- Academic Editor
Acute pericarditis is the most frequent pericardial disease characterized by
inflammation of the pericardial layers resulting in pain, dyspnea and fatigue.
Often limited to an isolated event, up to 30% of patients experience one or more
recurrences. There is limited knowledge about the pathophysiology of this
disease, possibly due to the limited availability of animal models. More
recently, following seminal clinical trials with colchicine and interleukin-1
(IL-1) blockers and a novel murine model of acute pericarditis using zymosan A,
it has become clear that the NLRP3 (NACHT, leucine-rich repeat, and pyrin
domain-containing protein 3) inflammasome/IL-1
Acute pericarditis is the most frequent pericardial disease, and an increasingly recognized cause of chest pain, with an estimated incidence of 27.7 cases per 100,000 persons/year [1]. As a complication of acute pericarditis, recurrences may occur in up to 30% of cases within 18 months after a first episode, especially among patients not treated with colchicine [2, 3, 4].
The latest 2015 European Society of Cardiology (ESC) guidelines recommend
non-steroidal anti-inflammatory drugs (NSAIDs) or aspirin and colchicine as an
initial treatment either for the first episode or for recurrences [5]. Recently,
essential steps have been accomplished to enlighten the pathophysiology and
therapy for acute and recurrent pericarditis. Specifically, a focus has been
placed on the emerging role of both NACHT, leucine-rich repeat, and pyrin
domain-containing protein 3 (NLRP3) inflammasome and interleukin-1
This review summarizes recent evidence about pathophysiology, diagnosis, and therapy in acute and recurrent pericarditis. The advancements in this field appear of utmost importance, especially in managing patients experiencing recurrent episodes, as a specific treatment, i.e., rilonacept, is now approved for treating this condition [10].
For many years, acute pericarditis has been thought to be initiated by a virus, including the more recently emerged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [11]. However, when a precise etiology cannot be determined, it is usually considered to be “idiopathic” [12]. The small number of animal models and pathology studies may have accounted for a long time for a scarce understanding of the disease and the consequential absence of targeted therapies (Fig. 1).
 Fig. 1.
Fig. 1.Animal models that investigated acute pericarditis. A limited number of animal models has been available for many years, thus explaining the limited knowledge about pericarditis pathophysiology and the availability of very few therapies. Interestingly, most of the stimuli of these animal models had a direct, yet previously unknown, link with the NLRP3 inflammasome. Indeed, hydrated magnesium silicate (talc) and Freund’s adjuvant containing aluminum are canonical stimuli for the NLRP3 inflammasome activation. An innovative murine model of acute pericarditis is based on intrapericardial injection of zymosan A that generates an inflammatory reaction of the pericardium.
The first available evidence of an animal model of pericarditis dates back to 1980 when Matsumori et al. [13] proposed a mouse model of chronic perimyocarditis obtained through intraperitoneal injection of Coxsackie virus B3 (Table 1, Ref. [6, 13, 14, 15, 16, 17]). In this model, as the virus stimulated chronic inflammation, a buildup of inflammatory cells — histiocytes, lymphocytes, plasma cells, and a few polymorphonuclear leukocytes — was evident starting from day 14 with perimyocardial fibrosis appearing at day 28 [13]. In 1986 Pagé et al. [14] reproduced the features of acute pericarditis in animals by dusting talc powder on dogs’ pericardium with a gauze (Table 1). A year later, Leak et al. [15] further recreated acute pericarditis in sheep through intrapericardial injection of heat-killed staphylococci in addition to Freund’s adjuvant (Table 1). The authors established an exceptionally complete animal model, able to recapitulate step-by-step all stages of pericardial inflammation up to a complete resolution of the inflammatory process [15]. The early phases were indeed characterized by neutrophil infiltration, edema, and accumulation of flocculent material in the submesothelial space. This process became even more organized at day 7, with an abundance of neutrophils, macrophages, erythrocytes, and fibrin deposition. At this stage, denudation of the mesothelial lining was observed, which favored platelet adherence and accumulation of neutrophils and macrophages. A localized healing process was observed starting from the second week, which ended after nearly nine months when the pericardial layers recovered entirely. Alternatively, Afanasyeva et al. [16] proposed a mouse model of perimyocarditis leading to pericardial constriction through subcutaneous injection with cardiac myosin emulsified in complete Freund’s adjuvant and intraperitoneal injection of pertussis toxin (Table 1). This model was specifically created to evaluate the clinical consequences of constrictive pericarditis. Kojima et al. [17] generated their mouse model by mimicking the post-operative pericardial adhesions (Table 1). To achieve this, they tested low- and high-doses of talc, minocycline, picibanil (lyophilized mixture of group A Streptococcus pyogenes), and heparin-treated blood. Finally, they found that talc was indeed the agent able to induce pericardial adhesions to the greatest extent, confirming what was previously described by Pagé et al. [14].
| Authors | Animal | Condition | Technique | Major findings |
|---|---|---|---|---|
| Matsumori and Kawai [13] | Mouse | Chronic perimyocarditis | Intrapericardial injection of Coxsackie virus B3 (0.1 mL of virus suspension) | One-third of mice died after virus injection. |
| Initial histopathological changes occurred mainly in myocardial fibers with a limited amount of inflammatory cells, that increased by day 14. | ||||
| On day 28, perimyocardial fibrosis was more evident while cellular infiltration gradually decreased. Perimyocardial fibrosis was marked over days 90 to 180. | ||||
| Pagé et al. [14] | Dog | Acute pericarditis | After pericardiotomy, atrial surfaces were dusted with sterile talcum powder. No information about the quantity of the talcum powder was provided. | No details about histopathological alterations were discussed. |
| Leak et al. [15] | Sheep | Acute pericarditis | The pericardial sac was exposed through left thoracotomy at the 5th intercostal space. The pericardial cavity was inoculated with bacterial toxin (0.2 g of dried bacterial cells from Staphylococcus aureus), complete Freund’s adjuvant (3 mL), and sterile phosphate-buffered saline (3 mL) under sterile conditions. The intrapericardial injection was suspended in a syringe with a 21-gauge needle to create a smooth emulsion. Then, 5 mL of the emulsion were injected into the pericardial cavity. | |
| Afanasyeva et al. [16] | Mouse | Perimyocarditis evolving toward constriction | Subcutaneous injections of 200 to 250 g of cardiac myosin emulsified in complete Freund’s adjuvant on days 0 and 7, and intraperitoneal injection of 500 ng of pertussis toxin on day 0. | Cardiac myosin–induced experimental autoimmune myocarditis in IFN- |
| The cellular infiltrate of the pericardium included poly- and mononuclear cells including eosinophils. Mesothelial hyperplasia and mesothelial reaction (i.e., change to cuboidal morphology of mesothelial cells, typical of pericardial injury) was also described. | ||||
| Kojima et al. [17] | Mouse | Acute post-operative pericarditis | Following skin incision in the abdomen and cut of the peritoneum to reach the abdominal cavity, an intrapericardial injection of 500 |
Only talc-injected mice showed diffuse and marked pericardial adhesions over the whole heart within 2 weeks visible. |
| A large amount of macrophages and myofibroblasts, together with elastic fibers and myocardial erosion, were found. | ||||
| Mauro, Bonaventura et al. [6] | Mouse | Acute pericarditis | After an incision on the left part of the thorax in the region of the 3rd–4th and fourth rib, muscle layers were dissected to expose the interosseous space and access the thoracic cavity. By carefully lifting the pericardial sac, zymosan A (1 mg dissolved in 50 |
Seven days after the intrapericardial injection of zymosan A, mice developed typical stigma of local inflammation: |
| After surgery, mice were randomly treated to the following pharmacological agents through intraperitoneal injection (over a period of 1 week): ibuprofen (100 mg/kg/day), colchicine (100 |
||||
| Pharmacological treatments: | ||||
| Transthoracic echocardiography was performed at day 3 and 7 to measure the amount of pericardial effusion. At day 7, mice were sacrificed and hearts harvested to get the pericardium for hematoxylin and eosin staining to measure pericardial thickness and immunofluorescence and immunohistochemistry stainings to look for the 3 components of the NLRP3 inflammasome (the sensor protein NLRP3, the scaffold protein ASC, and the effector protein caspase-1). | ||||
Legend. IFN, interferon; KO, knockout; NLRP3, NACHT, leucine-rich repeat, and pyrin domain-containing protein 3; NLRP3 inh, NLRP3 inhibitor.
Although previously unknown, most of the stimuli used in the previously
described animal models of pericarditis have a direct link to the NLRP3
inflammasome. Indeed, hydrated magnesium silicate — talc — was shown to
induce NLRP3 inflammasome activation [18]. Similarly, in the model by Leak
et al. [15], bacterial products of the Freund’s adjuvant, which also
contains aluminum, are considered canonical stimuli for the NLRP3 inflammasome
activation [19]. This evidence suggests an inflammasome-dependent model of acute
pericarditis. A novel murine model of acute pericarditis secondary to NLRP3
inflammasome activation has been recently developed [6] (Table 1). This mouse
model is based on intrapericardial injection of zymosan A that generates a local
inflammatory reaction (Fig. 2, Ref. [6]). This technique shares similarities to
what was previously done in animal models to cause peritonitis or arthritis [20, 21]. Zymosan A is a cell wall extract derived from the yeast
Saccharomyces cerevisiae and an agonist of the toll-like receptor-2 that
activates the NLRP3 inflammasome [22, 23]. As expression of pericardial
inflammation, we were able to observe the following manifestations: pericardial
effusion (83% increase at the time of sacrifice compared to sham mice),
pericardial thickness (45% increase compared to sham), and ASC
(apoptosis-associated speck-like protein containing a COOH-terminus caspase
activation and recruitment domain) expression (a 60-fold increase compared to
sham) [6]. ASC represents the scaffold for NLRP3 inflammasome assembly [24],
hence increased expression of ASC through formation of dense areas of aggregation
— termed specks — is indicative of NLRP3 inflammasome oligomerization [25, 26]. In addition, pharmacological blockade of the NLRP3 inflammasome improved
pericardial inflammation. Mice were treated with ibuprofen (an NSAID), colchicine
(an indirect blocker of the NLRP3 inflammasome), 16673-34-0 (an experimental
NLRP3 inflammasome inhibitor) [27], anakinra (a recombinant human IL-1 receptor
antagonist), and a recombinant murine IL-1 trap (able to bind and block
IL-1
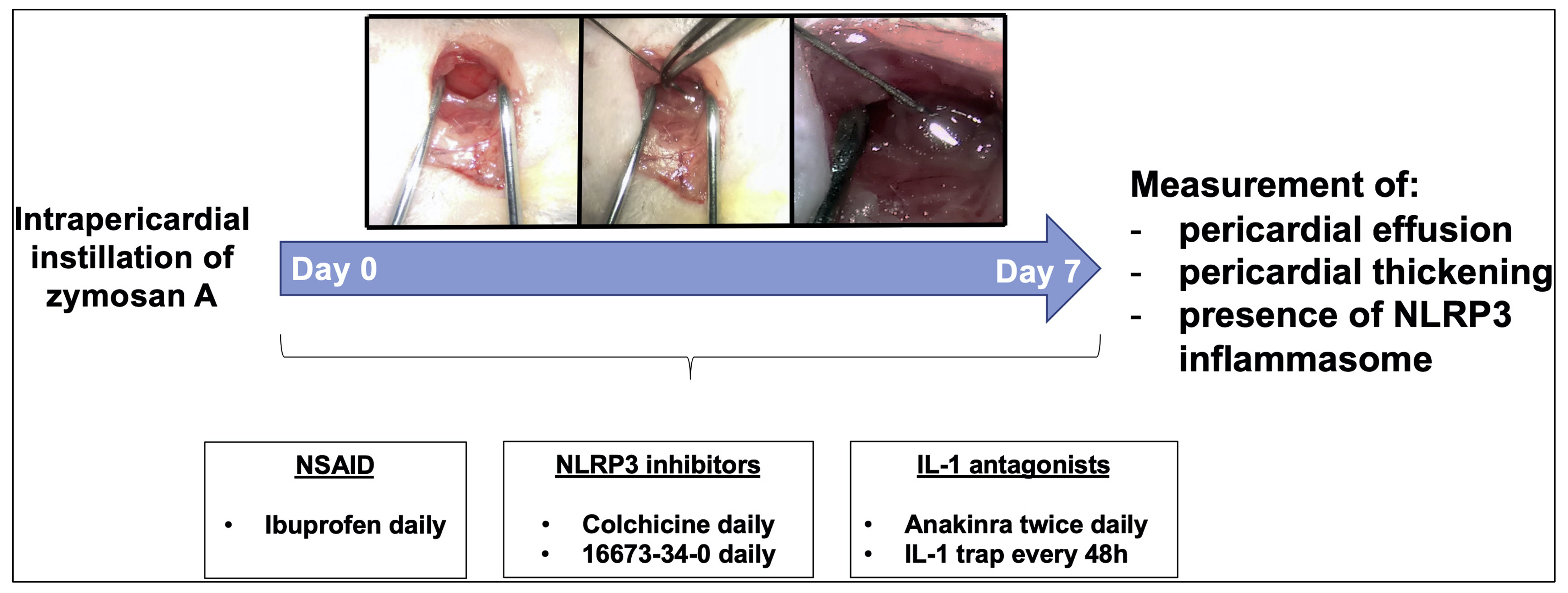 Fig. 2.
Fig. 2.A novel murine model of acute pericarditis induced by zymosan A. Injection of zymosan A into the pericardial sac induces a local inflammatory reaction. Following intrapericardial zymosan A injection, augmented pericardial effusion and thickness were observed in parallel with NLRP3 inflammasome activation. Reproduced with permission from “The Role of NLRP3 Inflammasome in Pericarditis: Potential for Therapeutic Approaches”, Mauro AG and Bonaventura A et al., JACC Basic Transl Sci. 2021 Feb 22; 6 (2): 137–150 [6].
Although large animals (sheep or dogs) might better recapitulate the pathogenetic mechanisms occurring in human pericarditis and be easier to work with from a practical standpoint (Fig. 1), they also carry numerous disadvantages, such as higher housing and handling costs. On the contrary, using a mouse model may allow a more straightforward and wider replication at limited costs. Additionally, using genetically modified mice may enable the study of alternative molecular pathways involved in the pathophysiology of pericarditis.
The innovative murine model strongly supports a pivotal role of the NLRP3
inflammasome/IL-1
According to 2015 ESC guidelines, pericarditis is classified as acute, incessant, recurrent, or chronic [5]. Acute pericarditis is diagnosed in the presence of at least two out of the following four criteria: (i) chest pain; (ii) pericardial rubs; (iii) electrocardiogram (ECG) changes; (iv) new or worsening pericardial effusion [5]. Sharp chest pain with rapid onset, typically worsened by inspiration or coughing, and alleviated by leaning forward or sitting up, is characteristic of acute pericarditis. Additional manifestations may include a dull or throbbing chest pain radiating to the trapezius ridge, low-grade fever or sinus tachycardia, which may be accompanied by non-cardiac manifestations (e.g., arthritis, rash, weight loss, night sweats) when pericarditis is associated with a systemic disease [30]. Regarding ECG changes, PR segment depression is rather sensitive and specific for pericarditis, along with diffuse ST-segment elevation. However, PR segment depression may often be the only ECG modification, while nondiagnostic or atypical changes are found in up to 40% of patients [30]. More than 30% of patients with pericarditis exhibit elevation in serum troponin I or T, or signs of myocardial involvement without new-onset abnormalities in left ventricular function upon imaging. Inflammatory biomarkers such as erythrocyte sedimentation rate (ESR), white blood cells (WBC), and C-reactive protein (CRP) are increased in approximately 80% of patients having acute pericarditis, however overall sensitivity and specificity are low. Acute pericarditis can progress to recurrent pericarditis in up to 30% of cases [3, 4]. Recurrent pericarditis is defined in the presence of signs and symptoms of acute pericarditis after a symptom-free window of at least 4–6 weeks after a prior episode of pericarditis [5, 31].
Echocardiography, cardiac computed tomography (CCT), and cardiac magnetic
resonance (CMR) are the most commonly used imaging techniques to assess and
characterize pericardial pathology and associated myocardial involvement [32, 33]. Major findings at cardiac multimodality imaging and their clinical relevance
in patients with pericarditis are reviewed in detail elsewhere [34].
Echocardiography is considered a first-line imaging test, whereas CCT and CMR are
generally used in case of inadequate echocardiographic images or diagnostic
uncertainty and/or to determine the severity of illness. Although normal in about
40% of cases, transthoracic echocardiography is essential to evaluate
ventricular dysfunction or possible complications (e.g., constrictive
pericarditis, cardiac tamponade), to quantify pericardial effusion, and to
monitor response to medical treatments. CCT provides morphological information,
being the most accurate for the measurement of pericardial thickness and the most
sensitive in identifying pericardial calcifications [34]. While CCT is not
primarily suggested for the diagnosis of cardiac tamponade, it may be more
informative to investigate constrictive pericarditis. CCT requires iodinated
contrast, exposes patients to ionizing radiations, and provides minimal
hemodynamic information, which limit its clinical usefulness and make it
unsuitable for serial evaluations [34]. CMR has emerged as the most comprehensive
imaging technique to interrogate the pericardium and adjacent myocardium as it
offers both morphological and hemodynamic information by integrating several
sequences within the same study [34, 35]. Late gadolinium enhancement (LGE)
provides accurate information regarding the presence and degree of pericardial
inflammation with very high sensitivity, and it is positively associated with
histological inflammatory and neovascularization markers [36] (Fig. 3, Ref.
[30]). Patients with multiple recurrences and LGE achieve significantly lower
clinical remission rates [37]. Pericardial thickening at CMR and CRP elevation
have been shown to predict the occurrence of adverse events, while the presence
of LGE conferred lower risk [38]. In addition, in patients with recurrent
pericarditis, CMR-guided management was associated with lower recurrence and
pericardiocentesis rates together with decreased use of glucocorticoids [39].
Pericardial inflammation, as evaluated through LGE measurement combined with the
assessment of pericardial edema in T
 Fig. 3.
Fig. 3.Main findings at CMR in patients with acute pericarditis. Reproduced with permission from “Management of Acute and Recurrent Pericarditis: JACC State-of-the-Art Review”, Chiabrando JG and Bonaventura A et al., J Am Coll Cardiol. 2020 Jan 7; 75 (1): 76–92 [30].
As the amount of information for a timely diagnosis of acute or recurrent pericarditis is progressively increasing, these elements must be considered as a whole for a tailored therapy according to two different phenotypes (Fig. 1).
Currently available agents targeting the NLRP3 inflammasome and/or IL-1 are colchicine, anakinra, and rilonacept [40, 41, 42].
The first description of colchicine in acute pericarditis dates back to 1987.
Based on previous experiences in patients with recurrent polyserositis in the
context of familial Mediterranean fever [43], three patients with
glucocorticoid-dependent recurrent pericarditis (two idiopathic and one
associated with systemic lupus erythematosus) were treated with colchicine 1 mg
daily [44]. Indeed, they experienced a long recurrence-free period lasting 15 to
36 months and were able to stop glucocorticoids after two months while on a 0.5
mg daily dose [44]. In 2005, the first two randomized, open-label trials using
colchicine for the treatment of acute and recurrent pericarditis were published,
namely the COPE (Colchicine for acute Pericarditis) and CORE (Colchicine for
Recurrent pericarditis) trials [45, 46] (Table 2, Ref. [45, 46, 47, 48, 49, 50, 51, 52, 53, 54]).
Colchicine (loading dose 1 to 2 mg, maintenance 0.5 to 1 mg daily) was used
together with NSAIDs for 3 to 6 months, and it was shown to reduce symptoms after
72 h as well as first and following recurrences [45, 46]. Colchicine was further
investigated in other randomized, double-blind trials for patients with either
acute or recurrent pericarditis (Table 2). The CORP (Colchicine for recurrent
pericarditis), ICAP (Investigation on Colchicine for Acute Pericarditis), and CORP-2 trials tested weight-adjusted colchicine (0.5 mg once
daily for patients
| Study | Study design | Treatment | Patients | Key results |
|---|---|---|---|---|
| Acute pericarditis | ||||
| COPE trial [45] | Open-label | Aspirin | 120 patients (mean age 56.9 |
Colchicine significantly decreased recurrence rate (at 18 months: 10.7% vs. 32.3% for aspirin alone, p = 0.004, NNT = 5) and symptom persistence at 72 hours (11.7% vs. 36.7% for aspirin alone, p = 0.003). |
| vs. | ||||
| aspirin + colchicine: | ||||
| No SAEs were observed | ||||
| Colchicine was discontinued in 5 cases (8.3%) because of diarrhea. | ||||
| ICAP trial [48] | Double-blind | Aspirin/ibuprofen +Placebo | 240 patients (mean age 52.1 |
Colchicine reduced the occurrence of incessant or recurrent pericarditis (16.7% vs. 37.5% for placebo; RRR 0.56, 95% CI 0.30 to 0.72, p |
| vs. | ||||
| aspirin/ibuprofen + colchicine: | Colchicine reduced symptom persistence at 72 hours (19.2% vs. 40.0%, p = 0.001) and hospitalization (5.0% vs. 14.2%, p = 0.02). | |||
| GI disturbance was similar in the two groups. No SAEs were reported. | ||||
| Sambola et al. [53] | Open-label | Aspirin/NSAIDs alone | 110 patients (mean age 44 |
No differences in the rate of recurrences was found (13.5% vs. 7.8%, p = 0.34). |
| vs. | ||||
| aspirin/NSAIDs + colchicine: | ||||
| Recurrent pericarditis | ||||
| CORE trial [46] | Open-label | Aspirin | 88 patients (55.2% males) | Colchicine reduced the recurrence rate (at 18 months 24% vs. 50%, p = 0.02; NNT = 4) and symptom persistence at 72 hours (10% vs. 31%, p = 0.03). |
| vs. | ||||
| aspirin + colchicine: | ||||
| Colchicine allowed for a longer symptom-free interval (17.2 | ||||
| Diarrhea led to drug discontinuation in 7% of colchicine-treated patients. No SAEs were reported. | ||||
| CORP trial [47] | Double-blind | Aspirin/ibuprofen +Placebo | 120 patients (52.5 males) | Colchicine decreased the recurrence rate at 18 months (24% vs. 55%, p |
| vs. | ||||
| aspirin/ibuprofen + colchicine: | ||||
| GI intolerance was the main side effect and was balanced between groups. No SAEs were observed. | ||||
| CORP-2 trial [49] | Double-blind | Aspirin/NSAIDs + placebo | 240 patients (mean age 48.7 |
Colchicine reduced recurrences (21.6% vs. 42.5%; RR 0.49, 95% CI 0.24 to 0.65, p |
| vs. | ||||
| aspirin/NSAIDs + colchicine: | ||||
| Colchicine was effective in inducing remission at 1 week (83.3% vs. 59.2%, p | ||||
| GI intolerance was the main side effect and was similar between groups. No SAEs were observed. | ||||
| Post-pericardiotomy syndrome | ||||
| Finkelstein et al. [50] | Open-label | On 3rd post-operative day, placebo or colchicine (0.5 mg three times daily for 1 month | 111 patients (73% males) | No difference was observed for the occurrence of PPS was diagnosed between colchicine and placebo groups (10.6.% vs. 21.9%, p |
| COPPS [51] | Double-blind | On 3rd post-operative day, | 360 patients (mean age 65.7 |
Colchicine reduced PPS incidence at 12 months (8.9% vs. 21.1%, p = 0.002; NNT = 8) and the secondary endpoint including PPS-related hospitalization, cardiac tamponade, constrictive pericarditis, and relapse at 18 months (0.6% vs. 5.0%, p = 0.024; NNT = 22). |
| Standard therapy + placebo | ||||
| vs. | ||||
| standard therapy + colchicine: | ||||
| GI disturbance occurs most frequently in the colchicine group. No SAEs were observed. | ||||
| COPPS-2 [52] | Double-blind | From 48 to 72 hours before surgery, placebo | 360 patients (mean age 67.5 |
Colchicine decreased PPS occurrence (19.4% vs. 29.4%, p |
| vs. | ||||
| colchicine: | ||||
| Meurin et al. [54] | Double-blind | Placebo | 197 patients (86.3% males) | Colchicine failed to reduce pericardial effusion or late cardiac tamponade (7% vs. 6%). |
| vs. | ||||
| colchicine: | Diarrhea was frequently occurred among patients on colchicine. No SAEs were recorded. | |||
Legend. AF, atrial fibrillation; CI, confidence interval; GI, gastrointestinal; NNT, number needed to treat; PPS, post-pericardiotomy syndrome; RRR, relative risk reduction; SAE, serious adverse effect.
In sum, colchicine in addition to standard anti-inflammatory therapy demonstrated to reduce recurrences up to 50% in acute and recurrent pericarditis as well as in post-pericardiotomy syndrome [55]. Over the past years, several meta-analyses supported the benefits of colchicine [56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66]. The most recent one [67], including all 6 available randomized clinical trials in acute and recurrent pericarditis (914 patients in total), demonstrated a significant lower recurrence and treatment failure rate with colchicine when compared to control (odds ratio [OR] 0.37, 95% confidence interval [CI] 0.27 to 0.51, and OR 0.29, 95% CI 0.21 to 0.41, respectively).
Nineteen case reports or series described a total of 65 patients (including both adults and children) treated with anakinra for a variable number of recurrences [68]. Among them, 77% (50/65) were treated with NSAIDs, while 92% (60/65) were both on colchicine and glucocorticoids. Recurrences occurred in 3% (2/65) of patients receiving full-dose anakinra (i.e., 100 mg once daily), 61% (21/34) experienced recurrent pericarditis following anakinra interruption, and 56% (35/62) still had recurrences after anakinra therapy was instituted [68].
In a small clinical proof-of-concept study by Wohlford et al. [69], five patients with acute pericarditis (three with a first episode and two with recurrent pericarditis) already treated with colchicine and NSAIDs (but not glucocorticoid-dependent) and experiencing moderate-to-severe pericarditis-related chest pain (initial or subsequent episode) were prescribed anakinra 100 mg subcutaneously within 24 hours of presentation. Anakinra significantly reduced pain, and no patients required rescue pain medication. In addition, IL-6 levels were also reduced considerably, and no treatment-related adverse events occurred [69] (Table 3, Ref. [7, 9, 69, 70]).
| Study | Study design | Treatment | Patients | Key results |
|---|---|---|---|---|
| Wohlford et al. [69] | Prospective open-label study | Anakinra 100 mg subcutaneously within 24 hours of hospital admission | 6 patients with acute pericarditis with moderate-to-severe chest pain | Anakinra was administered a median of 20 h after hospital admission. Pain score decreased from a baseline of 6 (6–7.5) to 4 (2.5–4) after 6 h and to 2 (1.5–2.5) after 24 h (p = 0.012 and p = 0.002, respectively). |
| IL-6 levels reduced within 24 h (95.3 [24.2–155.1] | ||||
| Pain reduction at 24 h was correlated with IL-6 reduction at 24 h (r = +0.966, p = 0.007). | ||||
| No AEs were described. | ||||
| AIRTRIP study [7] | Double-blind, placebo-controlled, randomized withdrawal trial | Anakinra at 2 mg/kg daily (up to 100 mg) subcutaneously for 2 months. | 21 patients (n = 11 anakinra, n = 10 placebo) with recurrent pericarditis ( |
In the open-label phase, all patients had a complete response to anakinra at day 8 as well as CRP normalized and pain rapidly reduced. All patients were able to stop glucocorticoids within 6 weeks. |
| Patients who responded (i.e., resolution of pericarditis) were randomized to anakinra or placebo for 6 months or until pericarditis recurrence | During the double-blind treatment phase, pericarditis recurrence was experienced by 90% (n = 9/10) patients in the placebo group vs. 18.2% (n = 2/11) patients in the anakinra group (incidence rate, 0.11% of patients per year). | |||
| Median time-to-flare was 72 (64–150) days after randomization in the placebo group, whereas it could not be computed in patients randomized to anakinra (p | ||||
| The most common AE in patients treated with anakinra was a local skin reaction at the injection site (95% patients). | ||||
| IRAP study [70] | Multicenter observational cohort study | Anakinra 100 mg daily subcutaneously | 224 patients with glucocorticoid-dependent and colchicine-resistant recurrent pericarditis | Recurrences occurred in 35% (n = 78/224) patients with a median flare-free of 10 months (5–18). |
| After anakinra treatment, a median of zero recurrences occurred with an 83% reduction in recurrence rate (RR 0.17, 95% CI 0.14–0.20, p | ||||
| A reduction of 91% for ED admissions (RR 0.09, 95% CI 0.06–0.13, p | ||||
| During follow-up, 8.9% were admitted to the hospital for pericardiectomy and discontinued anakinra. | ||||
| After anakinra treatment, glucocorticoids were tapered and NSAIDs suspended in most patients without recurrences (27% and 24% still on glucocorticoid and NSAID therapy, respectively; 58% on colchicine). | ||||
| Transient skin reaction at the injection site was the most frequent AE (38% of patients). Arthralgias and myalgias were found in 6% of patients, while 3% experienced infections during follow-up. | ||||
| RHAPSODY study [9] | Phase 3 multicenter, double-blind, event-driven, randomized-withdrawal trial | Rilonacept as a loading dose of 320 mg (or 4.4 mg/kg in patients |
86 patients in the 12-week run-in period. | Rilonacept greatly lowered risk of recurrences compared to placebo (HR 0.04, 95% CI 0.01–0.18, p |
| 61 patients who experienced clinical response during the run-in period (CRP |
Injection-site skin reactions and upper respiratory tract infections were the most common AEs. |
Legend. AE, adverse event; AIRTRIP, Anakinra-Treatment of Recurrent Idiopathic Pericarditis; CI, confidence interval; CRP, C-reactive protein; ED, emergency department; HR, hazard ratio; IL-6, interleukin-6; IRAP, International Registry of Anakinra for Pericarditis; NSAID, non-steroidal anti-inflammatory drug; RHAPSODY, Rilonacept inHibition of interleukin-1 Alpha and beta for recurrent Pericarditis, a pivotal Symptomatology and Outcomes stuDY; RR, rate ratio.
The first clinical trial of anakinra in recurrent pericarditis is the
double-blind, placebo-controlled medication withdrawal trial AIRTRIP
(Anakinra-Treatment of Recurrent Idiopathic Pericarditis) [7] (Table 3).
Twenty-one colchicine-resistant, glucocorticoid-dependent patients with recurrent
pericarditis and systemic inflammation were given anakinra for 60 days and then
randomized to either anakinra 100 mg daily, or placebo for another 6 months. All
patients had a complete response to anakinra by day 8 that persisted until
randomization (day 60) [7]. Patients assigned to anakinra were able to
successfully discontinue glucocorticoids within six weeks. Flares of pericarditis
were significantly reduced, occurring in nine out of 10 (90%) patients in the
placebo arm, and two out of 11 (18.2%) patients in the anakinra arm during the
double-blind treatment withdrawal phase. After randomization, median flare-free
survival was 72 days in the placebo group, whereas it could not be calculated in
the anakinra group (p
The IRAP (International Registry of Anakinra for Pericarditis) is a registry of
224 colchicine-resistant, glucocorticoid-dependent patients (46
First data with rilonacept in recurrent pericarditis derive from the phase II, multicenter, single-arm, open-label clinical trial RHAPSODY. In this study, either patients with at least a second recurrence and glucocorticoid-dependent recurrent pericarditis (no active recurrence, but at least two previous episodes) received subcutaneous rilonacept 320 mg (loading dose) with 160 mg weekly (maintenance dose) for 5 additional doses followed by an optional 18-week on-treatment extension period (option to wean background therapy) when already receiving conventional therapies (colchicine, NSAIDs, glucocorticoids) [71]. In symptomatic patients, chest pain was reduced, and CRP rapidly normalized in all patients. In addition, prednisone was successfully discontinued in 11 out of 13 patients (84.6%), with no patient experiencing recurrent pericarditis during this time [71]. Notably, the number of pericarditis episodes per year was nearly zero. The positive results of this phase II study found rilonacept to be safe, with most of the adverse events being mild-to-moderate in severity, primarily injection site reactions [71]. Among additional endpoints, a general improvement in the health-related quality of life was seen in symptomatic patients with increased CRP levels [72], while the exploratory cardiac magnetic resonance imaging substudy (11 patients) showed improvement in pericardial inflammation [73].
These promising results were confirmed in the phase III RHAPSODY study, a
double-blinded, placebo-controlled, multicenter randomized-withdrawal trial in
colchicine-resistant, glucocorticoid-dependent patients with symptomatic
recurrent pericarditis [9] (Table 3). After an initial 12-week run-in period,
during which all patients received rilonacept as a subcutaneous injection
(loading dose 320 mg followed by 160 mg weekly thereafter), patients who
responded favorably to rilonacept monotherapy (in terms of improvement in CRP and
chest pain) were eligible to enter the randomized-withdrawal period, where they
were randomly assigned in a 1:1 ratio to continue rilonacept or a matching dose
of placebo each week. During the run-in phase, a quick and persistent improvement
of chest pain and systemic inflammation was observed, with a median time to pain
response of 5 days, and a median time to CRP normalization of 7 days. The median
time required for patients to discontinue background therapy and continue with
rilonacept monotherapy was 7.9 weeks. Of note, all patients on glucocorticoids
were able to stop them and started receiving rilonacept monotherapy during the
run-in period. A recent post-hoc analysis has shown that the transition
from background therapies to rilonacept monotherapy occurred without recurrences,
irrespective of a sequential or concurrent tapering approach [74]. Regarding the
randomized-withdrawal period, rilonacept strikingly decreased the risk of
pericarditis recurrence compared with placebo (hazard ratio 0.04, 95% confidence
interval 0.01 to 0.18, p
In sum, the rapid resolution of pain (median five days), CRP normalization (median time of seven days), effective withdrawal of glucocorticoids, and the lack of recurrences in the treatment group following a randomized-withdrawal period provide confirmatory evidence that rilonacept monotherapy is sufficient to maintain disease control [42]. Hence, rilonacept is not only able to provide a rapid resolution of the acute flare of pericarditis but warrants successful maintenance of remission during rilonacept monotherapy. As of March 2021, rilonacept was approved by the FDA for the treatment of recurrent pericarditis [10] following the results of the RHAPSODY trial [9].
Evidence on the benefits of canakinumab in recurrent pericarditis is unclear, and described only in case reports and relatively small case series [77, 78, 79, 80]. Moreover, recurrences have been described after switching from anakinra to canakinumab in patients with good response to anakinra [81].
A subset of patients experiences multiple recurrences (
 Fig. 4.
Fig. 4.Suggested flowchart for the use of IL-1 inhibitors in patients
with recurrent pericarditis. In patients experiencing
In the past years, substantial advances in the understanding of acute and
recurrent pericarditis have been accomplished. As an etiologic diagnosis is often
unfeasible or fails, in most cases, acute pericarditis has been regarded to as
“idiopathic”. This has probably prevented the medical community for many years
from more in-depth mechanistic research aimed at pathophysiology and targeted
therapies. Thanks to clinical and pre-clinical studies conducted in recent times,
it is now clear that acute pericarditis is an inflammatory condition that can be
triggered by infectious or non-infectious stimuli, except for those cases due to
an autoimmune disease (e.g., systemic lupus erythematosus, rheumatoid arthritis).
In most cases, the acute inflammation of the pericardium completely resolves.
Almost 30% of patients may, however, experience recurrences, that result from a
rapid tapering of anti-inflammatory drugs or alternatively from a not adequately
controlled autoinflammatory phenomenon. The latter is likely to depend on a
sustained production of IL-1
An additional important point deals with patients’ phenotyping in order to provide a tailored therapy. Recent studies identified a central role for CMR in the diagnosis of acute pericarditis (e.g., LGE sequence and edema-weighted T2-weighted short-tau inversion recovery sequence) in patients experiencing multiple recurrences [34, 38, 86]. CMR can be coupled with the measurement of inflammatory biomarkers to recognize patients at higher risk for complications [87, 88]. This allows a more cautious tapering of anti-inflammatory therapies which should take place when both inflammatory biomarkers are lowering or negative, and pericardial LGE has resolved [37, 39].
Given the increased pathophysiological understanding of acute and recurrent
pericarditis and recent solid evidence from clinical studies, it is time for
guidelines to incorporate novel treatments targeting the NLRP3 inflammasome/IL-1
AB, NP, and AA conceived the structure of the manuscript. GKT, MG, AGM, AV, and MGDB drafted the first version of the manuscript. AB drafted Figures 1 and 4 and obtained permissions to reproduce Figures 2 and 3. AB, NP, AA, GKT, MG, AGM, AV, MGDB, and ST provided critical revisions of the first draft of the manuscript. AB, NP, AA, GKT, MG, AGM, AV, MGDB, and ST read and approved the final version of the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
Aldo Bonaventura and Alessandra Vecchié received a travel grant from Kiniksa Pharmaceuticals Ltd. to attend the 2019 AHA Scientific Sessions and honoraria from Effetti s.r.l. (Milan, Italy). Marco Giuseppe Del Buono received honoraria from Effetti s.r.l. (Milan, Italy). Nicola Potere received a training fellowship from the International Society on Thrombosis and Hemostasis. Antonio Abbate has served as a consultant to Applied Clinical Intel, AstraZeneca, Cardiol, Cromos Pharma, Effetti s.r.l, Eli Lilly, Implicit Biosciences, Janssen Pharmaceuticals, Kiniksa, Novo Nordisk, Olatec, Sanofi, and Serpin Pharma. Antonio Abbate is serving as Guest Editor of this journal. We declare that Antonio Abbate had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Yan Topilsky.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
