1. Impact Statement
Fractional CO laser is a valid therapeutic choice for vulvo-vaginal
atrophy treatment in menopausal women. Previous studies have a limited number of
patients and limited results focused on safety. In this original article the
sample is considerable, and the evaluation of efficacy is accurate, including all
the main validated questionnaires and scales to evaluate genitourinary syndrome
and sexual life: Female Sexual Functional Index (FSFI), Female Sexual Distress
Scale (FSDS), severity of Most Bothersome Symptoms (MBS) and Vaginal Health Index
Score (VHIS). In this way the results are more precise and structured to assess
vaginal and sexual health.
A significant improvement in all domains was recorded. Contrary to other
studies, attention was paid to safety and no severe adverse events were reported,
with attention also to mild adverse effects such as vaginal infections.
For the future research, this work is an important milestone and these results
about laser efficacy and safety can only be taken for granted if studied with
precision.
2. Introduction
Genitourinary syndrome of menopause (GSM) majorly caused by the physiological
decline in estrogen, affects up to 90% of menopausal women [1]. The syndrome
includes vulvovaginal atrophy (VVA), as well as urinary and sexual disorders,
compromising quality of life (QoL) in as many as 50% of postmenopausal women
[2, 3]. Several therapeutic options have been proposed for the relief of GSM
symptoms, including both hormonal and non-hormonal products [4, 5, 6, 7, 8]. The main
therapeutic goal remains the relief of associated symptoms and signs, which could
be ideally achieved through the restoration of the urogenital physiology.
Hormonal therapy seems to be an effective treatment, often not executable for
contraindication or patient’s low compliance to local or systemic medical
therapy. Moreover, in the majority of oncologic patients, the use of hormonal
therapy is generally not accepted, and women are skeptical.
Fractional CO laser therapy is an emerging and effective choice for women
affected by VVA [9, 10]. Laser therapy treatment, applied vaginally, promotes
tissue regeneration of vaginal wall through the production of collagen and
elastic fibers. In particular, the CO laser fractionally ablates the
tissue, causing immediate heat-induced collagen contraction and subsequent tissue
remodeling, improving elasticity of vaginal canal by increasing the extracellular
matrix of the mucosa and increasing the muscle tone. Different studies have
successfully evaluated the effectiveness of CO laser for the treatment of
VVA symptoms, however now, to our knowledge, there is no prospective study
performed who could confirm its effectiveness on a large number of patients
[4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. Recent studies have a longer follow-up, but on an extremely limited
number of patients [14]. The aim of this study, hence, is to evaluate the
efficacy of fractional CO Laser and its ability to modifying clinical
symptoms that are correlated with GSM, with sexual functioning and sexual
distress, among a large number of postmenopausal women suffering from VVA.
3. Materials and Methods
3.1 Clinical Intervention and Procedure
This prospective observational study was conducted at the Maternal and Child
Health and Urological Sciences Department, Policlinico Umberto I, Sapienza
University of Rome. The research was performed according to Good Practice
Guidelines, STROBE (STrengthening the Reporting of Observational studies in
Epidemiology) guidelines and IRB approved the study protocol n°
CD-1511/2017. Informed consent was obtained from all participants.
Inclusion criteria were as follows: menopausal women, the presence of
physiological/iatrogenic menopause that is manifested through one or more of the
following VVA’s symptoms (itching, burning, reduced lubrication, superficial
and/or severe dyspareunia), a desire to preserve sexual activity, negative urine
culture, negative pap smear, negative vaginal swabbing, signed informed consent,
absence of AUB (abnormal uterine bleeding), in accordance with our institution
regulations “Sapienza checklist” [15]. A gynecological exam including cervical,
vaginal and vestibular inspection, has been performed prior to each treatment.
Exclusion criteria defined as the following: the use of any hormone therapy
(systemic or local) in the six months prior to the enrolment, the use of vaginal
moisturizer or lubricants in the thirty days prior to the enrollment, the
presence of neurological bladder, urinary tract infection or any current vaginal
infection, any serious pathology or chronic condition that could interfere with
the study compliance, any psychiatric disorders that might potentially preclude
informed consent, any association of Pelvic Organ Prolapse beyond 2nd degree
(according to the pelvic organ prolapse quantification (ICS-POP-Q) system), and
the use of any anticoagulation medication once a week (or more) prior to and/or
during the treatment course. All women were treated by a CO laser (Lumenis
AcuPulse DUO, Lumenis, Yokne’am, Israel) in its fractionated mode, with a 28 mm
probe (FemTouch™, Lumenis, Yokne’am, Israel) with power setting of
10 microjoules and 10% density, for 3 consecutive times, with 4 weeks apart. The
steps of CO laser treatment have been described elsewhere and were
performed in an outpatient setting without the requirement of any specific
preparation such as analgesia or anesthesia [15]. No local therapy (e.g.,
lubricants or moisturizers) was recommended, neither for the 48 hours before the
treatment, nor after. To avoid vaginal irritation during the healing process,
patients were advised to avoid coital activity for at least 1 week following each
laser application.
3.2 Patients Reported Outcomes and Evaluation
At baseline (T0) and four weeks after the last CO laser treatment (T1),
women were asked to complete the following questionnaires:
The Female Sexual Functional Index (FSFI) questionnaire [16]; a 19-item
multidimensional self-reported questionnaire that is often used as an instrument
for the assessment of female Sexual Function. The maximum score for each domain
is 6.0, obtained by summing item responses and multiplying by a correction factor
(desire: 0.6; arousal: 0.3; lubrication: 0.3; orgasm: 0.4; satisfaction: 0.4;
pain: 0.4). The total composite sexual function score is a sum of the domain
scores, and ranges from 2.0 (not sexually active and no desire) to 36.0.
Female Sexual Distress Scale (FSDS) [17, 18]; a 13-item scale that aim to assess
and quantify sexually related distress associated with inadequate or impaired
sexual function.
Most Bothersome Symptoms (MBS) questionnaire [19]; a 3-point numerical scale
running from 0 to 3 (for ‘no symptoms’ and ‘worst possible symptoms’,
respectively), in which the severity of the most common VVA symptoms is recorded,
including vaginal itching, postcoital vaginal bleeding, vaginal dryness,
dyspareunia and dysuria.
The questionnaires were filled-out privately, without the presence of healthcare
professionals and/or with no time-limit or any other constrain, at two different
time points: at the first outpatient-visit and four weeks after the last CO
laser treatment session. In the same way, gynecological examination was performed
at baseline (T0) and four weeks after the last CO laser treatment (T1),
including the evaluation of Vaginal Health Index Score (VHIS) and vaginal wet
mount with microscopic evaluation and Whiff test [20]. For vaginal wet 1 drop of
0.9% NaCl was placed on a slide; a spatula is used to take a sample
of the discharge from the vaginal wall; the sample was carefully mixed with the
0.9% NaCl on the glass slide and carefully covered with a cover
slide, avoiding “smearing” and air trapping. Optical microscopical evaluation
involved count of number of epithelial cells and leucocytes per field and
resident flora evaluation in terms of lactobacilli or cocchi presence
(10 magnification). Number and characteristics of squamous cells
(typical/atypical) of the vaginal wet mount were evaluated if at least 5 cells
per field (10 magnification) were found. For Whiff test a second
discharge sample (prepared in the same way as the wet mount) was incubated with
one drop of 10% KOH solution without a cover slip. A sniff test was
therefore done immediately to evaluate the fishy amine odor. For the VHIS
evaluation five components where evaluated (elasticity, fluid volume, pH,
epithelial integrity and moisture). For the pH evaluation, vaginal
indicator strips (Auctions Sticks, Arkray Factory, Japan) were applied against
the lateral vaginal wall using sterile forceps, followed by a vaginal lavage for
wet mount.
3.3 Statistical Analyses
Internal consistency (relatedness of items within a factor) was determined using
the Cronbach’s alpha statistic, separately for the six domains as well as for all
of the individual items; reliability was determined for each of the domains and
for the full-scale score. The incidence of events was analyzed for statistical
significance by using the Fisher’s exact or test. The
t-test and Mann–Whitney U test were used to compare continuous
parametric and nonparametric variables, respectively. To assess the impact of
different treatments on our endpoints, repeated measures ANOVAs, incorporating
baseline scores, were used. Statistical analysis was performed by IBM-Microsoft
SPSS version 25.0 (Chicago, IL, USA) for Mac. Repeated measure ANOVA, with
Bonferroni corrected post-hoc tests, was used to evaluate the null hypothesis,
according to which there is no change in women’s pain scores, when measured after
each treatment.
4. Results
Ninety-two patients of the hundred and one enrolled in the study (91.1%)
completed the treatment with fractional CO laser and returned after 4 weeks
for the follow-up visit. Nine patients (8.9%) dropped out and were lost to
follow-up. The main characteristics of this study population are described in
Table 1.
Table 1.Characteristics of patients (n = 92).
| Age (mean SD) |
58.42 9.38 |
| Age at menopause |
47.39 7.41 |
| Years since menopause |
12.65 10.06 |
| BMI (mean SD) |
24.07 4.07 |
| Education |
|
|
None, n (%) |
1 (1.1%) |
|
Primary |
4 (3.4%) |
|
Secondary |
14 (14.9%) |
|
High school |
56 (62.1%) |
|
University |
17 (18.4%) |
| Marital status |
|
|
Married |
43 (46.7%) |
|
Having a companion |
4 (4.4%) |
|
Single |
8 (8.9%) |
|
Divorced |
30 (32.2%) |
|
Widow |
7 (7.8%) |
| Deliveries |
|
|
None |
22 (23.5%) |
|
One delivery |
29 (31.8%) |
|
Two deliveries |
34 (37.6%) |
|
Three deliveries or above |
7 (7.8%) |
|
Two deliveries |
34 (37.6%) |
|
Three deliveries or above |
7 (7.8%) |
| Comorbidities |
|
|
Cancer |
20 (21.7%) |
|
Hypertension |
8 (15.1%) |
|
Dyslipidemia |
3 (5.7%) |
| SD, Standard Deviation; BMI, Body Mass Index. |
The mean age of the participants was 58.42 9.38 years, the average onset
of menopause was 47.39 7.41 years, while the median duration of
menopausal status was 12.65 10.06 years. The mean BMI (Body Mass Index)
was 24.07 4.07. Twenty patients (21.7%) had previously diagnosed cancer
(within the six months prior to study’s inclusion), 8 patients (15.1%) were
previously diagnosed with hypertension, and 3 (5.7%) with dyslipidemia. Further
characteristics, including demographic data (i.e., educational background,
marital status and past deliveries) are elaborated in Table 1. No severe (G3-G4)
complications occurred after a median follow-up of six months (considering from
the first visit to the last one) nor did any of the patients complain severe
pain. One patient (1.1%) reported dizziness immediately after treatment, which
was successfully solved within 15 minutes. A minor bleeding (traces of blood on
the probe) probably related to the tip introduction and/or rotation occurred in
one patient (1.1%) with severe atrophy. Treatment was successfully completed in
this patient. One patient (1.1%) requested to abort the procedure for discomfort
upon probe introduction but decided to resume the procedure after two weeks. Two
patients (2.2%) reported symptoms of dysuria within 7 days from procedures,
which was treated with 3gr Fosfomycin trometamol, repeated after 24 h. One
patient (1.1%) reported symptoms of vaginosis 4 weeks after the procedure and
was treated with local therapy. No further adverse events have been reported. A
significant decrease of vaginal pH between T0 and T1 was observed (–0.53 0.24; 95% CI = 0.48–0.58; p 0.001). Moreover, a significant
increase in VHIS was registered (4.1 1.21; 95% CI = 3.84–4.35;
p 0.001). In vaginal wet mount, lactobacillus as predominant species
was identified in 27 patients (29.7%) at baseline and in 74 (81.3%) after
treatment (p 0.001). Moreover, a non-significant trend of reduction
in pathogenic cocci bacteria was observed. In addition, a significative increase
of normal vaginal epithelial cells counts 5 at T1 compared to baseline (T0)
was shown: Normal vaginal cell count 5 per field was detectable in 40 patients
at T0 (43.9%) and 61 patients at T1 (67.3%), p = 0.003. Changes in
MBS-score are described in Table 2.
Table 2.Most Bothersome Symptoms (MBS).
| MBS |
Mean SD |
95% CI |
ST Err. |
p-value |
| Dyspareunia |
–1.16 1.01 |
–1.45, –0.87 |
0.14 |
0.0001 |
| RUI |
–0.81 1.31 |
–1.13, –0.50 |
0.16 |
0.0001 |
| Dryness |
–1.30 1.15 |
–1.56, –1.02 |
0.14 |
0.0001 |
| Burning sensation* |
–0.60 1.06 |
–0.86, –0.35 |
0.13 |
0.0001 |
| Postcoital bleeding |
–0.42 0.93 |
–0.70, –0.16 |
0.13 |
0.002 |
| Vaginal pruritis |
–0.81 1.23 |
–1.11, –0.52 |
0.15 |
0.0001 |
| MBS, Most Bothersome Symptoms; SD, Standard Deviation; ST Err., Standard Error;
RUI, Recurrent urinary infections.
*Upon urinating. |
A significantly important improvement has been shown in all six parameters that
were evaluated: Dyspareunia (–1.16 1.01, 95% CI = –1.45, –0.87,
p 0.0001); RUI (–0.81 1.31, 95% CI = –1.13, –0.50,
p 0.0001; Dryness (–1.30 1.15, 95% CI = 1.56, –1.02,
p 0.0001); Burning sensation upon urination (–0.60 1.06,
95% CI = –0.86, –0.35, p 0.0001); Post coital bleeding (–0.42
0.93, 95% CI = –0.70, –0.16, p = 0.002); Vaginal pruritis
(–0.81 1.23, 95% CI = –1.11, –0.52, p 0.0001).
Changes in FSFI are described in Table 3, in Figs. 1,2.
Table 3.Female Sexual Functional Index (FSFI).
| FSFI |
Mean SD |
95% CI |
ST Err. |
p-value |
| Desire |
0.63 1.95 |
0.11–1.14 |
0.26 |
0.02 |
| Arousal |
1.58 5.01 |
0.25–1.15 |
0.66 |
0.02 |
| Lubrication |
2.23 5.45 |
0.77–3.70 |
0.73 |
0.003 |
| Orgasm |
1.43 3.99 |
0.39–2.50 |
0.53 |
0.009 |
| Satisfaction |
1.70 3.65 |
0.73–2.67 |
0.48 |
0.001 |
| Pain |
1.77 3.54 |
0.83–2.71 |
0.47 |
0.0001 |
| Total |
9.54 18.94 |
4.52–14.57 |
2.51 |
0.0001 |
| FSFI, Female Sexual Functional Index; SD, Standard Deviation; CI, Confidence
Interval; ST Err., Standard Error. |
 Fig. 1.
Fig. 1.
Changes in FSFI.
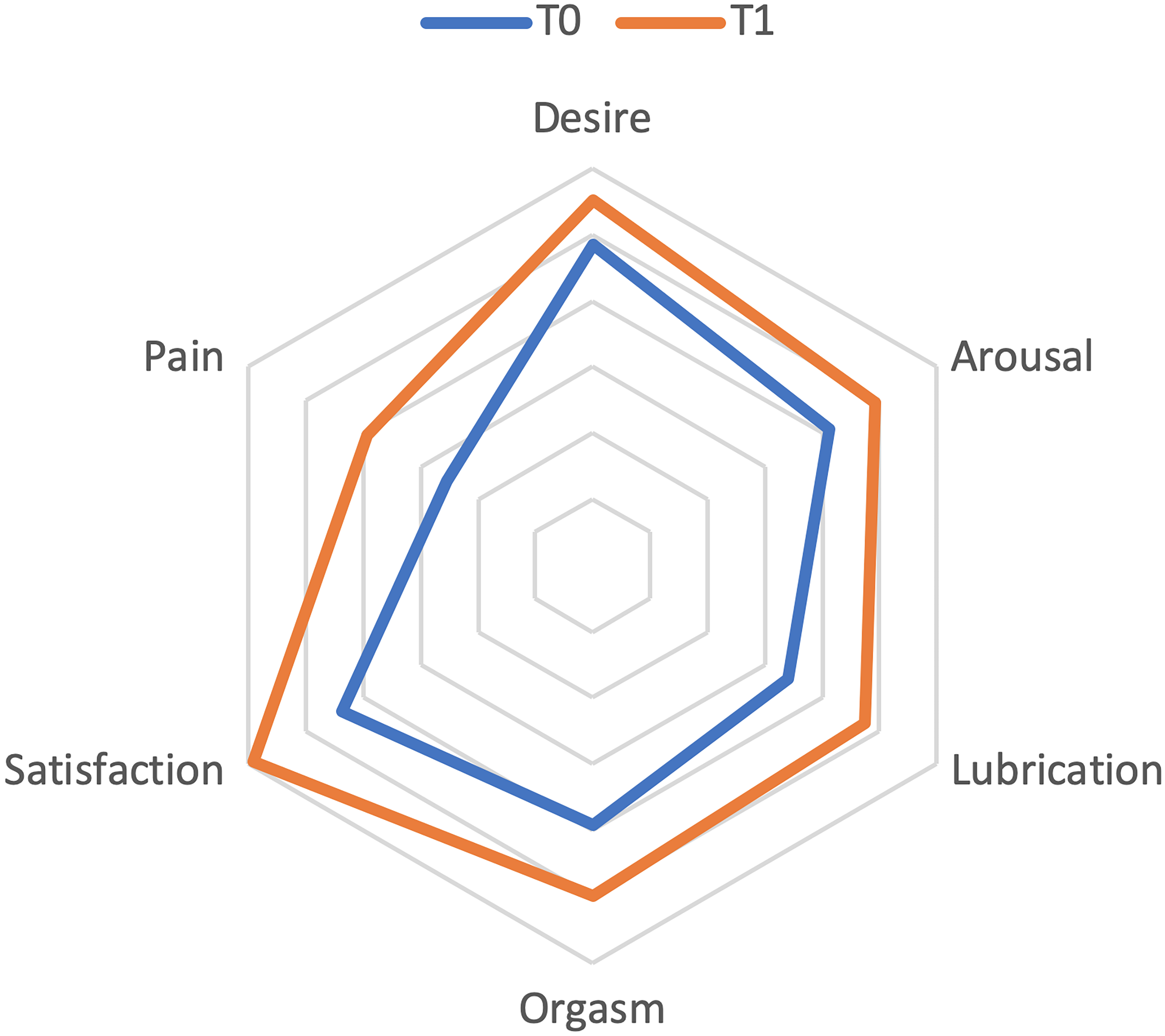 Fig. 2.
Fig. 2.
Changes in FSFI.
A significantly improvement of FSFI Total score (9.54 18.94, 95% CI =
4.52–14.57, p 0.0001) and of all 6 item evaluated were registered:
Desire (0.63 1.95, 95% CI = 0.11–1.14, p = 0.02); Arousal
(1.58 5.01, 95% CI = 0.25–1.15, p = 0.02; Lubrication (2.23
5.45, 95% CI = 0.77–3.70, p = 0.003); Orgasm (1.43
3.99, 95% CI = 0.39–2.50, p = 0.009); Satisfaction (1.70 3.65,
95% CI = 0.73–2.67, p = 0.001); Pain (1.77 3.54, 95% CI =
0.83–2.71, p 0.0001). As shown in Table 4, high inter-item
correlations were observed for all six domains (Chrombach’s alpha 0.925 or
0.810).
Table 4.Domain intercorrelation (Pearson’s r: range = –1.00 to
+1.00).
|
D |
A |
L |
O |
S |
P |
| T0 |
|
|
|
|
|
|
| D |
1.000 |
|
|
|
|
|
| A |
0.647 |
1.000 |
|
|
|
|
| L |
0.476 |
0.882 |
1.000 |
|
|
|
| O |
0.532 |
0.921 |
0.917 |
1.000 |
|
|
| S |
0.495 |
0.791 |
0.709 |
0.799 |
1.000 |
|
| P |
0.353 |
0.659 |
0.795 |
0.710 |
0.631 |
1.000 |
| T1 |
|
|
|
|
|
|
| D |
1.000 |
|
|
|
|
|
| A |
0.709 |
1.000 |
|
|
|
|
| L |
0.608 |
0.906 |
1.000 |
|
|
|
| O |
0.562 |
0.920 |
0.909 |
1.000 |
|
|
| S |
0.682 |
0.773 |
0.762 |
0.798 |
1.000 |
|
| P |
0.502 |
0.654 |
0.717 |
0.686 |
0.715 |
1.000 |
| D, desire; A, arousal; L, lubrication; O, orgasm; S, satisfaction; P, pain. |
Changes in FSDS are described in Table 5.
Table 5.Female Sexual Distress Scale (FSDS).
| FSDS Question |
Mean SD |
95% CI |
ST Err. |
p-value |
| Q1: Distress about sex life |
–0.28 1.02 |
–0.28–0.54 |
0.13 |
0.03 |
| Q2: Unhappy about sexual relationship |
–0.51 1.07 |
–0.24–0.78 |
1.35 |
0.0001 |
| Q3: Guilty about sexual difficulties |
–0.27 0.95 |
–0.03–0.51 |
0.12 |
0.03 |
| Q4: Frustrated about sexual problems |
–0.54 0.90 |
–0.31–0.76 |
0.11 |
0.0001 |
| Q5: Stressed about sex |
–0.48 0.88 |
–0.2–0.70 |
0.11 |
0.0001 |
| Q6: Unhappy about sexual relationship |
–0.16 1.05 |
0.10–0.42 |
0.13 |
0.235 |
| Q7: Worried about sex |
–0.36 0.90 |
–0.14–0.60 |
0.11 |
0.002 |
| Q8: Sexually inadequate |
–0.35 1.05 |
–0.08–0.61 |
0.13 |
0.010 |
| Q9: Regrets about sexuality |
–0.21 1.03 |
0.54–0.46 |
0.13 |
0.118 |
| Q10: Embarrassed about sexual problems |
–0.41 0.99 |
–0.16–0.70 |
0.12 |
0.002 |
| Q11: Dissatisfied with sex life |
–0.41 1.14 |
–0.12–0.70 |
0.14 |
0.006 |
| Q12: Angry about sex life |
–0.27 1.06 |
–0.00–0.54 |
0.13 |
0.049 |
| Q13: Bothered by low sexual desire |
–0.22 1.01 |
0.03–0.47 |
0.12 |
0.085 |
| TOTAL |
–4.45 8.73 |
–2.28–6.70 |
1.10 |
0.0001 |
| FSDS, Female Sexual Distress Scale; SD, Standard Deviation; ST Err., Standard
Error. |
A significantly improvement of Total FSDS have been demonstrated (–4.45
8.73, 95% CI = –2.28–6.70, p 0.0001). In particular, 11
parameters out of 13 of FSDS were statically reduced: Distress about sex life
(–0.28 1.02, 95% CI = –0.28–0.54, p = 0.03); Unhappy about
sexual relationship (–0.51 1.07, 95% CI = –0.24–0.78, p
0.0001; Guilty about sexual difficulties (–0.27 0.95, 95% CI =
–0.03–0.51, p = 0.03); Frustrated about sexual problems (–0.54
0.90, 95% CI = –0.31–0.76, p 0.0001); Stressed about sex
(–0.48 0.88, 95% CI = –0.2–0.70, p 0.0001); Unhappy
about sexual relationship (–0.16. 1.05, 95% CI = 0.10–0.42, p
= 0.235); Worried about sex (–0.36 0.90, 95% CI = –0.14–0.60,
p = 0.002); Sexually inadequate (–0.35 1.05, 95% CI =
–0.08–0.61, p = 0.010); Regrets about sexuality (–0.21 1.03,
95% CI = 0.54–0.46, p = 0.118); Embarrassed about sexual problems
(–0.41 0.99, 95% CI = –0.16–0.70, p = 0.002); Dissatisfied
with sex life (–0.41 1.14, 95% CI = –0.12–0.70, p = 0.006);
Angry about sex life (–0.27 1.06, 95% CI = –0.00–0.54, p =
0.049); Bothered by low sexual desire (–0.22 1.01, 95% CI = 0.03–0.47,
p = 0.085).
5. Discussion
The present large prospective observational study shows that CO fractionated LASER is effective and safe treatment in reducing symptoms related
to VVA/GSM, improving sexual function and QoL in postmenopausal women. Many
studies in literature have assessed the efficacy of CO Laser treatment of
VVA but most of them included a small number of women [12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22]. Literature on long
term effect of laser therapy is lacking; Alexadies et al. [14]
evaluated a series of three fractional CO laser treatments to the vulva and
vagina with a 1-year follow-up in a postmenopausal population but considering 18
postmenopausal females with atrophic vaginitis [15]. The present study
demonstrates a significant decrease in incidence of all Most Bothersome Symptoms
(MBS) evaluated. This improvement of women’s symptomatology is of great
importance since GSM, in menopausal population is underreported and
underestimated because of limited propensity of patients to discuss with
practitioner of this discomforts. Indeed, only 4% of women were able to
attribute vulvovaginal symptoms to GSM and only half of women discuss their
sexual health with practitioners when asked, while 33% do not discuss it at all
[23, 24]. The perceived reduction of all the most bothersome symptoms after laser
therapy are translated into a perceived better sexual function and increased
self-confidence. This is confirmed through a significant improvement of each item
and total score of FSFI. Interestingly, the outcome that showed to be
particularly improved was the perception of pain (1.77 3.54; p 0.0001). One of the potential explanations of this finding could be effect of
the laser on the vaginal mucosa that seems to increase vaginal thickness and
decreasing the exposure of surface area of the nerve endings, reducing the pain
threshold [13, 25, 26, 27, 28]. Another interesting change that occurs because of the
laser therapy is the increased vaginal lubrication that could be related to the
increase in mucosal growth of capillaries and, consequently, vaginal blood flow
[8]. Sexual satisfaction (1.70 3.65; p = 0.001) and the
possibility of experiencing an orgasm (1.43 3.99; p = 0.009)
significantly increased as well. In addition, women have demonstrated a lesser
fear of experiencing pain and a greater conviction that engaging in sexual
intercourse is possible again. Consistently, Desire (p = 0.02), Arousal
(p = 0.02) significantly increased and sexual distress domain’s indexes
(FSDS) significantly decrease after CO laser treatment. Literature’s
resources regarding the latter issue are limited and are somehow controversial,
probably because they are mostly, conducted among cancer survivors [29, 30].
Distress, on the other hand, seems to be increased in cases where therapeutic
efficacy does not meet patients’ goals and expectations. Our wet microscopy
results confirmed the improvement, in terms of vaginal microbial colonization and
pH progressive lowering and are consistent with literature evidence [31]. All
these benefits (MBS, FSFI, FSDS, VHI) were attributable CO laser treatment
since the patients’ population was accurately selected by excluding confounding
factors such as lubricants-use or ongoing hormonal therapies. Our findings stand
in line with recent literature [2, 5, 7, 8, 9, 10, 11, 12, 13, 32].
Nevertheless, the major limitation of the present study is the lack of control
arm, for the potential risk of placebo effect and follow- up should be longer.
For this reason, we have planned confirm our result with a double-blinded
randomized controlled trial with a large group of patients who would either go
through the full protocol of laser therapy or undergo a sham procedure. One of
the future goals should also be to better evaluate how many times could it be
possible to safely repeat the treatment. Recently, a randomized study, have
compared CO laser treatment versus sham procedure, enrolling a total of 88
patients (44 per each group). In that study sham procedure was able to produce a
non-statistically significant improvement in VHI (p = 0.06) and ICIQ
(p = 0.07) scores compared to baseline, while it has demonstrated a
significantly higher improvement of both VHI and VAS scores of patients treated
by laser compared with sham procedure. It is still unclear whether the
association of CO laser and usual medical options could exert a synergistic
effect on VVA. A randomized study has evaluated efficacy of fractional CO
vaginal laser treatment and compared it to local estrogen therapy and the
combination of both treatments (Laser and Estriol), for the treatment of VVA but
it found no significant difference in term of FSFI total scores between arms
[33]. Therefore, it could be considered as a first option for patients especially
those who have previous history of hormone-dependent cancer. A recent
retrospective study evaluated fractional CO vestibular laser treatment
combination with oral ospemifene in postmenopausal women presenting with
dyspareunia and vulvar pain showing a synergistic effect on clinical
effectiveness and long-term effect [34].
A recent paper reviewed the short-term effects and safety of vulvovaginal
fractional microablative CO laser therapy on atrophy symptoms using
validated questionnaires pre- and post-treatment, confirming the efficacy of this
treatment [35].
The considerable number of patients makes this article useful for literature,
for the low complication rate. We are continuing to collect data to have a longer
follow up. Another strength of the following study is to use validated tests to
evaluate clinical improvements.
6. Conclusions
Fractional CO laser improves vaginal health as well as signs and symptoms
associated with GSM, while significantly elevating QoL and sexual functionality
among postmenopausal symptomatic women.
Author Contributions
All authors contributed to the study conception and design. The first draft of
the manuscript was written by VDD, OD and AG and all authors commented on
previous versions of the manuscript. Material preparation—GP and IP. Data
collection—MS, CS and MF. Analysis was performed by VDD. Review &
editing—MM, LM and PBP. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
This prospective observational study was conducted at the Maternal and Child
Health and Urological Sciences Department, Policlinico Umberto I, Sapienza
University of Rome. IRB approved the study protocol n° CD-1511/2017.
Informed consent was obtained from all participants.
Acknowledgment
Not applicable.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest. We further confirm that any aspect
of the work covered in this manuscript that has involved either experimental
animals or human patients has been conducted with the ethical approval of all
relevant bodies and that such approvals are acknowledged within the manuscript.
All named authors have contributed significantly to the work, have read the
manuscript, attested to the validity and legitimacy of the data and its
interpretation, and have agreed to its submission. OD and AG are serving as one
of the Guest editors of this journal. LM is serving as one of the Editorial Board
members of this journal. We declare that OD, AG and LM had no involvement in the
peer review of this article and has no access to information regarding its peer
review. Full responsibility for the editorial process for this article was
delegated to PA and SM.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2.