- Academic Editor
†These authors contributed equally.
Coronary artery disease (CAD), caused by coronary artery occlusion, is a common cardiovascular disease worldwide. Long non-coding RNAs (lncRNAs) are implicated in the regulation of endothelial cell injury, angiogenesis, plaque formation, and other pathological mechanisms in CAD by acting on different cell types. Some lncRNAs are significantly upregulated in CAD patients; however, other lncRNAs are significantly downregulated. Differential expression of lncRNAs in CAD patients enables them to be exploited as potential biomarkers to evaluate disease progression and diagnosis/prognosis in CAD patients. In this study, we reviewed the role of lncRNAs in the development of different clinical subtypes of CAD.
Coronary artery disease (CAD) is one of the most deadly diseases worldwide [1]. Clinically, CAD is divided into stable angina pectoris, unstable angina pectoris, and acute myocardial infarction (AMI). AMI is associated with inflammation, fibrosis, and angiogenesis, and can lead to heart failure in severe cases [2]. The typical mechanism of AMI is the formation and progression of intracoronary thrombus, which in turn produces varying degrees of thrombotic stenosis/occlusion, ultimately leading to myocardial necrosis and the formation of circumferential myocardial scarring in the area of coronary artery supplied [3, 4]. Molecular mechanisms such as mitochondrial dysfunction, inflammation, oxidative stress, and excessive fibrosis can lead to adverse cardiac remodeling in the late stage of an AMI, which results in increased morbidity and mortality [5]. Studies have found that several long non-coding RNAs (lncRNAs) are abnormally expressed in biological samples extracted from CAD patients. LncRNAs can regulate pathological mechanisms and disease progression through different target genes or signaling pathways, making them essential biomarkers [6]. LncRNAs, a non-coding RNA larger than 200 nucleotides, play a biological role in CAD by acting on downstream target molecules such as microRNAs (miRNAs, miRs), mRNAs, or transcription factors [7, 8]. Most lncRNAs act as competing endogenous RNAs (ceRNAs) and regulate the expression and activation of downstream mRNAs by competitive binding with miRNAs, thus affecting the cardiovascular system’s biological functions, such as cell proliferation, migration, and apoptosis. Therefore, lncRNAs may have an important regulatory role in the pathophysiology of CAD [9]. The lncRNA-miRNA-mRNA pathway is a classic ceRNA mechanism. Learning more about lncRNAs and their role in CAD will help to develop new treatment and diagnostic methods for CAD patients. In this review, we aim to explore the biological role of lncRNAs in various subtypes of CAD.
The mechanism and function of lncRNAs in CAD have been widely studied in various cell types, most extensively in endothelial cells. Wang et al. [10] demonstrated that lncRNA p21 acts on miR-221 through the ceRNA mechanism, forming the miR-221/SIRT1/Pcsk9 axis. LncRNA p21 overexpression can inhibit endothelial cell apoptosis and promote endothelial cell proliferation, migration, and tube formation, thus reducing subcutaneous lipid deposition to prevent the progression of atherosclerosis (AS). LncRNA has been downregulated in AS patients and AS mouse models. Similarly, compared with healthy subjects, the expression level of lncRNA TONSL-AS1 in the plasma of CAD patients is also downregulated. Forced overexpression of this lncRNA in primary human coronary artery endothelial cells has been shown to promote proliferation and inhibit apoptosis by upregulating B-cell lymphoma-2 (BCL-2) expression levels through the negative regulation of miR-197 in these cells [11]. Furthermore, Kai et al. [12] have reported that lncRNA NORAD (NORAD, non-coding RNA activated by DNA damage) expression levels were significantly upregulated in CAD patients and oxidized low-density lipoprotein (ox-LDL)-treated human umbilical vein endothelial cells (HUVECs). LncRNA NORAD is closely related to the occurrence and development of AS. NORAD recruits HDAC6 by enriching for FUS (FUS RNA binding protein), then HDAC6 binds to the promoter region of the VEGF gene, enhancing the level of H3K9ac deacetylation in this region and thereby inhibiting VEGF gene transcription. Li et al. [13] have shown upregulation of lncRNA uc003pxg.1 and downregulation of miR-25-5p in peripheral blood mononuclear cells from CAD patients. LncRNA uc003pxg.1 has been shown to promote the proliferation and migration of HUVECs by upregulating cyclinD1 and CDK6 via negatively downregulating miR-25-5p in an in vivo study. Interestingly, two different transcripts of lncRNA ANRIL exert diametrically opposing effects on CAD endothelial cells, which highlights the molecular characteristics of lncRNAs and their diverse biological roles [14]. It is unknown whether other CAD-related lncRNAs have multiple transcripts and different functions. Table 1 (Ref. [10, 11, 12, 13, 15, 16, 17, 18, 19, 20, 21, 22]) shows the functional roles of lncRNAs, as assessed in endothelial cells, in the occurrence and development of CAD.
| LncRNA | Sample | Expression pattern | Assessed cell lines | Gene/Protein interactions | Signaling pathway | Function | Ref |
| p21 | Peripheral blood samples from 25 patients with AS and 18 health controls | Downregulated | HAECs | miR-221 | - | Inhibits HAEC proliferation, migration, and tube formation by decreasing miR-221 | [10] |
| TONSL-AS1 | Peripheral blood samples from 60 patients with CAD and 60 health controls | Downregulated | HCAECs | miR-197 | - | Increases migration and suppress apoptosis of HCAECs through regulating miR-197/BCL2 axis | [11] |
| NORAD | Peripheral blood mononuclear cells samples from 15 patients with CAD and 15 health controls | Upregulated | HUVECs | FUS | - | Its knockdown attenuates vascular endothelial injury through increasing VEGF gene transcription via enhancing H3K9 deacetylation by recruiting HDAC6 | [12] |
| uc003pxg.1 | Peripheral blood mononuclear cells samples from 80 patients with CAD and 80 health controls | Upregulated | HUVECs | miR-25-5p | - | Its knockdown attenuates migration and proliferation of human umbilical vascular endothelial cells through increasing miR-25-5p | [13] |
| HIF1A | Peripheral blood samples from 80 ApoE |
Upregulated | HCAECs/ECs | USF1 | - | Its knockdown inhibits the ox-LDL induced atherosclerotic inflammation in ECs and HCAECs by downregulating ATF2 via binding USF1 | [15] |
| KCNC3-3:1 | Peripheral blood mononuclear cells samples from 93 patients with CAD and 48 health controls | Upregulated | HUVEC | - | JAK1/STAT3 signaling pathway | Its knockdown attenuates human umbilical vascular endothelial cell migration by suppressing the JAK1/STAT3 signaling pathway | [16] |
| THRIL | Peripheral blood samples from 20 patients with CAD and 20 health controls | Upregulated | EPCs | FUS | AKT signaling pathway | Its knockdown attenuates apoptosis and autophagy by targeting FUS and activating AKT signaling pathway | [17] |
| NEAT1 | Peripheral blood samples from 40 patients with CAD and 40 health controls | Upregulated | HCAECs | miR-140-3p | - | Promotes apoptosis of HCAEC through regulating NEAT1/miR-140-3p/MAPK1 axis | [18] |
| ANRIL | Patients with CAD between January 2018 and July 2018 and sex-matched health control | Upregulated | HUVECs | let-7b | TGF-βR1/Smad signaling pathway | Its knockdown inhibits inflammation response and regulates endothelial | [19] |
| Dysfunction by inhibiting let‐7 and targeting TGF‐βR1/Smad signaling pathway | |||||||
| AK136714 | Male C57BL/6J mice and C57BL/6J, ApoE |
Upregulated | HUVECs | HuR and FOXO3 | - | Its knockdown inhibits ECs apoptosis and inflammatory responses by binding to FOXO3 | [20] |
| EZR-AS1 | Blood samples from 35 patients (24 men and 11 women; 50–75 years of age) with CHD and 38 individuals without CHD (22 men and 16 women; 50–75 years of age) | Upregulated | - | SMYD3 | - | Downregulation of EZR-AS1 inhibits the proliferation, migration, and apoptosis of HUVECs via SMYD3 | [21] |
| CASC11 | Plasma samples from 82 patients with CAD and 82 healthy controls | Downregulated | HCAECs | - | - | - | [22] |
| Abbreviations: HAECs, human aortic endothelial cells; EPCs, endothelial progenitor cells; HUVECs, human umbilical vein endothelial cells; HCAECs, human coronary artery endothelial cells; ECs, endothelial cells; CHD, coronary heart disease. | |||||||
Kang et al. [23] have reported overexpression of lncRNA AL355711 in atherosclerotic plaques and animal models of AS, and matrix metalloproteinase-3 (MMP3) has been associated with vascular smooth muscle cell (VSMC) migration in cardiovascular disease. Furthermore, knockdown of lncRNA AL355711 has also been demonstrated to inhibit AS progression by regulating VSMC migration through the ABCG1/MMP3 pathway. Upregulation of lncRNA CDKN2B-AS1 has been proven to upregulate PTPN7 through competitive binding with miR-126-5p, attenuating VSMC proliferation and promoting apoptosis. However, this lncRNA showed decreased expression in ox-LDL-induced VSMC models and serum samples from CAD patients. Transfection of pcDNA-CDKN2B-AS1 into ox-LDL-induced VSMCs has been demonstrated to result in the upregulation of lncRNA CDKN2B-AS1, which may play an important biological role; however, these findings have not been studied in vivo [24]. In addition, lncRNA Kcnq1ot1 has been found to stimulate the expression of Tead1 by competitively acting on miR-466k and miR-466i-5p, thus promoting injury and apoptosis of cardiomyocytes. However, this specific mechanism still needs to be clarified in further studies [25].
Knockdown of lncRNA Mirt2 has been shown to exacerbate hypoxia/reoxygenation-induced H9C2 myocardial cell damage and promote the progression of ischemic myocardial infarction in AMI rat models, while there was no significant effect on the normal or sham group. Upon further study of this mechanism, it was found that overexpression of Mirt2 could upregulate PDK1 levels through the negative regulation of miR-764 and inhibit myocardial apoptosis and injury. Thus, a signaling axis of lncRNA Mirt2/miR-764/PDK1 protecting cardiomyocytes was formed [26]. Table 2 (Ref. [15, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34]) lists those CAD-related lncRNAs whose functions have been evaluated in cardiomyocytes or VSMCs. Some studies have shown that vascular aging is a specific risk factor for CAD. VSMCs play an important role in the pathological processes of vascular remodeling and stiffness associated with vascular aging [35, 36]. Therefore, there may be some potential correlations between the effects of lncRNAs on VSMC functions and vascular aging that have yet to be explored.
| LncRNA | Sample | Expression pattern | Assessed cell lines | Gene/Protein interactions | Signaling pathway | Function | Ref |
| HIF1A | Peripheral blood samples from 80 ApoE |
Upregulated | SMCs | USF1 | - | Its knockdown inhibits the ox-LDL-induced atherosclerotic inflammation in SMCs by downregulating ATF2 via binding USF1 | [15] |
| CDKN2B-AS1 | Venous blood samples from 15 patients with CHD and 15 healthy controls | Downregulated | VSMCs | miR-126-5p | PI3K/Akt signaling pathway | Inhibits VSMC proliferation and inflammation and boosts apoptosis through targeting miR-126-5p | [24] |
| Kcnq1ot1 | 200–250 g male SD rats (AMI group included 35 mice and sham group included 35 mice) | Upregulated | H9c2 | miR-466k and miR-466i-5p | - | Triggers cardiomyocyte apoptosis by elevating Tead1 via sponging miR-466k and miR-466i-5p | [25] |
| Mirt2 | 20 adult male SD rats aged 12–14 weeks (AMI group included 10 mice and sham group included 10 mice) | Upregulated | H9c2 | miR-764 | - | Modulates cardiomyocyte injury by regulating the miR-764/PDK1 axis | [26] |
| LINC00261 | 100 male C57B/L6 mice aged 8–10 weeks old and weighed 18–25 g (MI group included 80 mice and the sham group included 20 mice) | Upregulated | H9c2 | miR-522-3p | - | Its knockdown inhibits cardiomyocytes apoptosis by targeting miR-522-3p | [27] |
| MIAT | 24 male C57BL6/J mice (weight 17–25 g) were established as ischemia/reperfusion (I/R) models | Upregulated | HCM | miR-181a-5p | JAK2/STAT3 signaling pathway | Its knockdown inhibits cell apoptosis and inflammation by regulating the JAK2/STAT3 signaling pathway via targeting miR-181a-5p | [28] |
| MIAT | 70 male SPF C57BL/6J mice (MI group included 60 mice and sham group included 10 mice) | Upregulated | HL-1 | miR-10a-5p | - | Promotes cardiomyocyte apoptosis by regulating the miR-10a-5p/EGR2 axis | [29] |
| MALAT1 | 32 male SD mice aged 14 weeks established as acute myocardial infarction (AMI) models | Upregulated | HL-1 | miR-125b-5p | - | Promotes cardiomyocyte apoptosis through regulating miR-125b-5p/NLRC5 axis | [30] |
| SNHG14 | Clean C57BL/6J mice and ApoE |
Downregulated | HA-VSMC | miR-19a-3p | - | Its overexpression promotes proliferation and inhibits apoptosis of VSMCs by regulating the miR-19a-3p/ROR |
[31] |
| NR_045363 | Neonatal ICR/CD1 mice established as myocardial infarction (MI) models | Upregulated | HA-VSMC | - | p53 signaling pathway | Alleviates cardiomyocyte apoptosis by inhibiting the p53 pathway | [32] |
| GAS5 | Mice (MI group included 15 mice and sham group included 15 mice) | Downregulated | H9c2 | miR-21 | PI3K/AKT signaling pathway | Its overexpression promotes cardiomyocyte apoptosis and inhibits cardiomyocyte proliferation by elevating PDCD4 via sponging miR-21 | [33] |
| FOXC2-AS1 | Samples from 35 patients with AS and 35 healthy controls | Upregulated | HVSMC | miR-1253 | - | Promotes proliferation and inhibit apoptosis of VSMCs via targeting the miR-1253/FOXF1 signaling axis | [34] |
| Abbreviations: HVSMCs, human vascular smooth muscle cells; VSMCs, vascular smooth muscle cells; HA-VSMC, human aortic vascular smooth muscle cells; HCM, human cardiomyocytes. | |||||||
Compared with the control group, lncRNA MHRT levels in the border
region of myocardial infarction in AMI mice were significantly increased, and
miR-3185 was proven to be a direct target gene of MHRT
regulating fibrosis. Overexpressed lncRNA MHRT promotes myocardial
fibrosis after myocardial infarction by negatively regulating miR-3185
and increasing TGF-
| LncRNA | Sample | Expression pattern | Assessed cell lines | Gene/protein interactions | Signaling pathway | Function | Ref |
| MHRT | Healthy male C57 BL/6 mice (20–25 g) were established as MI model | Upregulated | CFs | miR-3185 | - | Its knockdown inhibits CFs collagen production and proliferation | [37] |
| ZFAS1 | Male Wistar rats (weighting 240–260 g) were established as MI model | Upregulated | CFs | - | Wnt/ |
Its knockdown increases the proliferation, migration, and invasion of CFs by regulating the Wnt/ |
[38] |
| SNHG7 | 10 male C57BL/6J mice aged 10 weeks (23–25 g) (MI group included 5 mice and sham group included 5 mice) | Upregulated | CFs | miR-455-3p | - | Its knockdown inhibits Ang-II-induced apoptosis, collagen synthesis, and inflammation in CFs by regulating miR-455-3p/PTAFR axis | [39] |
| lncRNA 554 | Male C57BL/6 mice aged 8–10 weeks (20–30 g) were established as MI model | Upregulated | CFs | - | TGF- |
Its knockdown attenuates cardiac fibrosis by regulating the TGF- |
[40] |
| Ang362 | 60 male SD mice weighing 250–300 g (control group included 20 mice, sham-operated group included 20 mice, MI group included 20 mice) | Upregulated | CFs | Smad7 | - | Contributes to cardiac fibrosis post-MI by inhibiting Smad7 expression | [41] |
| MALAT1 | Male C57BL/6 mice aged 12–16 weeks (weighting 21–25 g) were established as MI model | Upregulated | NMCFs | miR-145 | TGF‐ |
Its knockdown attenuates cardiac fibrosis and alleviates AngII‐induced cell proliferation, collagen production, and myofibroblast transdifferentiation by regulating the TGF‐ |
[42] |
| Abbreviations: CFs, cardiac fibroblasts; PTAFR, platelet-activating factor receptor; NMCFs, neonatal mouse cardiac fibroblasts. | |||||||
Changes in circulating lncRNA expression levels in patients with CAD make them potential biomarkers for diagnosis and prognosis. Liu et al. [45] found that compared with a normal coronary artery group, there were 98 differentially expressed lncRNAs in peripheral blood mononuclear cells of unstable angina patients. A ROC curve was used to reflect the relationship between the sensitivity and specificity of these lncRNAs. The AUC, which was between 0.1 and 1, can be used to directly evaluate the diagnostic value of the lncRNA; the larger the value, the greater the diagnostic potential.
Among 98 differentially expressed lncRNAs, the AUC values of MALAT1 and
LNC_000226 were 0.81 and 0.799, respectively. Both have very high
diagnostic values, distinguishing unstable angina patients from the normal group.
However, the specificity of LNC_000226 was not very high [45]. In
addition, compared with healthy subjects, lncRNA MIAT was significantly
overexpressed in the peripheral blood of CAD patients, which is likely to be an
important biomarker for the diagnosis of CAD. LncRNA MIAT had an AUC of
0.908 and sensitivity and specificity of 0.700 and 0.714, respectively, and is
one of the independent risk factors for CAD patients [46]. Studies have shown
that the inflammatory mediators tumor necrosis factor-alpha (TNF-
| LncRNAs | Expression pattern | Sample | Diagnostic/Prognostic role | ROC curve analysis | Ref | ||
| Sensitivity | Specificity | AUC | |||||
| FA2H-2 | Downregulated | Blood samples from 316 patients with coronary heart disease | Diagnostic biomarker | 0.850 | 0.820 | 0.834 | [47] |
| MALAT1 | Upregulated | Blood samples from 140 patients with coronary heart disease and 90 controls | Distinguishing CHD patients from normal subjects | - | - | 0.837 | [49] |
| FAF | Downregulated | A serum sample from patients with 97 coronary heart disease and 97 controls | Diagnostic and prognostic biomarker | - | - | 0.935/0.916 | [48] |
| ENST00000416361 | Upregulated | Blood samples from 187 patients with CAD and 150 controls | Diagnostic biomarker | - | - | 0.790 | [50] |
| CASC11 | Downregulated | Plasma samples from 82 patients with CAD and 82 controls | Diagnostic biomarker | - | - | 0.900 | [22] |
| LNC_000226 | Upregulated | PBMC from 44 patients with UA and 44 NCA | Diagnostic biomarker | 0.957 | 0.587 | 0.810 | [45] |
| MALAT1 | Upregulated | PBMC from 44 patients with UA and 46 NCA | Diagnostic biomarker | 0.705 | 0.848 | 0.799 | [45] |
| MIAT | Upregulated | Peripheral venous blood samples from 155 patients with CAD and 76 controls | Diagnostic biomarker | 0.700 | 0.714 | 0.908 | [46] |
| NEAT1 | Upregulated | Peripheral venous blood samples from 47 patients with STEMI and 24 patients with UA and 27 controls | Distinguishing STEMI patients from normal subjects and UA patients | 0.638 | 0.882 | 0.822 | [51] |
| SOCS2-AS1 | Downregulated | Blood samples from 111 patients with CAD and 48 patients with mild coronary artery stenosis (mCAS) and 68 controls | Discriminating CAD patients from controls | 0.714 | 0.634 | 0.704 | [52] |
| HULC | Downregulated | Blood samples from 50 patients with CAD and 50 normal subjects | Discriminating CAD patients from normal subjects | - | - | 0.900 | [53] |
| DICER1-AS1 | Downregulated | Blood samples from 50 patients with CAD and 50 normal subjects | Discriminating CAD patients from normal subjects | - | - | 0.870 | [53] |
| Abbreviations: UA, unstable angina; STEMI, ST-elevation myocardial infarction; PBMC, peripheral blood mononuclear cells; ROC, receiver operating characteristic; AUC, area under the curve; NCA, normal coronary artery. | |||||||
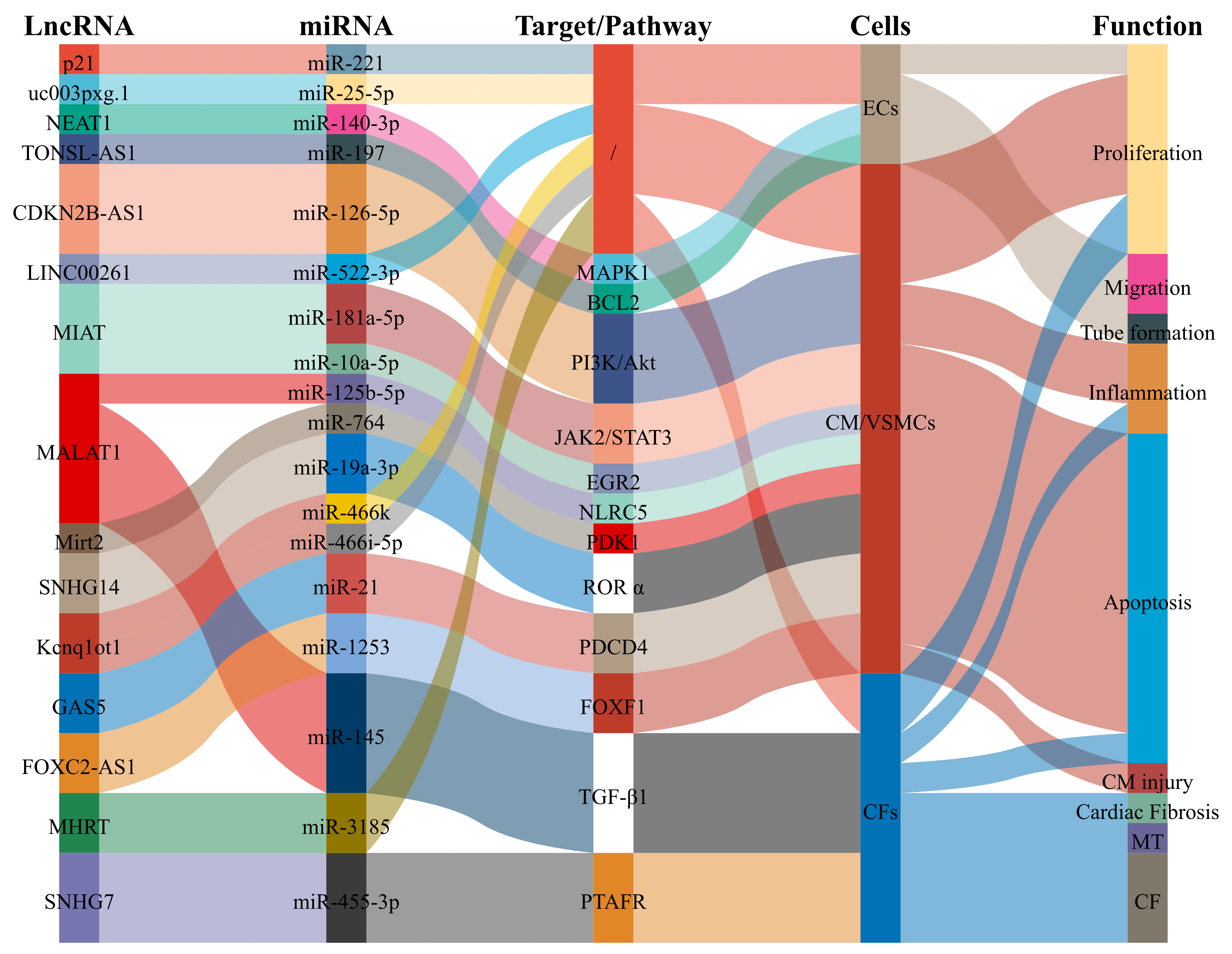 Fig. 1.
Fig. 1.LncRNAs as biomarkers of CAD participate in pathological processes and then affect the occurrence and development of CAD. Abbreviations: ECs, endothelial cells; VSMCs, vascular smooth muscle cells; CM, cardiomyocyte; CF, cardiac fibrosis.
Our review has found that most lncRNAs act as ceRNAs, competing with downstream
target miRNAs to regulate the pathophysiological process of CAD through different
mechanisms, such as regulation of vascular endothelial cells, regulation of
vascular smooth muscle cell activity, regulation of myocardial cell proliferation
and apoptosis, collagen fiber production, and myocardial fibroblast function.
The JAK1/STAT3 signaling pathway, AKT signaling pathway, and
other signaling pathways are all lncRNA signaling pathways involved in CAD.
lncRNAs affect different aspects and different targets in this process. For
example, lncRNA MIAT regulates the expression of miR-181a-5p
and miR-10a-5p; both are involved in cardiomyocyte apoptosis. The
knockdown of both lncRNA MALAT1 and lncRNA 554 can inhibit
myocardial fibrosis through the TGF-
The mortality rate in CAD remains high, largely because of the lack of effective drugs to prevent or decrease myocardial ischemic necrosis in clinical practice. LncRNAs may become the prototype to create effective drugs. In-depth exploration of the complex splicing process, differential transcriptional processing, differential intracellular expression, and subcellular localization of lncRNAs will contribute to the understanding of the pathogenesis and regulatory mechanism of CAD and lead to improvements in the clinical diagnosis and treatment of CAD. In summary, lncRNAs are involved in regulating many aspects of the pathogenesis of CAD and can be used as specific/sensitive markers for this disease. The diagnostic/prognostic/therapeutic role of lncRNAs in CAD will need to be explored in future studies.
All authors made significant contributions to the manuscript, and their contributions were acknowledged by all participating authors. The specific scientific contributions of each author are listed below. XW and JL wrote the manuscript and created the figures; GS collected the original data; JY and YP were responsible for the first review of the manuscript; XB and LW provided the overall design of the manuscript and the final review of the manuscript. All authors participated in the editorial revision of the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This study was supported by the National Natural Science Foundation of China (81860073 and 82160439); Special Foundation Projects of Joint Applied Basic Research of Yunnan Provincial Department of Science and Technology with Kunming Medical University [2019FE001(-138)]; Yunnan Provincial Department of Science and Technology (202001AT070039); Yunnan Health Training Project of High Level Talents (H-2018032); 100 Young and Middle-aged Academic and Technical Backbones of Kunming Medical University (60118260106); Young Talents of Yunnan Thousand Talents Plan (YNQR-QNRC-2019-006, RLQN20200002) and Clinical Medical Center for Cardiovascular and Cerebrovascular Disease of Yunnan Province (ZX2019-03-01).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
