Academic Editor: Michael H. Dahan
Background: It has been suggested that hypovitaminosis D is associated
with the development of preeclampsia. This study aimed to study the relationship
between preeclampsia and various vitamin D serum biomarkers including 25-hydroxyl
vitamin D [25(OH)D], vitamin D-binding protein (VDBP), and bioavailable and free
25(OH)D. Methods: This prospective study was conducted with 17 patients
with preeclampsia and 38 normal pregnant women as the control group. Total serum
25(OH)D and VDBP concentrations were measured. The levels of bioavailable 25(OH)D
and free 25(OH)D were also calculated. Two single nucleotide polymorphisms
(rs4588 and rs7041) of the GC gene encoding VDBP were analyzed.
Results: VDBP was significantly increased in the preeclampsia group
compared to the normal pregnancy group (454.2 vs. 403.4 ng/mL; p =
0.036). When the preeclampsia patients were analyzed by dividing them into
early-onset and late-onset, there was no significant difference in the serum
vitamin D biomarkers levels. Also, when preeclampsia patients were classified
into three subgroups of
Preeclampsia is a pregnancy-related syndrome that can affect almost any organ and is accompanied by newly developed high blood pressure during pregnancy. This syndrome occurs in 4–5% of all pregnancies and is one of the three leading causes of maternal death, along with bleeding and infection [1]. Preeclampsia is diagnosed if patients have gestational hypertension and proteinuria. Gestational hypertension is empirically diagnosed when properly measured blood pressure exceeds 140 mmHg systolic or 90 mmHg diastolic after 20 weeks of gestation in a previously normotensive woman. However, even in the absence of proteinuria, preeclampsia can be diagnosed if there is definite evidence of multiorgan involvement, including thrombocytopenia, renal dysfunction, hepatocellular necrosis, central nervous system perturbations, or pulmonary edema [1].
Various risk factors known to increase the incidence of preeclampsia include
chronic hypertension, a history of preeclampsia, diabetes, a high body mass index
(BMI) over 30 kg/m
Vitamin D is a pleiotropic fat-soluble hormone [3]. In recent years, vitamin D functions have been demonstrated that extend beyond the regulatory functions of skeletal system metabolism. Vitamin D has been reported to play critical roles in autocrine, paracrine, and endocrine functions in various organs and systems, particularly the reproductive system [4]. In addition, it has been reported that serum vitamin D levels are associated with the development of gynecological diseases such as endometriosis [5]. Previous studies showed that vitamin D deficiency during pregnancy was associated with maternal pregnancy-related complications, including preeclampsia, gestational diabetes, labor obstruction, and infectious diseases [6].
In addition to total 25-hydroxy vitamin D [25(OH)D], which is currently used as
a biomarker for vitamin D status, vitamin D-binding protein (VDBP), a transporter
of vitamin D, and bioavailable vitamin D have been suggested as serum factors
affecting the action and the metabolism of vitamin D or reflecting a more
accurate vitamin D status [7, 8]. Vitamin D requires two hydroxylation steps for
conversion to its active form, 1
Serum VDBP levels are increased drastically during pregnancy due to elevated estrogen levels [14]. Thus, it can be postulated that vitamin D metabolism in pregnancy might be different from that in the non-pregnancy state. Bioavailable vitamin D or VDBP may affect the function and status of vitamin D in pregnant women and ultimately affect pregnancy outcomes [15, 16]. Consistent with this hypothesis, a limited number of studies have been conducted recently on the relationship between serum 25(OH)D levels and pregnancy, as well as other serum vitamin D markers and pregnancy [17, 18, 19]. However, the association of vitamin D with maternal outcomes has not yet been extensively investigated and the previous studies occasionally showed contradictory results. Therefore, the present study attempted to study the relationship between preeclampsia and various serum vitamin D biomarkers including 25(OH)D, VDBP, and bioavailable and free vitamin D.
All the study subjects in this study were patients who visited the Department of
Obstetrics and Gynecology in Gyeongsang National University Hospital, Jinju,
Korea from February 2017 to May 2018. We prospectively enrolled 17 preeclampsia
patients using the diagnostic criteria described in the American College of
Obstetricians and Gynecologists (ACOG) classification [20]. According to the
criteria, the preeclampsia group included women with the new onset of
hypertension after 20 weeks of gestation with proteinuria; thrombocytopenia;
elevated creatinine levels; elevated liver enzyme levels; cerebral symptoms such
as headache, visual disturbances, and convulsions; or pulmonary edema. The normal
pregnancy group included 38 pregnant women who visited the outpatient clinic
without any obstetrical complications. At the time of study enrollment, whole
blood and serum samples were collected from pregnant women with preeclampsia and
normal pregnant women during the prenatal period and stored at –80
The measurement of serum total 25(OH)D concentrations were performed by the Elecsys Vitamin D Total Kit with the Cobas e602 module (Roche Diagnostics, Mannheim, Germany). The assay principle of the Elecsys Vitamin D Total Kit is electrochemiluminescent method using ruthenium-labeled VDBP, biotin-labeled vitamin D, and streptavidin-coated microparticles. Serum VDBP concentrations were measured using ELISA methods with the Human Vitamin D BP Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA). All the measurements and analysis were perfomred according to the manufacturer’s protocol.
Bioavailable and free 25(OH)D concentrations were calculated using the equations from previous studies. Serum 25(OH)D, VDBP, albumin concentrations, and the results of GC genotyping were included as variables in the calculation equations [13, 21].
Real-time polymerase chain reaction (PCR) SNP analysis was employed for GC genotyping after DNA was isolated from peripheral blood leukocytes using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). The TaqMan SNP Genotyping Assay (Thermo Fisher Scientific, Waltham, MA, USA) and the ABI ViiA 7 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) were utilized for SNP assay.
Statistical analyses were conducted using R software version ‘4.0.3’ (R Core Team. R Foundation for Statistical Computing, Vienna, Austria, 2020). In the quantitative data comparison test, the T-test or ANOVA analysis was performed if the distribution assumption was satisfied. If the distribution assumption was not satisfied, the Kruskal-Wallis test or Mann-Whitney U-test (Wilcoxon rank-sum test) was performed. In the qualitative data comparison test, the Chi-squared test was performed if the distribution assumption was satisfied, and Fisher’s exact test was performed if the distribution assumption was not satisfied.
The following analyses were performed to compare differences between the groups. For quantitative data, ANOVA, T-test, and the Kruskal-Wallis test and Mann-Whitney U-test (Wilcoxon rank-sum test) were performed for dissatisfied distribution assumptions. In qualitative data analysis, the Chi-squared test was performed when the distribution assumption was satisfied, and Fisher’s exact test was used when the distribution assumption was dissatisfied.
The general gestational characteristics and laboratory results of the patients enrolled in this study are shown in Table 1. Among the general gestational characteristics, there were no significant differences between the preeclampsia and normal pregnancy groups in age, height, parity, gestational weight gain, and gestational age at delivery. However, body weight and BMI were higher in the preeclampsia group than in the normal pregnancy group (p = 0.048 and p = 0.005, respectively).
| Variables | Preeclampsia | Normal pregnancy | p value |
| (n = 17) | (n = 38) | ||
| Age (years) | 33.2 |
32.9 |
0.828 |
| Height (cm) | 159.4 |
160.9 |
0.353 |
| Body weight (kg) |
65.5 |
56.8 |
0.048 |
| BMI (kg/m |
25.6 |
21.9 |
0.005 |
| Parity | 0.6 |
0.8 |
0.456 |
| Gestational weight gain (kg, at delivery) | 13.0 |
13.4 |
0.805 |
| Gestational age at delivery (weeks) | 35.5 |
36.7 |
0.064 |
| Hemoglobin | 12.7 |
12.0 |
0.063 |
| Platelets | 216.5 |
234.8 |
0.383 |
| AST | 36.0 |
17.2 |
0.033 |
| ALT | 35.8 |
12.1 |
0.232 |
| Creatinine | 0.6 |
0.5 |
0.022 |
| Uric acid | 6.3 |
4.2 |
|
| LDH | 258.1 |
184.7 |
0.003 |
| Albumin | 3.2 |
3.7 |
|
| Cholesterol | 251.6 |
248.4 |
0.733 |
| Calcium | 8.6 |
9.1 |
0.040 |
| Phosphate | 3.9 |
3.7 |
0.317 |
| Urine protein | 2.6 |
0.2 |
|
Values are presented as the mean Abbreviations: BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase. | |||
In the laboratory findings, the aspartate aminotransferase (AST), creatinine,
uric acid, lactate dehydrogenase (LDH), urine protein levels were significantly
higher in the preeclampsia group than in the normal pregnancy group (p =
0.033, p
When the serum concentrations of various vitamin D biomarkers were compared between the two groups, VDBP levels were significantly increased in the preeclampsia group compared to the normal pregnancy group (p = 0.036) (Table 2). The concentrations of other biomarkers such as total 25(OH)D, bioavailable 25(OH)D, and free 25(OH)D tended to be lower in the preeclampsia group than in the normal pregnancy group, but there was no statistical significance. In addition, no statistical significance was observed between the two groups in the evaluation of vitamin D status by total 25(OH)D concentrations.
| Serum vitamin D biomarkers | Preeclampsia | Normal pregnancy | p value | |
| (n = 17) | (n = 38) | |||
| Total 25(OH)D (ng/mL) | 16.8 |
22.2 |
0.138 | |
| Vitamin D status based on total 25(OH)D level |
0.345 | |||
| - deficiency ( |
13 (76.5%) | 21 (55.3%) | ||
| - insufficiency (20 |
3 (17.6%) | 10 (26.3%) | ||
| - sufficiency ( |
1 (5.9%) | 7 (18.4%) | ||
| VDBP (ng/mL) | 454.2 |
403.4 |
0.036 | |
| Bioavailable 25(OH)D (ng/mL) | 1.3 |
1.6 |
0.610 | |
| Free 25(OH)D (pg/mL) | 4.4 |
4.8 |
0.757 | |
Values are presented as the mean Abbreviations: VDBP; vitamin D-binding protein. | ||||
The preeclampsia patient group was divided into two subgroups based on the onset of symptoms and signs. The symptoms and signs that developed at less than 34 weeks of gestational age were classified as early-onset preeclampsia, and those that developed at 34 weeks or more of gestational age were classified as late-onset preeclampsia. While late-onset preeclampsia showed a tendency toward lower total 25(OH)D concentrations than early-onset, VDBP, bioavailable 25(OH)D, and free 25(OH)D concentrations tended to be higher in the late-onset group than in the early-onset group. However, the differences were not statistically significant (Table 3).
| Serum vitamin D biomarkers | Early onset preeclampsia | Late onset preeclampsia | p value | |
| (n = 12) | (n = 5) | |||
| Total 25(OH)D (ng/mL) | 18.6 |
12.4 |
0.161 | |
| Vitamin D status based on total 25(OH)D level |
0.653 | |||
| - deficiency ( |
8 (66.7%) | 5 (100.0%) | ||
| - insufficiency (20 |
3 (25.0%) | 0 (0.0%) | ||
| - sufficiency ( |
1 (8.3%) | 0 (0.0%) | ||
| VDBP | 449.3 |
466.2 |
0.649 | |
| Bioavailable 25(OH)D (ng/mL) | 1.2 |
1.6 |
0.187 | |
| Free 25(OH)D (pg/mL) | 3.9 |
5.5 |
0.160 | |
Values are presented as the mean Abbreviations: VDBP, vitamin D-binding protein. | ||||
The preeclampsia patient group was divided into three subgroups based on the
time interval from preeclampsia diagnosis to delivery: 2 days before (
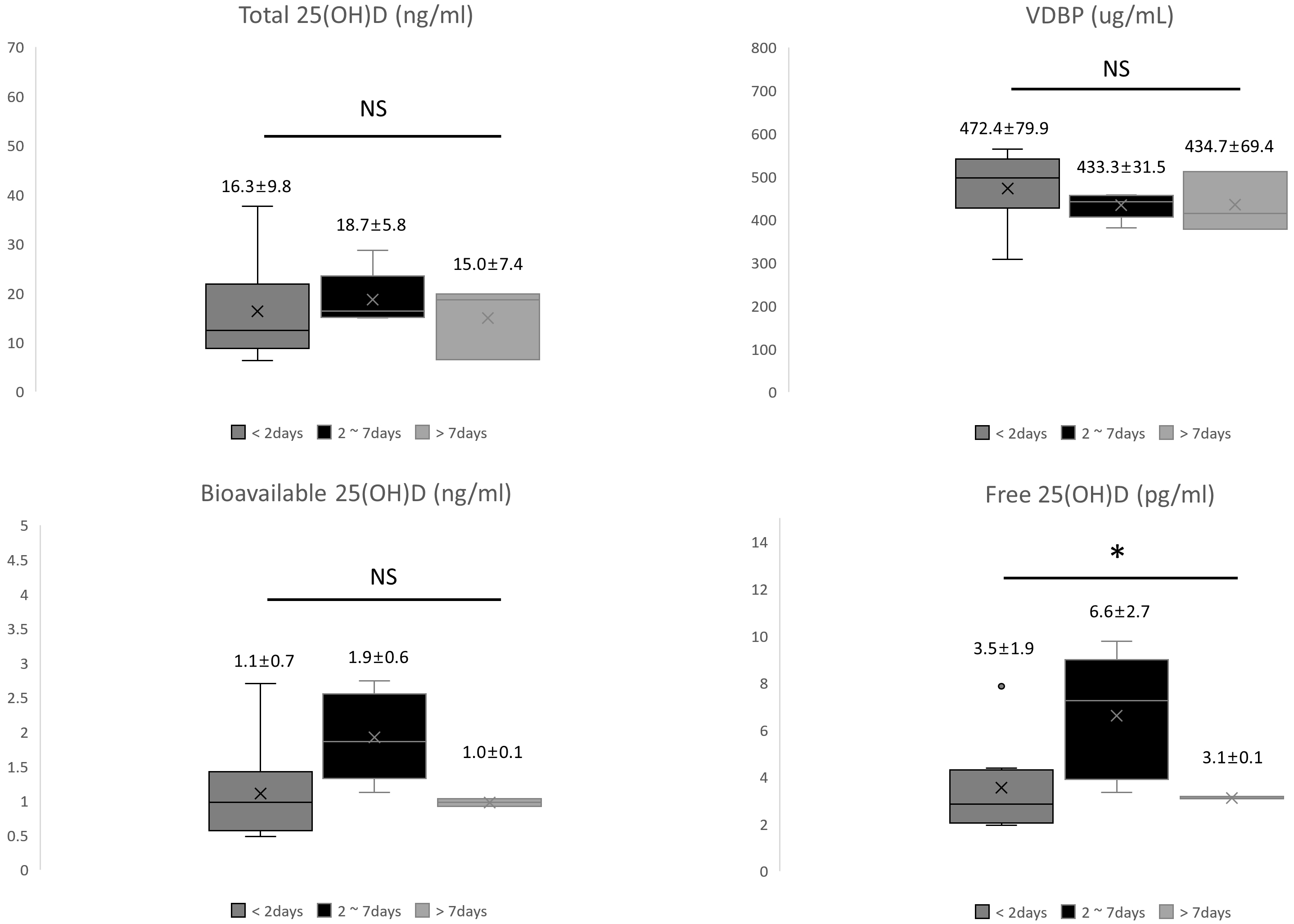 Fig. 1.
Fig. 1.Comparison of vitamin D biomarkers by the time interval from preeclampsia diagnosis to delivery. Abbreviations: VDBP, vitamin D-binding protein; NS, statistically not significant. *p = 0.032.
The genotype frequencies of GC in patients with preeclampsia and normal pregnancies are summarized in Fig. 2A,B. In the preeclampsia group, Gc1s-1f (29.0%), Gc2-1f (24.0%), and Gc1f-1f (23%) were detected in the order of the genotype with the highest frequency. In the normal pregnancy group, the most frequent genotype was Gc2-1f (29.0%), followed by Gc2-1s (21%). Three were no statistical differences in the genotype frequencies of GC between the preeclampsia and normal pregnancy groups (p = 0.397).
 Fig. 2.
Fig. 2.Genotype and Allele Frequencies of the GC gene. Analysis of GC genotype frequency in pregnant women with preeclampsia (A) and normal pregnancies (B). Analysis of GC allele frequency in pregnant women with preeclampsia (C) and normal pregnancies (D). Values are presented as numbers (%).
Among the three VDBP alleles, the most frequent allele in the preeclampsia group was Gc1f (50.0%), followed by Gc2 (26.0%), and Gc1s (24.0%). In the normal pregnancy group, Gc1f (36.0%), Gc1s (34.0%), and Gc2 (30.0%) were detected in the order of the allele with the highest frequency in Fig. 2C,D. The allele frequency was not significantly different between the two groups (p = 0.622).
Preeclampsia has serious consequences for both the mother and fetus, but there is still no effective way to predict and prevent it. Placental growth factor and a soluble fms-like tyrosine kinase 1 (sFlt-1)/placental growth factor (PlGF) ratio have been applied as major biomarkers for prediction of preeclampsia and indicating a poor prognosis for preeclampsia, but clinical usefulness is limited due to differences between several studies [22]. Several previous studies reported that vitamin D deficiency was related to the onset of preeclampsia. [2, 6]. Vitamin D deficiency has been associated with an increased incidence of pregnancy complications [23]. A recent meta-analysis demonstrated an increased risk of preeclampsia in women with hypovitaminosis D [24]. It has been reported that the relationship between vitamin D deficiency and adverse maternal outcomes might be due to the absence of vitamin D immunosuppressive function [4]. A recent meta-analysis study from 27 randomized controlled trials (RCTs) concluded that vitamin D supplementation during pregnancy may help prevent preeclampsia [25]. Therefore, it can be considered that vitamin D could be related to the development of preeclampsia. However, the results of vitamin D studies are not consistent. The reason can be found in the difference in the vitamin D measurement methods as some researchers measured only total 25(OH)D levels. Therefore, in the present study, various serum biomarkers including total 25(OH)D, VDBP, and bioavailable and free 25(OH)D levels were investigated in patients with preeclampsia to elucidate the association of vitamin D and preeclampsia.
In the present study, we observed that among various vitamin D biomarkers, serum VDBP concentrations were significantly increased in the preeclampsia patient group compared to the normal pregnancy group through a patient-controlled study. In addition, we classified preeclampsia patients according to the clinically important onset time and time interval from diagnosis to delivery and also compared and analyzed the concentrations of various vitamin D biomarkers in those classified subgroups.
As a result of the general gestational characteristics and laboratory findings of the subjects enrolled in this study, the preeclampsia group showed a higher body weight and BMI before pregnancy compared to the normal pregnancy group. Based on these findings, it can be inferred that high weight and BMI may play a role as a risk factor for the development of preeclampsia. In preeclampsia, water retention in the body occurs, resulting in decreased albumin and increased serum creatinine and uric acid levels. These general characteristics and laboratory findings mean that the subjects of this study were appropriately recruited to the preeclampsia patient group and the normal pregnancy control group.
Due to the high estrogen status of pregnant females, serum VDBP levels are known to be higher than in non-pregnant conditions [15, 16, 26]. Therefore, pregnant women are expected to have different vitamin D metabolism than non-pregnant women. VDBP is mainly known to act as a vitamin D transporter and play an important role in the metabolism and role of vitamin D. It is also known to play various other physiologically important roles such as extracellular actin scavenging and immune modulation. Unlike previous studies, in our study, no association was found with obvious hypovitaminosis D in the preeclampsia patient group compared to the normal pregnancy group. Hypovitaminosis D was observed not only in the total 25(OH)D concentrations, but also in the bioavailable and free 25(OH)D not bound to VDBP. In our study, higher serum VDBP concentrations were observed in the preeclampsia group than in the normal pregnancy group. The high concentration of VDBP alone in the absence of differences in other vitamin D biomarker concentrations may suggest that VDBP was associated with preeclampsia in relation to other functions of VDBP as well as that of a vitamin D transporter. In fact, it was suggested that VDBP may be implicated in the pathogenesis of preeclampsia via its actin scavenging role and the scavenging of extracellular actin released by damaged cells [27, 28]. A previous study of 126 pregnancies, including 100 normal patients and 26 with preeclampsia showed that the concentration of extracellular actin was increased in preeclampsia and the concentration of the extracellular actin-VDBP complex was also high [27]. Since the ELISA kit we used to measure the serum VDBP concentration in the present study measures total VDBP, it is thought that the extracellular actin-VDBP complex was actually measured as well.
In our study, we analyzed vitamin D biomarkers that would have divided the preeclampsia patient group into three subgroups based on the time interval from diagnosis to delivery. Although there was no statistical significance, tendencies toward decreased VDBP levels and increased bioavailable 25(OH)D levels were observed while the total 25(OH)D remained unchanged in the 2–7 days subgroup (Fig. 1). Furthermore, in the 2–7 days subgroup, free 25(OH)D was significantly increased (p = 0.032). These findings may suggest that the increase in bioavailable and free 25(OH)D in patients with preeclampsia was related to the changes in VDBP concentrations. And the lack of statistically significant differences in VDBP and bioavailable 25(OH)D, with significance only in free 25(OH)D levels, was probably because the number of preeclampsia patients enrolled in our study was insufficient. Therefore, taking these results together, it can be inferred that the increase in VDBP levels in preeclampsia was related to changes in vitamin D metabolism as a vitamin D transporter function and partially related to other VDBP functions such as extracellular actin scavenging and immune modulation. It may not be possible to draw a clear conclusion based on the results of our study alone, and more follow-up studies are necessary to prove this possibility.
Vitamin D concentrations are known to correlate with several parameters, including local latitude. Previous research has shown that people living in high latitudes have lower vitamin D levels due to reduced vitamin D effective UV irradiation [29]. Even in the same region, the synthesis and metabolism of vitamin D vary by race, and it is known that blacks have lower blood levels of vitamin D than whites. This difference in vitamin D metabolism according to race is presumed to be related to the genetic polymorphism in GC gene encoding VDBP [21]. However, there are no studies on the comparison of serum vitamin D concentrations in mothers of various races.
Polymorphisms in GC gene encoding VDBP are known to affect vitamin D
activity. Two well-known SNPs, rs7041 (c.1296T
There were several limitations to this study that may affect the interferences derived from the data. First, there was a limit to finding statistical meaning because the number of enrolled preeclampsia patients and normal pregnant women included in the test was small. Second, this was a cross-sectional study. Blood sampling of the enrolled study subjects was performed only once. Thus, follow-up observations were not performed. Third, vitamin D-related environmental factors such as food intake, the outdoor activity period, and vitamin D supplement intake, were not investigated in study participants. Despite these limitations, the strengths of our research were that it assessed not only total 25(OH) D concentrations but also various forms of vitamin D biomarkers to elucidate the association between vitamin D and preeclampsia.
Since the number of subjects enrolled in our study is small, a large-scale study with sufficient number of subjects is necessary to more clearly elucidate the relationship between preeclampsia and serum vitamin D markers. Therefore, we plan to pursue a large-scale multicenter study based on the results of this study. VDBP, a vitamin D biomarker associated with preeclampsia proposed in the present study, is known as a multipotent protein. In addition to the mechanism of regulating the bioavailability of vitamin D by binding to vitamin D metabolites, it has various functions such as immune modulation and actin-scavenging. However, it is not known exactly how VDBP is involved in the pathophysiology of preeclampsia. Thus, additional in vitro experiments would be needed to further elucidate the role of VDBP and its relationship to preeclampsia.
Serum VDBP levels were significantly higher in patients with preeclampsia than in those with normal pregnancies in the present study. However, other vitamin D biomarkers including total, bioavailable, and free 25(OH)D concentrations and the genotype and allele frequency of VDBP did not differ. Thus, we suggest that among various serum vitamin D biomarkers, increased VDBP could be associated with the onset and pathogenesis of preeclampsia.
Among the authors in the list, WJC and MCC designed the research. IAC, JYJ, HCJ, JEP, JCB, and JKS performed the research and wrote the manuscript. SCK performed statistical analysis. WJC and MCC improved the draft. And all authors read and approved the final manuscript.
The study was approved by the Institutional Review Board of the Gyeongsang National University Hospital (IRB No. GNUH 2019-05-005). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Not applicable.
MCC was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A3067635). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
