Academic Editor: Luca Roncati
Background: Procalcitonin can effectively differentiate between
bacterial sepsis and a systemic inflammatory response syndrome of noninfectious
origins in the adult. However, the usefulness of procalcitonin in perinatal field
is not determined. Thus, the purpose of this study was to determine whether
procalcitonin levels in the umbilical blood reflect the severity of
chorioamnionitis and to assess their correlation with perinatal outcomes.
Methods: A retrospective study was conducted in 145 pregnant women with
singleton pregnancies and their neonates at a tertiary center between September
2010 and March 2013. Procalcitonin levels in the umbilical blood were measured by
an immunoluminometric assay. The severity of chorioamnionitis was classified by
the histological stage and grade for maternal and fetal inflammatory responses.
Procalcitonin levels were evaluated according to the severity of
chorioamnionitis; and the association with neonatal sepsis, intraventricular
hemorrhage (IVH), neonatal death within 28 days of life, and periventricular
leukomalacia was investigated. Results: In total, 28 women (19%) had
chorioamnionitis, of which 21 (75%) delivered at less than 34 weeks of
gestation. Fetal response in those with chorioamnionitis was correlated with
maternal response. Procalcitonin levels in the group with fetal stage 2 and 3 or
grade 2 responses were significantly higher than those infants with any other
stage or grade. The frequency of neonatal sepsis, IVH stage III or IV, and
neonatal death significantly increased (p
The term chorioamnionitis is used to describe a clinical symptom complex suggestive of intrauterine infection. There is now considerable evidence supporting the relationship between placental inflammation and important clinical outcomes such as neurological impairment and chronic lung disease [1, 2, 3]. This requires a more active clinical approach to the management of chorioamnionitis. Once chorioamnionitis is diagnosed, the fetus may benefit from immediate delivery rather than intrauterine treatment with antibiotics. Unfortunately, there are no useful clinical signs or biomarkers that reflect histological chorioamnionitis to help determine the appropriate timing of delivery in the presence of chorioamnionitis.
Procalcitonin (PCT) can effectively differentiate between bacterial sepsis and a systemic inflammatory response syndrome of noninfectious origins [4]. PCT has several advantages over other potential biomarkers such as a wide biological range, short induction time after bacterial stimulus, and long half-life [5]. However, the use of PCT as a marker of sepsis during pregnancy is controversial. Because, the general reference values for PCT in pregnancy, which are essential for clinical decision-making, have not been established [6]. Additionally, pregnancy conditions such as elevation of progesterone and immune state can lead to a resistant to lipopolysaccharide-induced production of PCT [6, 7]. On the other hand, the serum PCT levels in pregnant women are significantly higher than those in non-pregnant women [8], probably due to the physiological synthesis by trophoblast and stromal cell of the decidua [6, 9].
Histological chorioamnionitis is most commonly associated with intrauterine bacterial infection. It is believed that the fetal inflammatory response syndrome (FIRS), as a result of infection pathways and increasing cytokines activate an inflammatory response that potentially lead to fetal vasculitis, fetal death, preterm labor, or preterm rupture of the membranes [10, 11]. It is considered that this cytokine activation lead to production of PCT in the fetus.
With respect to the sequence of maternal and fetal inflammation, histological chorioamnionitis has repeatedly been shown to be both sensitive and specific for infection, and it is considered the gold standard against which other clinical predictors of infection should be measured [10, 11, 12, 13, 14]. Recently, Redline et al. [15] proposed a clinically relevant and pathologically reproducible grading and staging system for acute chorioamnionitis.
The objective of this study was to determine the changes in PCT levels in the umbilical cord blood according to the histological progression of the disease (stage) and gradations of intensity (grade) for maternal and fetal inflammatory responses in chorioamnionitis and assess these correlations with perinatal outcomes.
This retrospective study was conducted at a tertiary perinatal center, the University of Miyazaki, between September 1, 2010 and March 31, 2013. The pregnant women with singleton pregnancies and their neonates, who were managed at our center in this period, were enrolled in this study. The mothers delivered the neonates with chromosomal abnormalities, life-threatening structural anomalies, and hydrops were excluded from the study. The gestational age was based on the last menstrual period and was confirmed by ultrasonographic examination. We used medical charts and discharge forms to investigate the following items: (1) maternal and neonatal characteristics, (2) clinical signs of maternal infection such as fever and any other maternal complications during the intrapartum period, (3) presence of premature rupture of membranes (PROM), (4) culture for pathogens in the amniotic fluid or umbilical cord blood, and (5) clinical signs of neonatal infection.
Early-onset neonatal bacterial infection was diagnosed during the first 72 h of
life based on the presence of three or more of the following categories of
clinical signs in the neonate: (1) skin color (pallor, jaundice, cyanosis); (2)
respiratory (apnea, tachypnea
The umbilical cord bloods were routinely collected in a standardized fashion at the time of delivery to measure PCT levels for all patients. The measurement of PCT levels were conducted at the laboratory in University of Miyazaki. The umbilical cord blood was collected into collection tubes containing ethylenediaminetetraacetic acid. All tubes were immediately centrifuged and assayed within 1 h after collection. An immunoluminometric assay was performed to measure the PCT levels in the umbilical cord blood using a PCT kit (Roche Diagnostics Japan, Tokyo, Japan) and a fully automated chemiluminescence analyzer (cobas®, Roche, Germany). Using this method, the lowest detection limit of PCT was 0.02 ng/mL.
Following admission of neonate to the center, all placentas were subjected to histological analysis by pathologists blind to this study including the umbilical cord PCT levels. Placental findings consistent with chorioamnionitis were defined by the presence of an inflammatory neutorophilic infiltrate at two or more sites on the chorionic plate and extraplacental membrane. The histological progression of disease (stage) and gradations of intensity (grade) for the maternal and fetal inflammatory responses were classified according to the definition reported by Redline et al. [15]. This scheme utilized three-tiered staging and two-tiered grading of both the maternal and fetal inflammatory responses, respectively.
We investigated whether the PCT levels of the umbilical cord blood reflected the severity of placental inflammation. We also investigated the relationship between PCT levels in the umbilical cord blood and neonatal outcomes such as early-onset sepsis, intraventricular hemorrhage (IVH), frequency of intubation, death within 28 days of life, and cystic periventricular leukomalacia (PVL).
Between-group differences were assessed using the Mann–Whitney U test,
A multiple logistic regression analysis was performed to identify any
independent predictive factors of the neonatal outcomes. Only predictive
variables with a p value
A total of 145 pregnant women with singleton pregnancies and their neonates were enrolled in this study. The maternal and neonatal characteristics of this study group are shown in Table 1. Ten women (6.9%) were febrile within 2 h before or during delivery, but only four women had histological chorioamnionitis. Twenty-eight (19%) women had PROM, 10 of which had histological chorioamnionitis. Twenty-eight (19%) had histological chorioamnionitis. The gestational age at delivery varied from 22 to 41 weeks of gestation, and 21 (75%) women delivered at less than 34 weeks of gestation. Two of 28 women did not show the fetal inflammatory response in the placenta.
| Patients | |||
| total n = 145 | |||
| Age (year) | 30.9 | ||
| Primipara (%) | 68 (47) | ||
| G.W at delivery | 32.3 | ||
| Cesarean section (%) | 102 (70) | ||
| Maternal fever ( |
10 (6.9) | ||
| PROM (%) | 28 (19) | ||
| Male (%) | 61 (42) | ||
| Birth weight (g) | 1816 | ||
| Histological CAM (%) | 28 (19) | ||
| Positive culture (%) | 7 (25) | ||
| Pathogen | |||
| GBS | 1 | ||
| Bacteroides ureolyticus | 1 | ||
| Candida | 2 | ||
| Fusobacterium nucleatum | 1 | ||
| Capnocytophaga | 1 | ||
| Hemophilus influenzae | 1 | ||
| GW, gestational week; PROM, premature rupture of membranes; CAM,
chorioamnionitis; GBS, group B streptococcus. Data shown mean | |||
PCT level and CRP level in chorioamnionitis group were significantly higher than
those in no chorioamnionitis group (p
| CAM (n = 28) | non CAM (n = 117) | p value | |
| PCT (ng/mL) | 8.4 |
0.22 |
|
| CRP (mg/dL) | 0.34 |
0.08 |
|
| Gestational age at birth (weeks) | 28 |
33 |
|
| Birthweight (g) | 1283 |
1916 |
|
| CAM, chorioamnionitis; PCT, procalcitonin; CRP, c-reactive protein. | |||
The relationship between the histological progression of the disease and maternal and fetal inflammatory responses is shown in Fig. 1. The fetal inflammatory response in chorioamnionitis progressed as the maternal response progressed. However, the maternal response was equal to or more severe than the fetal response.
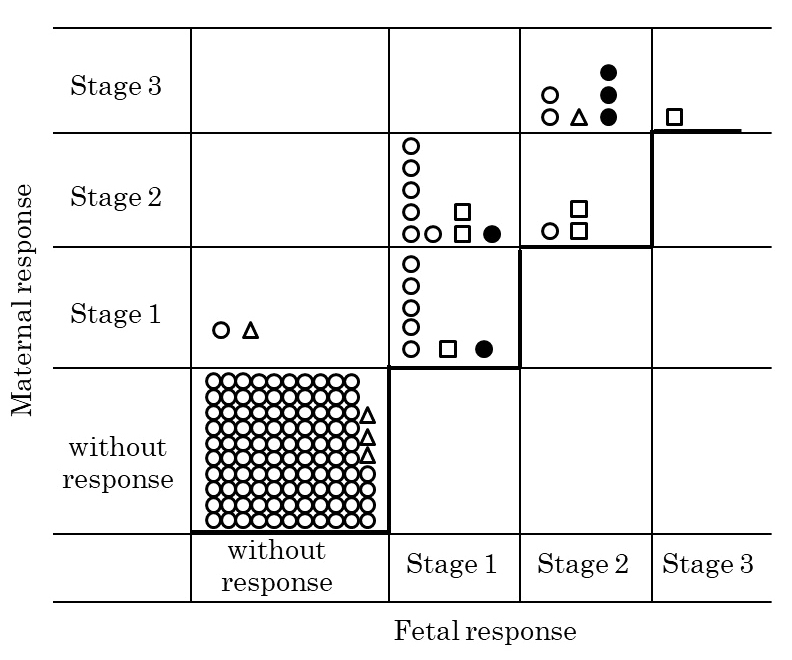 Fig. 1.
Fig. 1.Relationship between the maternal inflammatory response and fetal inflammatory response in chorioamnionities.
Circle,
The PCT levels in the umbilical blood according to the stage (S) and grade (G) for maternal and fetal inflammatory responses in chorioamnionitis are shown in Table 3. Seven cases with the most severe maternal inflammatory response (S3/G2) showed variable PCT levels, but four (57%) had PCT levels higher than 2 ng/mL. Two of the seven cases showed low PCT levels (0.43 and 0.44 ng/mL) and both of these had Candida neonatal sepsis. In nine women showing moderate S1/G1 responses, two had PCT levels higher than 2 ng/mL. One (PCT, 7.76 ng/mL) of the two delivered at 27 weeks of gestation had neonatal Haemophilus influenzae sepsis. The remaining one (PCT, 43.22 ng/mL) delivered at 40 weeks of gestation with an umbilical arterial blood of pH 6.9 and base excess of –14.8 mmol/L. Of the 117 women without maternal inflammatory responses, 114 (97%) showed PCT levels lower than 0.5 ng/mL.
| Placental response | PCT level (ng/mL) | ||||
| <0.5 | 0.5–2 | 2–10 | |||
| total | 129 | 5 | 6 | 5 | |
| 145 | n (%) | n (%) | n (%) | n (%) | |
| Maternal response | |||||
| Stage 3, Grade 2 ; n (%) | 7 | 2 (1.6) | 1 (20) | 1 (16.7) | 3 (60) |
| Stage 2, Grade 2 ; n (%) | 2 | 0 | 0 | 2 (33.3) | 0 |
| Stage 2, Grade 1 ; n (%) | 10 | 7 (5.4) | 0 | 2 (33.3) | 1 (20) |
| Stage 1, Grade 1 ; n (%) | 9 | 6 (4.7) | 1 (20) | 1 (16.7) | 1 (20) |
| without response; n (%) | 117 | 114 (88.4) | 3 (60) | 0 | 0 |
| Fetal response | |||||
| Stage 3, Grade 2 ; n (%) | 1 | 0 | 0 | 1 (16.7) | 0 |
| Stage 2, Grade 2 ; n (%) | 5 | 1 (0.8) | 0 | 1 (16.7) | 3 (60) |
| Stage 2, Grade 1 ; n (%) | 4 | 2 (1.6) | 1 (20) | 1 (16.7) | 0 |
| Stage 1, Grade 1 ; n (%) | 16 | 11 (8.5) | 0 | 3 (50.0) | 2 (40) |
| without response; n (%) | 119 | 115 (89.1) | 4 (80) | 0 | 0 |
Similarly, the PCT levels were analyzed according to the fetal inflammatory responses in the placenta (Table 3). There was only one case that showed a fetal S3/G2 response with a PCT level higher than 2 ng/mL. Two Candidal sepsis cases showed S2/G2 and S2/G1 responses. In the 16 women showing fetal S1/G1 responses, two had PCT levels higher than 10 ng/mL; one had 43.22 ng/mL (the term asphyxia case) and the other had 13.09 ng/mL (22 weeks of gestation with diffuse chorioamniotic hemosiderosis). Of the 119 women without maternal inflammatory responses, 114 (97%) showed PCT levels lower than 0.5 ng/mL and the remaining four had PCT levels between 0.5–2 ng/mL (0.57–1.07 ng/mL).
PCT levels of the umbilical blood were compared according to the maternal and fetal inflammatory responses (Table 4). In the maternal inflammatory responses, the PCT levels in S3 or G2 cases were significantly higher than those of any other stages or grades. Because only one case had a S3 fetal inflammatory response, the PCT levels were analyzed after combined S2 and S3, which were significantly higher than those at any other stage. Similarly, as the fetal grades advanced, the PCT levels also increased.
| Inflammatory response | PCT (ng/mL) | |
| Maternal stage | ||
| without response | 0.22 | |
| Stage 1 | 5.97 | |
| Stage 2 | 2.75 | |
| Stage 3 | 29.45 | |
| Maternal grade | ||
| without response | 0.22 | |
| Grade 1 | 4.15 | |
| Grade 2 | 22.16 | |
| Fetal stage | ||
| without response | 0.23 | |
| Stage 1 | 4.84 | |
| Stage 2 | ||
| 3 | 21.36 | |
| Fetal grade | ||
| without response | 0.23 | |
| Grade 1 | 4.30 | |
| Grade 2 | 36.34 | |
| * p | ||
The frequencies of neonatal complications were compared among the four PCT levels (Table 5). The frequency of early-onset sepsis in cases with PCT levels higher than 2 ng/mL was greater than that in those with PCT levels lower than 2 ng/mL. There were nine neonates with IVH grades III or IV, six of which had PCT levels higher than 2 ng/mL; this was a significantly higher incidence than in cases with PCT levels higher than 2 ng/mL. There were five deaths within 28 days of life, four of which were cases that had PCT levels higher than 2 ng/mL. There was no relationship between the PCT level and PVL frequency.
| PCT (ng/mL) | |||||
| 0.5–2 | 2–10 | ||||
| Outcome | total | n = 129 (%) | n = 5 (%) | n = 6 (%) | n = 5 (%) |
| Early-onset sepsis | 13 | 2 (1.6) | 1 (20) |
6 (100) |
4 (90) |
| Intubation | 21 | 6 (4.7) | 5 (100) |
6 (100) |
5 (90) |
| IVH III or IV | 9 | 2 (1.7) | 1 (20) | 3 (50) |
3 (60) |
| Death |
5 | 1 (0.8) | 0 (0) | 3 (50) |
1 (20) |
| PVL | 7 | 6 (4.7) | 0 (0) | 1 (17) | 0 (0) |
| IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia. * p | |||||
The study subjects were divided into the poor infant outcome (neonatal death and
abnormal ultrasonography findings) and good infant outcome groups (Table 6). PCT
and CRP levels in umbilical venous blood in the poor outcome group were
significantly higher than that in good outcome group (p
| Variables | Neonatal outcome | ||
| poor (n = 18) | good (n = 127) | p value | |
| PCT (ng/mL) | 12.72 |
0.25 |
|
| CRP (mg/dL) | 0.33 |
0.11 |
|
| CAM n (%) | 16 (88.9) | 12 (9.4) | |
| Early-onset sepsis n (%) | 12 (66.7) | 1 (0.8) | |
| Gestational age at birth (week) | 26 |
33 |
|
| Birthweight (g) | 984 |
1908 |
|
| Male n (%) | 8 (44.4) | 53 (41.7) | 0.85 |
The findings from the multiple logistic regression analysis are shown in Table 7. Six predictive factors, namely PCT level, CRP level, chorioamnionitis, early-onset sepsis, gestational age at birth and birthweight, were entered into the multivariate model. As a result, two predictive factors, namely early-onset sepsis and chorioamnionitis, were found to be independent factors of poor infant outcome.
| B | p value | OR | 95% CI | ||
| variable | Lower | Upper | |||
| Early-onset sepsis | 3.15 | 0.002 | 23.38 | 3.31 | 165.2 |
| Chorioamnionitis | 3.26 | 25.91 | 4.53 | 148.32 | |
| Constant | –2.23 | 0.011 | |||
As mentioned above, one infant who was delivered at term had birth asphyxia and mild chorioamnionitis and showed a high PCT level (43.22 ng/mL). MRI revealed that this infant had white matter injury with neurological impairment.
We evaluated the PCT levels in the umbilical cord blood according to the maternal and fetal inflammatory responses in the placenta and investigated the relationship between PCT levels and neonatal outcomes. We found that the umbilical PCT level reflected the histological progression of disease (stage) and the gradations of intensity (grade) for both maternal and fetal inflammatory responses. Furthermore, the prevalence of early-onset sepsis, IVH grades III or IV, and neonatal death significantly increased when the umbilical PCT levels became higher than 2 ng/mL.
Intrauterine infection induces fetal inflammatory responses, resulting in elevated levels of proinflammatory cytokines and an increased incidence of preterm birth and neonatal lung and brain injury [1, 2, 16, 17]. The fetal inflammatory response syndrome (FIRS) is characterized by the activation of the innate fetal immune system after in utero exposure to infection/inflammation [18]. FIRS was originally defined on the basis of increased cord blood concentrations of interleukin (IL)-6, which was shown to be associated with adverse neonatal outcome [19]. However, it is difficult to clinically diagnose chorioamnionitis. Clinical chorioamnionitis, variably defined by the presence of maternal fever, tachycardia, leukocytosis, foul-smelling vaginal discharge, or fetal tachycardia, is present only in a minority of cases with either histologically, microbiologically, or biochemically proven chorioamnionitis [20, 21, 22, 23]. Moreover, there are no highly sensitive and specific biomarkers for detecting chorioamnionitis.
PCT is ubiquitously produced in response to endotoxin or mediators released
during bacterial infections (for example, tumor necrosis factor-
The most important feature of a biomarker is its potential to change clinical decision making. Huetz et al. [29] reported the potential impact of umbilical cord blood procalcitonin based algorithm on antibiotics exposure in neonates with suspected early-onset sepsis. They concluded that this algorithm could significantly help the clinicians in their antibiotic prescription decision to decrease neonatal antibiotics exposure as compared with current practice [29]. However, they could not show the usefulness of PCT in umbilical cord blood as a diagnostic tool. The chief advantage of cord blood is that it is available in relatively large quantities immediately upon delivery, its sampling is technically easy to perform and does not pose a risk of infection or hemorrhage for the neonate. High PCT level in umbilical venous blood reflected sever chorioamnionitis in this study. Additionally, the chorioamnionitis and early-onset sepsis were the predictive factors for the infant’s outcomes in this study. Delay in diagnosis and treatment of sepsis increases mortality, prolongs length of hospital stay. Thus, when PCT level in umbilical venous blood show the high value of more than 2 ng/mL, it might be required that antibiotics therapy is initiated immediately after birth. However, the precise cut-off value of PCT level is required to introduce PCT measurement in clinical practice. The limitation of this study is that we cannot show this cut-off value. Because, there are several influenced factors for PCT level in umbilical venous blood such as neonatal asphyxia and prematurity.
Seventy-five percent of our study population with chorioamnionitis delivered at less than 34 weeks of gestation. In a cohort study of 483 singletons with a mean gestational age of 34.5 weeks (range 24–42 weeks), histological chorioamnionitis significantly increased the odds ratio for early abnormal brain sonography findings such as increased periventricular echodensity or echolucency, severe IVH, or ventriculomegaly [21]. This result is consistent with our result. However, there remains a controversy about whether prematurity-independent effects of chorioamnionitis on neonatal outcomes exist [30]. It is difficult to assess chorioamnionitis as a risk factor for adverse outcomes in extremely premature infants because prematurity can itself cause adverse outcomes. Regarding this point, we could show that early-onset sepsis and chorioamnionitis were independent variables by using multivariable logistic regression analysis, although prematurity influenced on the neonatal outcome in between-group difference’s analysis.
In this study, we did not show a relationship between histological chorioamnionitis and PVL. However, our result is consistent with the report by Soraisham et al. [31], in which no association between clinical chorioamnionitis and PVL was observed in 477 infants born at less than 33 weeks of gestation.
Early-onset sepsis and histological chorioamnionitis were independent variables against the neonatal adverse outcomes according to multivariable logistic regression analysis. Umbilical PCT levels higher than 2 ng/mL reflected the severity of histological chorioamnionitis and were associated with poor perinatal outcomes.
MK made the conception and design, analyzed and interpreted the patient data, and wrote the manuscript. JM collected the data and analyzed the patient data. NY collected the data and analyzed the patient data. YK collected the data and analyzed the patient data. All authors read and approved the final manuscript.
The study conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Faculty of Medicine, University of Miyazaki, Miyazaki Japan (approval number 2013-077). Informed consent was obtained from all subjects involved in the study.
The authors would like to thank Enago (www.enago.jp) for the English language review.
This work was supported by JSPS KAKENHI Grant Number JP25462566.
The authors declare no conflict of interest. MK is serving as one of the Guest editors of this journal. We declare that MK had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to LR.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
