3 proteins. It was indicated that the abnormal expression of SMARCEI may have an impact on the pathological changes of cervical cancer and may play a key role in promoting the carcinogenesis and development of cervical cancer. which were in line with the previous literature reports. Zhang et al. [18] found that SMARCEI was a specific and sensitive marker of clear cell meningioma, and SMARCEI mutation could lead to the occurrence of clear cell meningioma. SMARCEI mutation causes the loss of SMARCEI function, leading to the loss of inhibition of SWI/SNF complex on tumor and participating in the occurrence and development of tumor [19].
Human CRISP3 is located on human chromosome 6 and is the third member of the cysteine rich secretory protein family and is widely distributed in human tissues. It is detected in human body fluid secretion including sweat, plasma, prostate, pancreas and salivary glands [20]. The study found that CRISP3 is low expressed in colon, thymus, ovary and epididymis tissues, but its specific function has not been clearly studied [21]. CRISP3 is also low expressed in various tumor tissues that Henriksen et al. [22] found that CRISP3 is low expressed in malignant ovarian epithelial cells. Volpert et al. [23] found that CRISP3 can be used as a prognostic marker of prostate cancer. The higher the expression level of CRISP3 in prostate tissue, the higher the risk of recurrence of prostate cancer patients. Wang et al. [24] found that the detection of CRISP3 level may be a new method to predict breast cancer. The low expression of CRISP3 in breast cancer patients is related to the overall survival rate and disease-free survival rate. The results of the study shown that the positive expression rate of CRISP3 in the observation group was significantly higher than the control group. It is indicated that CRISP3 is expressed in patients with cervical cancer and the abnormal expression of CRISP3 may participate in the occurrence and development of cervical cancer. The results of the study also shown that the positive expression rate of CRISP3 was statistically significant in different tumor differentiation degrees of cervical cancer patients, and the lower the tumor differentiation degree, the higher the positive expression rate of SMARCE1 and CRISP3 proteins. The abnormal expression of CRISP3 may have an impact on the pathological changes of cervical cancer, and may play a key role in promoting the carcinogenesis and development of cervical cancer which were in line with the previous literature reports.
Tumor markers refer to proteins, peptides or other biological substances are produced by the body in the process of tumor occurrence, development, invasion and metastasis of tumor cells which are synthesized, secreted or shed into body fluids or tissues by the tumor cells or the body in response to tumor cells [25]. The content of tumor markers in normal healthy people is extremely low, but it is obviously expressed at a high level in tumor tissues. Therefore, the determination of tumor markers presence or content could be used to diagnose the generation of malignant tumors, analyze the patient’s condition, monitor metastasis, and judge the prognosis of patients [26]. CEA is an acid glycoprotein isolated from embryonic colon mucosa and colon adenocarcinoma which is expressed on the surface of tissue cell membrane and is widely used in the differential diagnosis of malignant tumors [27]. CA125 is a mucin-like glycoprotein with high molecular weight which can promote cell metastasis and infiltration by influencing mutual recognition and adhesion among cells [28]. CA153 is a polymorphic epithelial mucin secreted by glands and exists in many kinds of adenocarcinoma. Studies have found that the increase rate of CA153 can reach about 70% when tumor cells metastasize so that it has good diagnostic value for the development and prognosis of the disease [29]. The results of the study shown that the level of the serum CEA, CA125, CA153 in the observation group were significantly higher than the control group. It is indicated that CEA, CA125 and CA153 are highly expressed in cervical cancer patients, and the changes are related to the occurrence and development of cervical cancer.
In addition, the study results also found that the ROC curve analysis showed that the AUC values of SMARCE1, SMARCE1 + tumor markers, CRISP3, CRISP3 + tumor markers, SMARCE1 and CRISP3 combined tumor markers in the diagnosis of cervical cancer were 0.760, 0.851, 0.739, 0.810, 0.944, respectively. It is indicated that the combined detection of SMARCE1 and CRISP3 combined tumor markers has high clinical diagnostic value for cervical cancer. The study has the following deficiencies including only a small sample, single center study, and does not clarify how SMARCE1 and CRISP3 participate in the occurrence and development of cervical cancer. Large sample, multi-center studies are still needed in the future, and more in-depth biological research is needed to further clarify the relevant pathways.
To sum up, SMARCE1 and CRISP3 are expressed in cervical cancer patients, These data may serve as the basis for clinical counselling and future discussions on this relevant topic.
All data generated or analysed during this study are included in this published article.
Conception and design—LH; Administrative support—HZ; Provision of study materials or patients—LH; Data analysis and interpretation—JW; Manuscript writing—All authors. All authors read and approved the final manuscript.
This study was approved by the Tianjin Fifth Central Hospital Ethics Committee (approval number: 20221120). We confirmed that informed consent was obtained from all patients and their families. We confirmed all methods were carried out in accordance with Helsinki declaration.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
