Background: Uterine fibroids are the most common benign gynaecological
tumours in women. When symptomatic, heavy menstrual bleeding is the principal
manifestation of uterine fibroids. Nowadays, several conservative procedures have
been utilized to discourage hysterectomy or myomectomy and great interest has
been focused on laser energy technology associated with mini-invasive approaches.
Diode lasers demonstrated many advantages in laparoscopic and hysteroscopic
surgery. Methods: To evaluate the Dual Wavelength Diode Laser System
(DWLS) ablation techniques for treating fibroids, we devised an experimental
system for characterizing the myolysis fibre ablation performance with a
particular fibre designed to create a thermal ablation in the centre of the
myoma. All the myomas were used for the experiment after a total hysterectomy and
their excision from the fresh uteri. The experiment setup was composed of two
beaker containers filled with isotonic saline water, temperature monitoring myoma
surface and water bath, a diode laser fibre and a diode laser energy deposition
system. Results: Good macroscopic results have been found for fibroids
of about 60 cm
Uterine fibroids (myomas, leiomyomas or leiomyomatas) are the most common benign gynaecological tumours in women, predominantly affecting women of late fertile age. Approximately 60–70% of women experience this disease at least once in their lifetime [1]. Common risk factors are reproductive age (premenopausal) and ethnicity, as black women, are more likely to develop fibroids in their lives [2].
Most women with fibroids are asymptomatic. Symptoms arise in relation to the size and number or location of the fibroids, and frequent symptoms include abnormal uterine bleeding with anaemia, dysmenorrhea, pelvic pain and/or discomfort (linked to the bulky myoma effect), such as urinary symptoms, fatigue, and constipation, which significantly deteriorates the quality of life for the patients [2, 3]. However, heavy menstrual bleeding (HMB) is the principal manifestation of uterine fibroids and it is typically related to a submucosal location or an impaired endometrial function [3, 4]. Like the uterine septum, uterine fibroids can also impair reproductive function by generating implantation defects (submucosal or large intramural fibroids) and/or altering the uterine cavity lining [5, 6, 7]. Many attempts have been made to avoid a negative impact on pregnancy outcomes and to restore fertility after a myomectomy [8, 9].
Symptoms, age, desire to maintain fertility and patient choice are the key factors in personalizing a patient’s treatment when suffering from uterine fibroids [10].
Hysteroscopic removal is the most effective treatment and is the standard minimally invasive surgical procedure for treating submucous fibroids [11, 12, 13]. Hysteroscopic myomectomy for G2 myomas is a complex procedure that should only be performed by surgeons experienced in hysteroscopy. This procedure is associated with a significant risk of complications proportional to the myoma’s degree of intramural development [14, 15].
During the past three decades, various methods were developed in a mini-invasive perspective to coagulate the fibroids instead of surgically removing them. This approach is known as “myolysis” and was first performed in 1987 by Mergui in France. He used a Nd:Yag Laser to perforate the fibroid pseudo capsule, burn/coagulate the tissue, destroy its blood supply, and provoke its shrinkage in laparoscopy [16]. In the early 1990s, myoma coagulation was performed using heat either by laser technology or a bipolar radiofrequency probe (120 Watts) in a process called thermomyolysis [17, 18]. Due to the difficulty of predicting the extent of local heat distribution, this technique had a limited diffusion, and later studies reported cases of uterine rupture during pregnancy after myoma thermomyolysis [19].
In 2004, magnetic resonance-guided Focused Ultrasound Surgery (MRgFUS) was
approved to treat uterine fibroids, liver, breast, pancreatic cancers and bone
metastasis [20]. MRgFUS is performed under magnetic resonance imaging (MRI)
guidance with a contrast medium and a transabdominal probe that focuses on 1.5
MHz frequency ultrasound (US) emissions on the target area (of about 0.5
cm
Other promising techniques include microwave and radiofrequency myolysis. Microwaves, originally developed for liver tumour ablation, are a novel technology used to treat submucosal myomas. Recently, Tsuda et al. [22] performed this type of myolysis in women with menorrhagia caused by submucosal myomas. The shrinkage reached about 70% of the myoma’s initial volume, in a safe and effective procedure.
Radiofrequency myolysis is another uterine-conserving technique. Radiofrequencies produce heat by high-frequency oscillations alternating within an electric field, causing coagulation and necrosis of the target area and inactivation of hormonal receptors on the myoma, which prevents regrowth of the fibroid tissue [23].
Recently, great interest has been focused on laser energy technology associated with mini-invasive approaches. Among laser energy technologies, currently, diode lasers demonstrated many advantages in laparoscopic [24, 25, 26] and hysteroscopic surgery (endometrial polyps, myomas and metroplasty) [27, 28, 29, 30, 31, 32, 33]. Lately, a new type of laser is available, which generates two wavelengths (980 + 1470 nm) from a diode semiconductor Dual Wavelength Diode Laser System (DWLS). The two wavelengths provide contemporary absorption in water (1470 nm) and haemoglobin (980 nm), with high performances in cutting, vaporizing and haemostasis [33].
Haimovich demonstrated how myolysis by laser-induced interstitial thermotherapy (LITT) with the Leonardo® diode laser DWLS (Biolitec®, Jena, Germany) using a laser fibre working through the working canal of the Bettocchi hysteroscope (Storz®, Tuttlingen, Germany) could be effective [32].
As the types of fibre and the safest device (Leonardo®, Biolitec®, Jena, Germany, DUAL 45) settings for performing effective but uncomplicate myolysis are not known, we performed ex vivo tests on uterine fibroids obtained from women undergoing hysterectomy. The Leonardo® DUAL 45 is a device that allows mixing the two wavelengths: 1470 nm (mainly used for cutting) with a power from 0 to 15 Watt (W) and 980 nm (mainly used for coagulation) with a power from 0 to 30 W up to a resulting maximum of 45 W obtained by mixing the two wavelengths. The time of using this type of laser with a specific power causes certain the energy to be measured in Joules (J) by the device.
To evaluate the diode laser DWLS ablation techniques for treating fibroids and aid in future treatment planning, we devised an experimental system for characterizing the myolysis fibre ablation performance with a particular fibre (Myolysis®, Biolitec®, Jena, Germany) (Fig. 1A,B), which has the characteristic of spreading the energy radially and not only in the tip that is explicitly designed to create a thermal ablation in the centre of the myoma.
 Fig. 1.
Fig. 1.Macroscopic and histopathological examination of the myoma after ex vivo myolysis. (A) Myolysis diode laser fibre. (B) Myolys diode laser fibre activated with the characteristic of spreading the energy radially and not only in the tip. (C) The experiment setup with the fibre mode off. (D) The experiment setup with the fibre mode on. (E) Myoma lowered into the saline water bath of the beaker container to evaluate its volume before starting the experiment. (F) Myoma lowered into the saline water bath of the beaker container to evaluate its volume after the ablation procedure.
The main goals of the study were:
(1) To ensure the treatment’s safety by keeping the high temperature in the external surface of the fibroids tissue.
(2) To estimate the efficacy of the treatment in terms of the percentage of volume ablation inside the myoma.
Women who underwent total laparotomic abdominal hysterectomy due to multiple uterine fibroids with diameters ranging from 1 to 8 cm of the maximum diameter at the Division of Obstetrics and Gynecology, Department of Surgical Sciences, University of Cagliari, were enrolled in this study and permitted post-operative testing of their uterine specimens. All the myomas were used for the experiment after a total hysterectomy and their excision from the fresh uteri. A total of three patients participated in the study, and measurements were obtained from 12 unique fibroids.
Written content was obtained for the informed consent of the participants. The institutional review board of the Azienda Ospedaliero Universitaria (AOU) of Cagliari approved the study, code Prot. PG/2020/10910 and the study was registered on ClinicalTrials.gov PRS code NCT04748978 as a preliminary part of the in vivo study.
Immediately following surgical removal of the uterus, each specimen was
transferred from the operating room to the laboratory centre (
The overall system consisted of:
(1) a diode laser energy deposition system (Leonardo®, Biolitec® DUAL 45),
(2) a diode laser fibre (Myolysis®, Biolitec®),
(3) temperature monitoring myoma surface and water bath with a digital thermocouple thermometer,
(4) tenaculum forceps as a holder template,
(5) two beaker containers filled with isotonic saline water maintained at 37
For mimicking the physiologic conditions, the diode laser fibre and the samples
were placed inside a water bath containing 0.9% isotonic saline water. The bath
was maintained at human body temperature (37
The sensor position of the thermocouple thermometer from the fibroid surface and
saline bath were logged before starting the experiment. Before starting the
ablation procedure, each myoma was lowered into the saline water bath of the
first beaker container to evaluate its volume (V), measuring the
The myolysis was performed as follows: a diode laser fibre was inserted parallel
(with 90
| Experimental set | Diameters of the fibroid (cm) | Fibroid Volume (cm |
Fibroid Volume (cm |
Volume of the fibroid ablation (%) | Energy (J) reached when the experiment was stopped | |
| Set 1 | Sample 1 | 4.1 |
114 | 86 | 24.56% | 8500 |
| Sample 2 | 3.2 |
56 | 45 | 16.07% | 5500 | |
| Sample 3 | 2.2 |
16 | 15 | 6.25% | 800 | |
| Set 2 | Sample 1 | 4.3 |
126 | 95 | 24.6% | 9000 |
| Sample 2 | 3.2 |
60 | 49 | 18.33% | 5000 | |
| Sample 3 | 2.1 |
17 | 16 | 5.88% | 900 | |
| Set 3 | Sample 1 | 4.4 |
122 | 85 | 30.32% | 10000 |
| Sample 2 | 3.1 |
60 | 32 | 46.6% | 5000 | |
| Sample 3 | 2.0 |
13 | 10 | 23.07% | 1500 | |
| Set 4 | Sample 1 | 3.8 |
111 | 82 | 26.12% | 12000 |
| Sample 2 | 2.8 |
51 | 33 | 35.29% | 5000 | |
| Sample 3 | 1.8 |
12 | 7 | 41.6% | 1000 | |
In contrast to other applications of thermal ablation, such as tumour ablation,
the maximum efficiency of ablation in fibroids would be limited by an additional
safety constraint, i.e., precluding collateral thermal damage in adjacent
tissues. The clinical objective in thermal ablation techniques is to expose the
fibroid tissue to ablative temperatures (
After the ablation procedure, each myoma was again inserted in the first beaker
container to evaluate the
Following the thermal property measurements, the specimens were delivered to the pathology lab for standard post-surgical processing and analysis. Macroscopic and Hematoxylin and Eosin (H&E) stained sections were reviewed by the pathologists (DF and GF) after the original histopathologic diagnoses were verified.
Table 1 shows the fibroid volume and energy achieved for each sample when the experiment was stopped.
Set 1 was set out with the maximum power of the device (30 W of 980 nm + 15 W of
1470 nm). Set 2 was set out with 15 W of 980 nm + 15 W 1470 nm. Set 3 was set out
with 10 W of 980 nm + 10 W of 1470 nm. Set 4 was set out with 5 W of 980 nm + 5 W
of 1470 nm. Each of the four sets was used on three fibroids samples ranging from
12 to 126 cm
Based on the results shown in Table 1, the ablation coverage of fibroids was
less than 100%. The maximum ablation coverage achieved with the set 3 on a myoma
of 60 cm
For sets 1 and 2, the powers are too high for these volumes of myomas, as the
experiment was stopped after a few minutes due to the reaching of the threshold
temperature on the surface or even due to the presence of a hole in the opposite
part of the entrance of the laser fibre. When the device was set up with such
high powers (sets 1 and 2) and used on myomas with a volume not exceeding 120
cm
Fig. 2 shows the macroscopic (Fig. 2A) and histopathological examination (Fig. 2B,C). In particular, Fig. 2A shows the macroscopic effect of the set 3 sample 2 ablation with the vaporization of the core of the myoma. The shrinkage of the myoma was not reported; however, we observed that the core of the fibroid was vaporized by reducing its volume by almost 50% and keeping its external surfaces intact.
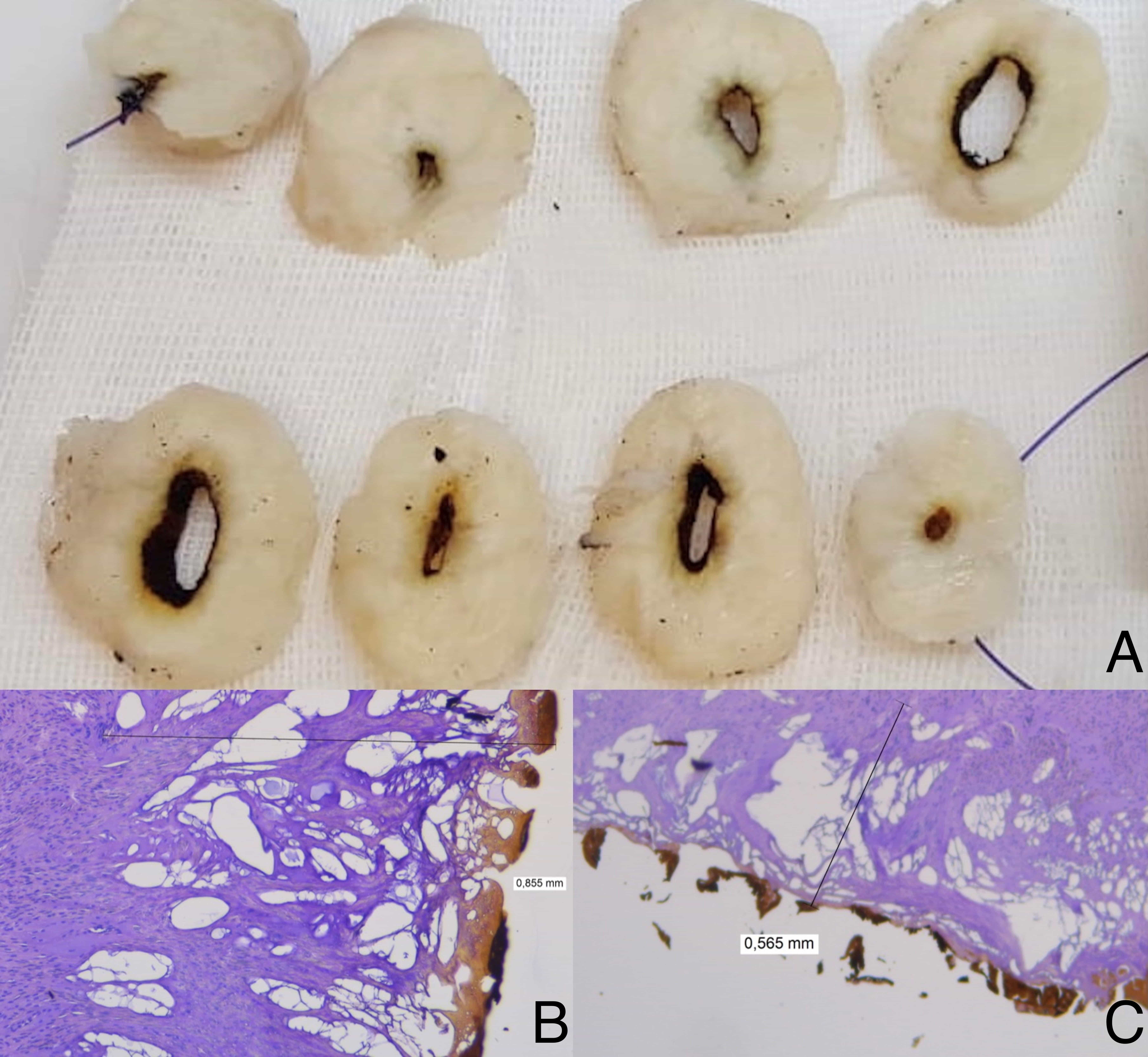 Fig. 2.
Fig. 2.Macroscopic and histopathological examination of the myoma after ex vivo myolysis. (A) The leiomyoma was freshly cut by creating slices proceeding from the fibre entry point to the opposite diametral point. (B) Histological picture of the burn feature in smooth muscle cells from a uterine leiomyoma. The cell nuclei disappeared, the cell shapes changed from fascicles to irregular tapes due to many vacuum artifacts, and the vessels look obliterated. The bar measures a length of 0.855 mm from where the tissue shows again its usual morphology (hematoxylin eosin stain). (C) Low power field of a leiomyoma showing the characteristic scorching artifacts from the surgical procedure. Huge and many irregular vacuums can be seen for the length of 0.565 mm measured by the black bar (hematoxylin eosin stain).
Women are constantly in need of minimally invasive solutions for the therapy of symptomatic fibroids. Laparoscopic or laparotomic hysterectomy, laparoscopic or laparotomic myomectomy, and hysteroscopic myomectomy are the standard procedures, many of which may trigger physical and mental distress [10]. The standard method of hysteroscopic myomectomy includes the use of a 10-mm surgical hysteroscope, which necessarily involves cervical dilatation, anaesthesia, and hospitalization. The treatment requires the myoma slicing with the cutting loop on the fibroid surface [14].
Myomas greater than 3 cm and/or positioned in the fundus or lateral walls have a higher risk of surgical failure than smaller fibroids as well as those placed in the anterior or posterior walls. Hysteroscopic surgical procedures can remove the myoma completely only in cases where the fibroid is mainly within the uterine cavity (G0 and G1) and focuses on removing tiny fibroid tissue fragments [36]. Therefore, in recent years, less invasive approaches have caught the interest of researchers. Several conservative procedures have been utilized to discourage hysterectomy or myomectomy, such as radiofrequency and MRgFUS or microwaves myolysis [20, 21, 22, 23].
The safety and feasibility of radiofrequency treatments in uterine fibroids have been reported in numerous studies [23, 37, 38]. This technique requires a probe insertion into the myoma by using ultrasonic real-time imaging to direct the procedure. Radiofrequency treatments were shown to reduce the volume of myomas by more than half [37]. Patients tolerate this form of myolysis well, and it has a variety of benefits, including no post-ischemic pain and a fast recovery [37]. However, there are some disadvantages in using radiofrequency effects, such as the complexity in providing a histopathological diagnosis of the tissue, performing transvaginal sonography, and the need for follow-up analyses, such as the inspection of the intrauterine tissue after treatment [38].
MRgFUS have the advantage of being able to identify the precise position of uterine myomas. The literature shows that fibroids react to MRgFUS treatment in a variety of ways. The amount of sonication power required for ablation varies significantly between patients and even within fibroids. Fibroids with a brighter T2-weighted signal strength (T2wSI) are more complicated to ablate, resulting in lower nonperfused volume ratios immediately after treatment and worse long-term outcomes. Increased vascularization, fluid-rich tissues, high cellularity, and/or degeneration have all been related to high T2wSI in fibroids [39, 40].
Microwaves, which were originally designed for liver tumour ablation, are a
novel treatment for submucosal myomas. One of the disadvantages of using
microwaves occurs when the myoma is localized in the sidewalls, and the
applicator’s angle of use is not 90
To overcome the limitations of the techniques described above, we thought it could be advantageous to use a DWLS diode laser working through the working canal of the Bettocchi hysteroscope (Storz®) to perform the myolysis allowing for the direct visualization and localization of the fibroid as well as the possibility of having a histological examination and performing the procedure in an office setting without anaesthesia. In fact, as we showed in previous studies, pain is minimized thanks to the progress in technical instruments (size and type of device, distension media) and with increased gynaecologist experience [41, 42, 43, 44], in addition to pre-operative hormonal therapies (gonadotropin-releasing hormone analogues or progestins), which provide better conditions during the operating session, due to the thinning of the endometrial thickness and volumetric reduction of the myoma [45].
The diode laser previously demonstrated goods results in hysteroscopic polypectomy and metroplasty demonstrating extreme precision of cutting, precise and constant control of tissue vaporization in the complete absence of bleeding, controlled power of penetration/deepening, a high capacity of hemostasis, the absence of electrical interferences, high safety and good compliance by the patients [27, 28]. Due to the physical characteristics of the two wavelengths, using the diode laser to perform myolysis could be revolutionary: the 1470 nm vaporizes tissues in the close surroundings, while the 980 nm, which achieves high absorption for haemoglobin, coagulates blood vessels over a larger area providing a high capacity for ablation [32]. Theoretically, from a scientific point of view, the wavelength of 1470 nm used for a long time inside the myoma could be enough to cause the tissue’s vaporization. Following the already known in vivo experiment of Hamovich, our aim will be to achieve HMB improvement of the treated patients and not to make the myoma disappear [32]. Therefore, the 980 nm, thanks to its affinity for haemoglobin, could be fundamental to reduce the vascularization of the myoma even if it can lead to a rapid increase in heat on the treated surface. Hence, even if the myoma has been not fully vaporized, the vascularity could be impaired.
This experimental study represents only an ex vivo and preliminary test for the in vivo study we plan to carry out in which the most important outcome will be the resolution of the symptoms (in particular HMB) for our patients who have no desire for pregnancy. In fact, while some authors support pregnancy after myolysis, other studies mentioning uterine rupture cases during pregnancy highly recommend that myolysis is only approved for women who do not desire future pregnancy [18, 19]. Another factor to be considered for the in vivo study, will be the possible damage that this technique may have on the endometrium’s basal layer, where the stem cells are most represented. Adult stem cells have been found in both human and mouse endometrium, indicating that epithelial progenitor cells and mesenchymal stem/stromal cells play essential roles in the cyclical regeneration of endometrial epithelium and stroma. Heat-induced inflammation may also inhibit the regeneration of traumatized endometrium by injuring stem/progenitor cells or causing their loss through the production of inflammatory mediators and fibrotic tissue formation [46].
Good macroscopic results have been found for fibroids of about 3 cm
(~60 cm
There were certain limitations to this study: (1) the anatomical heterogeneity
among and within uterine fibroids, as revealed by differences in total collagen,
collagen types, water component, gross appearance, and mechanical variations,
makes it very difficult for determinate the exact amount of time/energy that will
work for all the fibroids [47]; (2) blood perfusion effects were not considered;
(3) half of the fibroid is tipically inside the myometrium, and the other half is
in the uterine cavity, but in our study, it was not possible to take into account
the attenuation of the temperature on the myoma surface by the myometrium; and
(4) all of our tests were performed by inserting the fibre at a
90
To our knowledge, this is the first ex vivo study with a new DWLS, which made it possible to understand which one of the settings of the Leonardo®, Biolitec® DUAL 45 could be recommended for the safest and best macroscopic results in myolysis. The possibility of having a direct hysteroscopic visualization of the myoma, of being able to use this technique without narcosis and of having the possibility to stop when the patient feels hot leads us to think that this could be an alternative and safe technique in the execution of hysteroscopic myolysis. Further studies in vivo are necessary, however, to explore the feasibility and reproducibility of the technique.
CEM, Cumulative Equivalent Minutes; DWLS, Dual Wavelenghts System; H&E, Hematoxylin and Eosin; HMB, Heavy Menstrual Bleeding; kHz, kilo Hertz; LITT, Laser-induced Interstitial Thermotherapy; MHz, Mega Hertz; MRgFUS, Magnetic Resonance-guided Focused Ultrasound; MRI, Magnetic Resonance Imaging; Nd:YAG, Neodymium-doped Yttrium Aluminum Garnet; T2wSI, T2-weighted Signal strength; US, Ultrasound.
MND, FrSc, SA, FeSo and LN conceived and designed the experiment; MND and FrSc performed the experiment; DF, GF, and SA participated in coordination of the study; DF and GF analyzed the samples; MND, ASL and MN wrote the paper; Feso, LN, ASL and MN helped in drafting the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Written content was obtained with the informed consent of the participant. The institutional review board of the Azienda Ospedaliero Universitaria of Cagliari approved the study, code Prot. PG/2020/10910 and the study was registered on ClinicalTrials.gov PRS code NCT04748978 as a preliminary part of the in vivo study.
This publication was created as part of a research project financed with the resources of P.O.R. SARDEGNA F.S.E. 2014-2020 - Asse III “Istruzione e Formazione, Obiettivo Tematico: 10, Obiettivo Specifico: 10.5, Azione dell’accordo fi Partenariato: 10.5.12” Avviso di chiamata per il finanziamento di Progetti di ricerca – Anno 2017.
This research received no external funding.
The authors declare no conflict of interest.
