Academic Editors: Valerio Gaetano Vellone and Michael H. Dahan
Background: Numerous hypoechoic cysts in the placenta on prenatal ultrasonography with a live fetus may indicate twin pregnancy with a complete hydatidiform mole with a coexistent fetus, partial mole, confined placental mosaicism, or placental mesenchymal dysplasia (PMD). Ultrasonographic appearances of these are similar; however, the differential diagnosis should be made because maternal and fetal prognoses differ. Cases: We present two cases of twin pregnancies with numerous placental cystic lesions. The first case was a partial hydatidiform mole in monochorionic diamniotic twins with a diploid karyotype that underwent a spontaneous abortion at 20 gestational weeks. The second case was PMD in dichorionic diamniotic twins; live twin neonates were delivered at 34 gestational weeks. Emergency cesarean section delivery was performed due to severe preeclampsia and fetal growth restriction in twin A at 34 weeks of gestation. Conclusions: Numerous hypoechoic cysts in the placenta on prenatal ultrasonography with a normal live fetus warrants chromosomal analysis and serial ultrasonographic examination to differentiate between PMD and molar pregnancy with a coexisting normal fetus to avoid unnecessary termination.
Numerous small hypoechoic cysts within the placenta can be caused by various etiologies. The common condition is molar pregnancies, most of which involve a complete mole, but a partial mole may also develop. Moreover, if there is a live fetus with numerous cystic lesions within the placenta, various possible etiologies including a twin pregnancy with a complete mole and coexistent fetus, a partial mole, confined placental mosaicism, and placental mesenchymal dysplasia (PMD) should be considered. The ultrasonographic appearances of the placenta are similar, showing multicystic lesions, in these conditions; however, it is difficult but important to differentiate these diseases because the clinical course and outcome of the diseases are quite different [1, 2]. The genetics of complete and partial mole is different; complete mole is generally associated with diploid karyotype —46,XX —of paternal origin, whereas partial hydatidiform mole usually has a triploid karyotype —69,XXX, 69,XXY or less commonly 69,XYY — and is accompanied by an abnormal fetus. A partial molar pregnancy of diploid karyotype coexisting with normal living fetus is extremely rare but has been reported [3].
PMD is a rare placental disorder characterized by multiple hypoechoic cystic appearances on ultrasonography and mesenchymal stem villous hyperplasia on histopathology [1]. PMD usually has a diploid karyotype with characteristics of progressive varicose dilatation of fetal chorionic vessels and reduced cystic lesions in the placenta after the late second trimester, on serial ultrasonographic examination.
Here, we report two pregnancy cases diagnosed differently, with numerous hypoechoic cysts in the placenta on prenatal ultrasonography with live normal twin fetuses in the first trimester. The first case was a partial mole in monochorionic diamniotic twins with a diploid karyotype, and the second case was PMD in dichorionic diamniotic twins.
A 30-year-old, gravida 1, para 0, twin pregnant woman visited the obstetric
department with sonographic abnormal placental findings at 13 weeks of gestation.
She had no medical or obstetric history. Her pregnancy resulted from ovulation
induction using clomifen. She was referred to our hospital because of placental
focal honeycomb appearance lesion at prenatal ultrasonography at 11 weeks of
gestation. Ultrasonographic examination revealed a twin pregnancy with
monochorionic diamniotic (MCDA) twins, with crown-rump length of 7.22 cm (13.3
weeks size) in twin A and 7.01 cm (13.1 weeks size) in twin B. There were no
structural anomalies in either fetus (Fig. 1A–B). The single placenta was
located in the right lateral and posterior wall of the uterus with a dividing
thin membrane (Fig. 1A); however, the right side of the placenta from twin A,
showed multiple hypoechoic cystic appearances with the size of the cystic area
being 6
 Fig. 1.
Fig. 1.Sonographic image of the placenta of twin A at 13 weeks of gestation of Case 1. (A) Twin A and the thin inter-twin membrane (arrow) is seen, which divides the two fetuses. (B) Twin B. (C) The placenta shows a partially multiple hypoechoic cystic area (arrowheads) at the right side of the placenta.
We performed chorionic villous sampling at both normal placenta and abnormal
placenta sites at 13 weeks of gestation to confirm karyotype. The chromosomal
study revealed normal diploid karyotype —46,XY— in both normal and abnormal
placentas. We excluded confined placental mosaicism. We performed genetic
amniocentesis again at 16 weeks of gestation to re-confirm diploid karyotype,
even though partial molar pregnancies can exist with a living fetus. This also
revealed the same normal karyotype. With these findings, our preferential
prenatal diagnosis was a partial molar pregnancy with normal diploid karyotype in
MCDA twin pregnancy. At 20.5 weeks of gestation, the patient was admitted to the
emergency department with acute abdominal pain and vaginal bleeding, and
spontaneous abortion occurred. There was no gross abnormality in either neonate.
The placenta measured 17
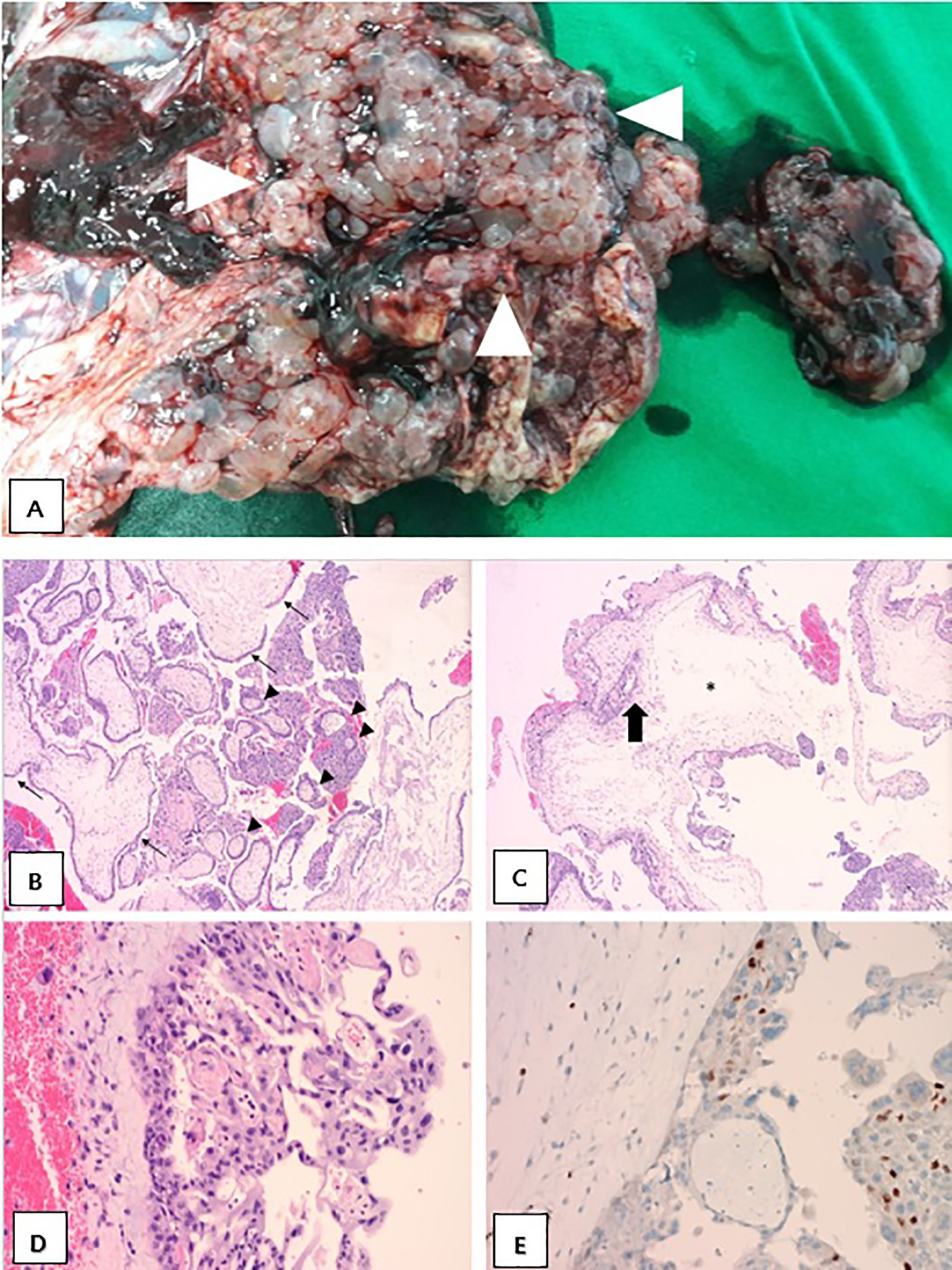 Fig. 2.
Fig. 2.Gross and histopathological findings of the placenta of Case 1.
(A) The disc was covered with multiple cysts (arrowheads) that contained yellow
serous fluid at the maternal surface. (B) Two intermixed villous populations,
consisting of small, fibrotic normal-appearing immature villi (arrow heads) and
larger, irregular, hydropic villi (arrows). (C) Enlarged villi with scalloped
borders (bold arrow) and central cistern formation (asterisk). (D) Moderate
trophoblastic hyperplasia. (E) Focal presence of p57 nuclear staining in
cytotrophoblast and villous stromal cells. ((B)–(D) H&E stain, (E) p57
immunohistochemistry; (B)
A 38-year-old, gravida 2, para 0, twin pregnant woman was referred to hospital
at 15 weeks of gestation due to abnormal placental lesion and fetal growth
restriction in twin A. Ultrasound examination revealed dichorionic diamniotic
twin fetuses. The estimated fetal weight (EFW) of twin A was 119 g (12
percentile) without structural abnormalities, and widespread multiple small
cystic lesions were seen in the placenta (Fig. 3A). Twin B fetus also showed
normal growth, EFW was 146 g (67 percentile) without structural abnormalities,
and there were no visible placental lesions (Fig. 3B). Chorionic villous sampling
had already been done at a local hospital at 11 weeks of gestation due to
abnormal ultrasonography finding of the placenta in twin A and cystic hygroma in
twin B. The result was 46,XX,t(11;22)(q23.2;q11.2) in twin A and
46,XY,t(11;22)(q23.2;q11.2) in twin B. With diploid karyotype results, we
suspected PMD rather than the partial hydatidiform mole. On serial
ultrasonography, placental multicystic lesions in twin A gradually reduced in
size and shape; however, numerous dilated and tortuous chorionic vessels abruptly
developed at 31 weeks of gestation (Fig. 3C–F). The fetal growth of twin A was
slow and showed small-for-gestation (EFW was 1446 g [9.3 percentile]) at 32 weeks
of gestation; however, there were no apparent fetal abnormalities in both
fetuses. At 33 weeks of gestation, the patient had a sudden headache and high
blood pressure. Emergency cesarean section delivery was performed due to severe
preeclampsia and fetal growth restriction in twin A at 34 weeks of gestation.
Neonate A was female and weighed 1400 g (
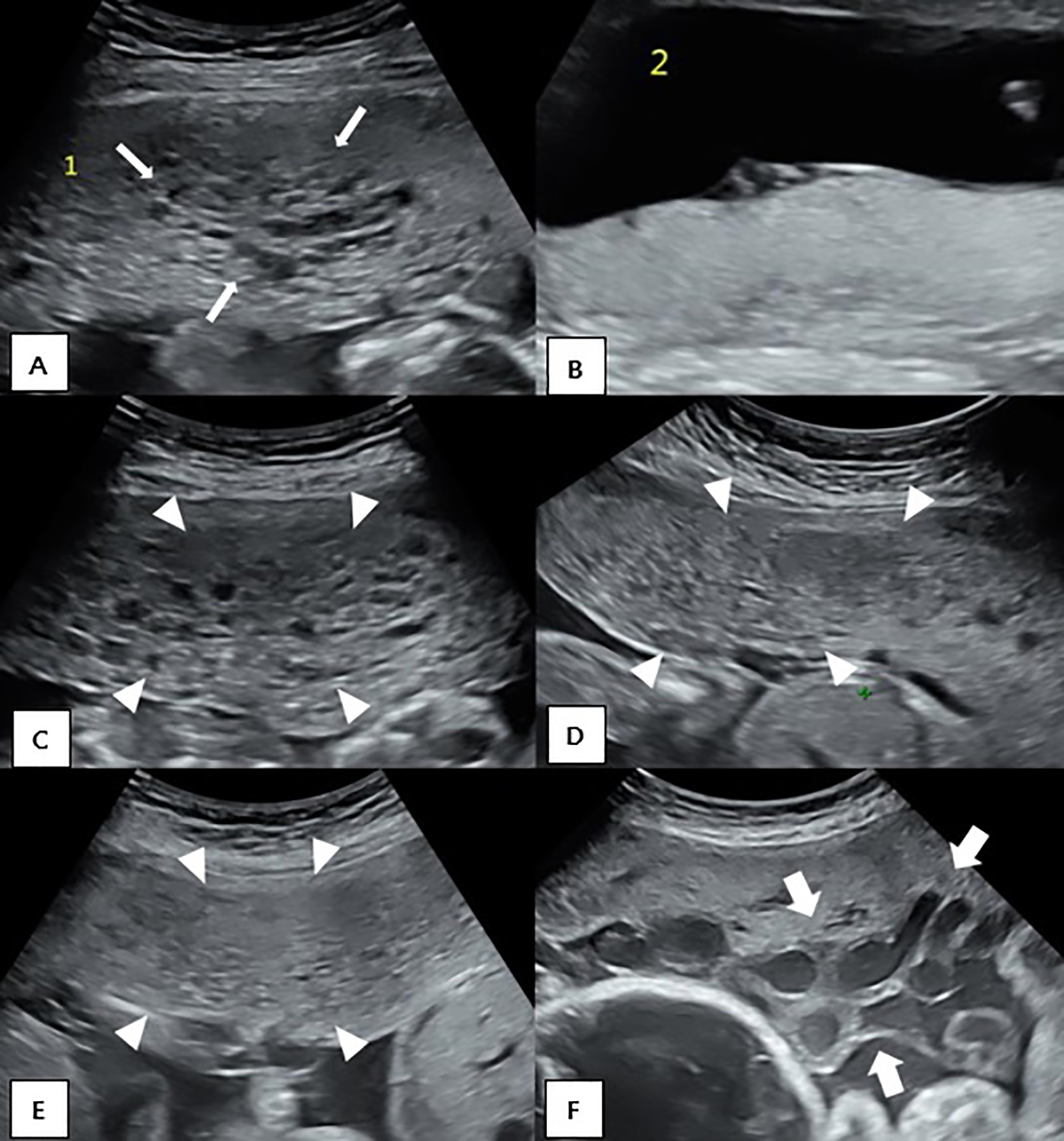 Fig. 3.
Fig. 3.Serial ultrasonographic images of the placentas of Case 2. (A) Numerous multiple cystic appearance (arrows) in the placenta of twin A at 15 weeks. (B) Normal placenta of twin B at 15 weeks. Serial ultrasonographic images of the placenta of twin A at 17.6 weeks (C), 23.1 weeks (D), 25.2 weeks (E) and 31.6 weeks (F). Small multiple cystic lesions became decreased (arrowheads); however, dilated chorionic vessels (bold arrows) became apparent at 31 weeks.
 Fig. 4.
Fig. 4.Gross findings of the placentas of Case 2. (A) On the fetal surface; the right side shows the PMD placenta with the fetal growth restriction infant. Numerous dilated and tortuous vessels (white arrow) were seen. (B) On the maternal surface; the left side shows the PMD placenta with the fetal growth restriction infant. The area of PMD lesion (yellow arrow) shows grape-like vesicle lesions. *PMD, placental mesenchymal dysplasia.
 Fig. 5.
Fig. 5.Histopathological findings of the placenta of twin A of Case 2.
(A) Markedly dilated chorionic plate vessels. (B) Abnormally enlarged stem villi
with thick vascular proliferation with thrombi (arrows). (C) Villous cysts in a
markedly myxomatous stroma of stem villi. Trophoblastic proliferation was not
observed. (D) Retention of p57 immunohistochemical staining in villous
cytotrophoblasts. ((A)–(C) H&E stain, (D) p57 immunohistochemistry; (A)
Hydatidiform mole is a trophoblastic neoplasm characterized by the proliferation of trophoblasts and hydropic degeneration of villi. Two types are observed; one is the complete hydatidiform mole, and the other is the partial mole [4]. In cases of hydatidiform mole, the coexistent live fetus is extremely rare but can be formed by either a complete mole with a live twin fetus or a partial mole with an abnormal triploid fetus. The outcome of a live fetus with a coexistent mole has a higher frequency of severe complications such as spontaneous abortion, intrauterine death, preterm delivery, fetal growth restriction (FGR) [5]. Veronica et al. [6] have reported prenatal diagnosis and pregnancy outcomes for 13 cases of twin pregnancies with complete hydatidiform mole and coexistent normal fetus. According to this study, five women delivered a live baby but preterm delivery with an average gestational age of 31 weeks (range 26–37 weeks) [6]. Also, there is a high risk of maternal complications such as: preeclampsia, thyrotoxicosis, and hemorrhages with severe anemia [5]. However, there can be differences between the complete and partial moles. The fetus with a coexistent complete mole has a chance of survival because the fetus usually has no anomalies, while the abnormal triploid fetus of partial mole tends to end in spontaneous abortion [4]. With a partial mole, the fetus may have growth restriction, a wide variety of structural abnormalities, and will die in the 1st trimester due to a lethal fetal abnormality [2]. Because a living fetus with a hydatidiform mole has poor outcomes, it is difficult to determine whether to provide immediate intervention or conduct expectant management. Most authors concluded that termination is not indicated in the cases of a partial mole if the fetus is normal [7]. Twin pregnancy with a complete mole with a live fetus also can be continued if there are no fetal anomalies or maternal complications [8].
PMD is a rare placental vascular anomaly without trophoblast proliferation, characterized as mesenchymal hyperplasia in stem villi. It was first described by Moscoso et al. [9] in 1991 as placentomegaly that produces the image of partial hydatidiform mole with elevated alpha feto-protein levels. Most PMD cases have normal karyotypes, but that was associated with preterm labor (33%), fetal growth restriction (33%), and fetal death in utero or neonatal death (13%) [1]. Twenty-three percent of the cases of PMD are associated with Beckwith-Wiedemann syndrome, characterized by macrosomia, macroglossia, omphalocele, exomphalos, internal visceromegaly, and increased incidence of childhood tumors [10].
With these various conditions of the placental molar lesion with a live fetus, the differential diagnosis is important. It is essential to recognize the detailed features of PMD and molar pregnancy and to distinguish the disease to prevent unnecessary termination of pregnancy. Pathology, karyotyping, and serial sonographic examination play a key role in distinguishing PMD and molar pregnancy. In pathologic evaluation, the proliferation of trophoblasts is seen in hydatidiform mole, but mesenchymal stem villous hyperplasia is seen in PMD. Twin gestations with complete mole and coexisting normal fetus can easily be misclassified as a partial mole because there are two populations of villi (molar villi from the complete mole and normal villi from the normal twin) [11]. Among them, P57 expression is very useful in the differential diagnosis of the complete mole and partial mole [12]. In chromosomal analysis, the fetal karyotype is usually normal (biparental diploid, 46 chromosomes, one set from each parent) in PMD, diploid karyotype (purely androgenetic) in the complete mole, and triploid karyotype (biparental) in the partial mole. However, a partial mole could coexist with a fetus with a diploid karyotype rarely reported [13]; In case 1 also revealed to have a diploid karyotype with a partial mole. We performed chorionic villus sampling (CVS) and amniocentesis for karyotype, but the possibility of mosaicism should be considered [14].
The serial ultrasonographic examination helps to diagnose PMD. The multiple cystic lesions in the placenta seen in early gestation gradually decreased with gestation; however, varicose dilatation of fetal chorionic vessels develop during the late second trimester in most cases of PMD [15].
In cases of partial mole with a diploid normal fetus, complete mole with a live fetus, and PMD pregnancy, the continuation of pregnancy can be an acceptable option because the fetus has a chance of survival, although the outcome of pregnancy can be poor. Once the suspected diagnosis has been established, the patient should be informed and advised about the potential fetal and maternal risks. Moreover, close monitoring during pregnancy and postpartum period is needed when continuation of pregnancy is desired.
Numerous hypoechoic cysts in the placenta on prenatal ultrasonography with a normal live fetus warrants chromosomal analysis and serial ultrasonographic examination to differentiate between PMD and molar pregnancy with a coexisting normal fetus to avoid unnecessary termination.
DHI, YNK, HJC, and MJK were involved in conceptualization and manuscript writing. HKY was involved in histologic staining of the tissue and manuscript editing. DHK, BJM, JMB, and DHJ were involved in coordination and manuscript editing. All authors read and approved the final version of the manuscript.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Inje University Busan Paik Hospital (approval number: 19-0208). This patient gave their informed consent they participated in the case report.
Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
