1. Introduction
Stroke, a leading cause of morbidity and mortality worldwide, is the third most
common cause of death in most Western countries, after coronary heart disease and
cancer [1]. New research shows that the incidence of stroke is increasing. One in
four people in the world has experienced a stroke in their lifetime. It is also a
major cause of disability worldwide, with 50 per cent of survivors suffering from
chronic disability [2]. In a county-level study, stroke mortality among adults
aged 35 to 64 in the United States increased from 14.7/100,000 in 2010 to
15.4/100,000 in 2016 [3]. At present, many researchers have explored the death
factors of stroke, the retrospective study of Wa á kowicz shows that
hypertension, smoking, coronary heart disease and previous stroke history are
risk factors leading to death of patients with acute stroke [4]. Given the high
incidence, disability, and mortality rates, and the high financial burden of
stroke [5, 6, 7], there is great interest in finding novel markers that identify
individuals at higher risk of stroke. One potential risk marker may be beta-2
microglobulin (2M). 2M, a non-glycosylated protein that exists in each nucleus,
which forms major histocompatibility complex (MHC) class I molecules on the cell
surface [8].
Serum 2M levels elevate with systemic inflammation and deteriorated glomerular
filtration rate (GFR), prompting 2M as a marker of renal disease and kidney
function [9]. 2M is closely associated to the incidence and death of a variety
of diseases, including cardiovascular (CVD) [10], Chronic Obstructive Pulmonary
Disease (COPD) [11], chronic kidney disease (CKD) [9], diabetes [12], cancer [13]
and hemodialysis mortality [14]. To further explore the role of 2M in overall
health, several previous epidemiological studies showed the associations between
2M and all-cause and cause-specific mortality, and found higher 2M was
significantly associated with higher risk of sudden cardiac death (SCD),
end-stage renal disease (ESRD), and infectious mortality [15, 16, 17]. In addition,
blood disease is one of the uncommon causes of cerebrovascular diseases such as
acute stroke. The detection of hematological markers will help correct diagnosis
of stroke. The positive correlation between 2M and acute stroke in this unusual
cause is still lacking [18]. Some studies showed the association between 2M with
CVD mortality [16, 19, 20] and stroke incidence [21, 22], however, no study further
reported the association between 2M and specific stroke mortality. The evidence
to determine the relationship between 2M and stroke mortality is still limited,
and no prior study has specifically evaluated the prognostic value of 2M in
adults aged 40 in U.S.
In this study, we aimed to examine the associations of 2M with stroke and
all-cause mortality, using over 20 years of follow-up in the National Health and
Nutrition Examination Survey III (NHANES III). Subgroup analyses were carried out,
considering potentially traditional risk factors associated with stroke,
including age, sex, ratio of family income to poverty, BMI, alcohol, smoking,
history of hypertension, and history of diabetes [23, 24].
2. Methods
2.1 NHANES III
The Third National Health and Nutrition Examination Survey (NHANES III) is a
large-scale, multistage, ongoing, nationwide probability sampling survey by
National Center for Health Statistics (NCHS) during the period 1988–1994. NHANES
III is a two-stage, six-year comprehensive survey, overall survey response rate
of participants (78%) has been described in previous studies [25, 26]. All
baseline data are available at
https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx.
2.2 Study Population
In NHANES III, blood samples from 7807 participants were measured for 2M
content. In this study, we performed a prospective cohort of 2M levels with risk
of stroke and all-cause mortality in the U.S. adult population. Participants of
this study had not a history of stroke at baseline and had mortality follow-up
information including underlying cause of death. Considering the onset age of
stroke, we excluded those aged 40 years (n = 1083). We further excluded
participants who had missing information on 2M (n = 670) and baseline
characteristics (n = 814), or if self-reported heart failure or stroke onset (n =
456). The final analytic cohort consisted of 4914 participants. All participants
provided written informed consent. The NHANES was approved by the NCHS Ethics
Review Board.
2.3 Exposure Measurement and Outcome Assessment
In 2009, beta-2 microglobulin (2M) levels were measured from stored surplus
serum samples of NHANES III participants in the University of Minnesota, stored
at –70 °C until the time of 2M measurement and was assayed
using the N Latex 2 microglobulin assay, Siemens Diagnostics, and IL.
The inter-assay coefficient of variation for the 2M assay was 2.7% (mean 1.757
mg/L) when 2M concentrations ranged from 0.253 to 61.700 mg/L. Given the
possible nonlinear relationship of 2M levels with mortality rates, participants
were divided into the following five categories based on quintiles of serum 2M
levels: 1.73 (reference group), 1.73 to 2.00, 2.01 to 2.33, 2.34 to 2.90, and
2.91 mg/L. Stroke mortality includes deaths caused by
ischemic stroke and hemorrhagic stroke, but there is no clear distinction between
stroke in the data of NHANES III. Participants were asked if and when they had a
stroke. For example, participants were asked: “Have doctors or other health
professionals ever told you about a stroke?”. All causes mortality
included stroke (codes I60–I69), heart disease (codes I00–I09, I11, I13, and
I20–I51), cancer (codes C00–C97), and other causes (codes C98–C99). The causes
of death were classified according to the codes of ICD-10 (International
Statistical Classification of Diseases, 10th Edition) [27, 28]. NHANES
participants were linked to the National Death Index through a probabilistic
matching algorithm to determine mortality status and special causes of death as
of December 31, 2015.
2.4 Covariates
Information on sociodemographic characteristics and potential biochemical
factors was collected at baseline (n = 4914), including age (we categorized into
60 and 60 years), sex (male and female), race/ethnicity (non-Hispanic
white, non-Hispanic black, Mexican American, and Other), marital status (married,
widowed, divorced, and single), ratio of family income to poverty (we categorized
into 1.58, 1.58–4.09, and 4.09), alcohol (we categorized into never
drinker, moderate drinker, and heavy drinker), smoking (we categorized into never
smoker, former smoker, and current smoker). At baseline, body mass index (BMI)
was calculated by the formula: BMI = weight/height height. Glycated
hemoglobin (%), serum creatinine (mg/dL), low-density lipoprotein (LDL)-cholesterol (mg/dL),
high-density lipoprotein (HDL)-cholesterol (mg/dL), c-reactive protein (mg/dL) were performed by the
Laboratory at the University of Missouri and the University of Minnesota. GFR was
estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)
2009 creatinine equation [29]. Histories of hypertension and diabetes were
recorded by participants’ self-reports.
2.5 Statistical Analysis
Trends of population characteristics across quintiles of 2M were examined by
using Anova for continuous variables, or Chi-square tests for categorical
variables. The hazard ratios and 95% confidence intervals between 2M with
stroke and all-cause mortality were investigated by using weighted Cox
proportional hazards regression, model 1: adjusted for age, sex, race/ethnicity,
and marital status; model 2: model 1 + ratio of family income to poverty, BMI,
alcohol, and smoking; model 3: model 2 + glycated hemoglobin (%), serum
creatinine (mg/dL), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), c-reactive
protein(mg/dL), GFR, history of hypertension and history of diabetes.
In addition, subgroup analyses were completed to assess the heterogeneity in the
associations of 2M with risk of stroke and all-cause mortality and tested for
interaction p value for age groups (60 and 60 years), sex (male and
female), ratio of family income to poverty (1.58, and 1.58), BMI
(25, 25–29.9, and 30 kg/m), alcohol (current drinker and
non-current drinker), smoking (current smoker and non-current smoker), history of
hypertension (no and yes), and history of diabetes (no and yes).
To further test the robustness and potential variations of associations of 2M
with stroke and all-cause mortality, we conducted several sensitivity analyses.
First, we repeated all analyses by excluding deaths during the first two years of
follow-up to reduce potential reverse causation. Second, we excluded individuals
with chronic kidney disease, diabetes, or hypertension, because both 2M and
stroke events could be influenced by major chronic diseases. Finally, we
estimated the association between 2M levels and risk of stroke among those with
GFR 60 mLmin 1.73 m, the cutoff for
abnormal renal function/chronic kidney disease. All analyses were conducted using
the SAS software version 9.4 (SAS Institute, Cary, NC, USA) and GraphPad Prism 8
software (GraphPad Software, San Diego, CA, USA). Two-sided p values
0.05 were considered statistically significant.
3. Results
3.1 Population Characteristics
Through sample selection, 4914 patients were finally included in the study (Fig. 1). Table 1 shows baseline characteristics of participants (mean age = 63.0
years, 44.3% male), by quintiles of serum 2M levels. At baseline, participants
with higher 2M were more likely to be females, older individuals (60 years),
drinkers, smokers, or individuals with hypertension and diabetes. They were less
likely to be Mexican American, non-married, obese, or with low glycated
hemoglobin (%), low serum creatinine, and low c-reactive protein level.
Moreover, they were more likely to have lower LDL-cholesterol, HDL-cholesterol,
and GFR. The Spearman correlations of 2M with age, BMI, and a range of
biochemical factors are shown in Table 2.
 Fig. 1.
Fig. 1.
Flowchart of the study.
Table 1.Baseline demographic characteristics of the study population,
according to quartiles of 2M.
| Characteristics |
2M |
p value |
| Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
| 1.73 |
1.73–2.00 |
2.01–2.33 |
2.34–2.90 |
2.91 |
| Total, N |
956 |
976 |
996 |
999 |
987 |
|
| Age |
|
|
|
|
|
|
|
60 |
64.6 |
40.2 |
24.5 |
12.6 |
9.1 |
0.001 |
|
60 |
35.4 |
59.8 |
75.5 |
87.4 |
90.9 |
|
| Sex |
|
|
|
|
|
|
|
Male |
45.7 |
46.1 |
43.5 |
45.2 |
39.8 |
0.001 |
|
Female |
54.3 |
53.9 |
56.5 |
54.8 |
60.2 |
|
| Race/ethnicity |
|
|
|
|
|
|
|
Non-Hispanic white |
74.9 |
83.1 |
84.3 |
85.2 |
85.2 |
0.001 |
|
Non-Hispanic black |
12.4 |
8.6 |
7.8 |
6.9 |
8.7 |
|
|
Mexican American |
4.3 |
2.8 |
2.8 |
2.2 |
1.9 |
|
|
Other |
8.4 |
5.5 |
5.1 |
5.6 |
4.2 |
|
| Marital status |
|
|
|
|
|
|
|
Married |
76.3 |
73.6 |
65.8 |
65.2 |
55.4 |
0.001 |
|
Widowed |
7.2 |
14.3 |
18.2 |
23.6 |
33.8 |
|
|
Divorced |
10.9 |
6.5 |
8.8 |
6.2 |
5.3 |
|
|
Single |
5.6 |
5.6 |
7.2 |
5.0 |
5.5 |
|
| Ratio of family income to poverty |
|
|
|
|
|
|
|
1.58 |
23.0 |
23.3 |
32.8 |
31.2 |
43.6 |
0.001 |
|
1.58–4.09 |
45.2 |
43.7 |
44.8 |
45.2 |
40.1 |
|
|
4.09 |
31.8 |
33.0 |
22.3 |
23.6 |
16.2 |
|
| BMI, kg/m |
|
|
|
|
|
|
|
Normal (25.0) |
44.3 |
37.0 |
33.3 |
28.5 |
37.0 |
0.001 |
|
Over weight (25.0–29.9) |
36.2 |
41.8 |
39.2 |
40.7 |
31.9 |
|
|
Obese (30.0) |
19.5 |
21.2 |
27.5 |
30.8 |
31.1 |
|
| Alcohol |
|
|
|
|
|
|
|
Never drinker |
44.0 |
51.9 |
58.4 |
64.1 |
68.1 |
0.001 |
|
Moderate drinker |
31.0 |
28.3 |
20.4 |
18.3 |
13.6 |
|
|
Heavy drinker |
24.9 |
19.4 |
19.8 |
15.8 |
15.2 |
|
|
Missing |
0.1 |
0.4 |
1.4 |
1.8 |
3.1 |
|
| Smoking |
|
|
|
|
|
|
|
Never smoker |
43.6 |
42.1 |
44.5 |
43.5 |
44.6 |
0.001 |
|
Former smoker |
32.6 |
35.8 |
36.6 |
43.4 |
38.8 |
|
|
Current smoker |
23.8 |
22.1 |
18.9 |
13.1 |
16.6 |
|
| Glycated hemoglobin (%) |
5.53 0.04 |
5.64 0.03 |
5.62 0.03 |
5.77 0.03 |
5.89 0.04 |
0.030 |
| Serum Creatinine (mg/dL) |
1.01 0.01 |
1.06 0.01 |
1.11 0.01 |
1.16 0.01 |
1.42 0.02 |
0.001 |
| LDL-cholesterol (mg/dL) |
159.02 3.46 |
150.36 1.66 |
158.95 3.01 |
154.94 2.62 |
153.38 2.50 |
0.444 |
| HDL-cholesterol (mg/dL) |
54.31 0.54 |
52.13 0.51 |
51.89 0.52 |
48.03 0.46 |
48.36 0.49 |
0.001 |
| C-reactive protein (mg/dL) |
0.35 0.01 |
0.37 0.02 |
0.46 0.02 |
0.50 0.02 |
0.90 0.04 |
0.001 |
| GFR |
117.53 0.96 |
106.51 0.83 |
99.02 0.86 |
90.20 0.72 |
75.29 0.97 |
0.001 |
| History of hypertension |
25.8 |
30.9 |
34.2 |
46.2 |
58.4 |
0.001 |
| History of diabetes |
5.4 |
7.4 |
7.5 |
9.7 |
15.7 |
0.001 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by
height in meters squared); 2M, 2-microglobulin; GFR, glomerular
filtration rate.
Values are weighted mean SE for continuous variables or weighted % for
categorical variables.
Table 2.Spearman correlations between 2M and other factors.
| Factors |
2M |
p value |
| Age |
0.537 |
0.001 |
| BMI |
–0.004 |
0.767 |
| C-reactive protein |
0.187 |
0.001 |
| LDL-cholesterol |
–0.022 |
0.115 |
| HDL-cholesterol |
–0.126 |
0.001 |
| Glycated hemoglobin |
0.098 |
0.001 |
| Serum Creatinine |
0.436 |
0.001 |
| eGFR |
–0.515 |
0.001 |
3.2 Stroke Mortality
Table 3 showed that participants with high 2M levels were at higher risk of
stroke mortality in unadjusted model (Q5 VS Q1; HR 6.83, 95% CI 3.10–15.04, p
for trend 0.001). After multivariable adjustment, 2M was still statistically
significant associated with stroke mortality in differently adjusted model (Q5 VS
Q1: HR 3.57 in the model 1; HR 3.50 in the model 2; HR 3.45 in the model 3).
Another, a linear dose–response association was observed between 2M levels and
risk of stroke mortality (Fig. 2). Stroke mortality risk increased monotonically
with 2M modeled continuously, with no indication of a threshold.
Table 3.The association of 2M with stroke and all-cause mortality.
|
2M |
p for trend |
| Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
| 1.73 |
1.73–2.00 |
2.01–2.33 |
2.34–2.90 |
2.91 |
| Stroke mortality |
|
|
|
|
|
|
|
Deaths, No. (%) |
28 (2.4) |
46 (3.0) |
43 (3.0) |
63 (7.0) |
74 (6.1) |
|
|
Deaths/person-years |
396/19076 |
648/17460 |
489/15964 |
603/12821 |
577/8633 |
|
|
Unadjusted |
1 [Reference] |
1.43 (0.69, 2.98) |
1.65 (0.80, 3.40) |
4.98 (2.34, 10.60) |
6.83 (3.10, 15.04) |
0.001 |
|
Model 1 |
1 [Reference] |
1.07 (0.52, 2.21) |
1.05 (0.51, 2.18) |
2.92 (1.28, 6.70) |
3.57 (1.70, 7.49) |
0.001 |
|
Model 2 |
1 [Reference] |
1.08 (0.53, 2.21) |
1.05 (0.49, 2.24) |
2.95 (1.27, 6.88) |
3.51 (1.58, 7.78) |
0.001 |
|
Model 3 |
1 [Reference] |
1.06 (0.50, 2.26) |
1.08 (0.48, 2.44) |
3.03 (1.18, 7.75) |
3.46 (1.34, 8.95) |
0.001 |
| All-cause mortality |
|
|
|
|
|
|
|
Deaths, No. (%) |
385 (32.5) |
560 (50.6) |
698 (65.1) |
842 (82.5) |
930 (91.6) |
|
|
Deaths/person-years |
5622/19076 |
7688/17460 |
9013/15964 |
9193/12821 |
7357/8633 |
|
|
Unadjusted |
1 [Reference] |
1.80 (1.49, 2.18) |
2.70 (2.32, 3.14) |
4.42 (2.57, 5.47) |
8.05 (6.42, 10.10) |
0.001 |
|
Model 1 |
1 [Reference] |
1.33 (1.11, 1.60) |
1.66 (1.42, 1.95) |
2.52 (2.11, 3.01) |
4.37 (3.47, 5.49) |
0.001 |
|
Model 2 |
1 [Reference] |
1.36 (1.14, 1.63) |
1.65 (1.43, 1.91) |
2.56 (2.15, 3.04) |
4.18 (3.33, 5.26) |
0.001 |
|
Model 3 |
1 [Reference] |
1.36 (1.14, 1.62) |
1.69 (1.45, 1.97) |
2.60 (2.17, 3.11) |
3.96 (3.04, 5.17) |
0.001 |
1. Percentages and mortality rates were estimated using U.S. population weights.
2. Values are n or weighted hazard ratio (95% confidence interval).
Model 1: adjusted for age, sex, race/ethnicity, and marital status.
Model 2: model 1 + Ratio of family income to poverty, BMI, alcohol, smoking.
Model 3: model 2 + Glycated hemoglobin (%), Serum Creatinine (mg/dL),
LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), C-reactive protein (mg/dL), GFR,
history of hypertension and history of diabetes.
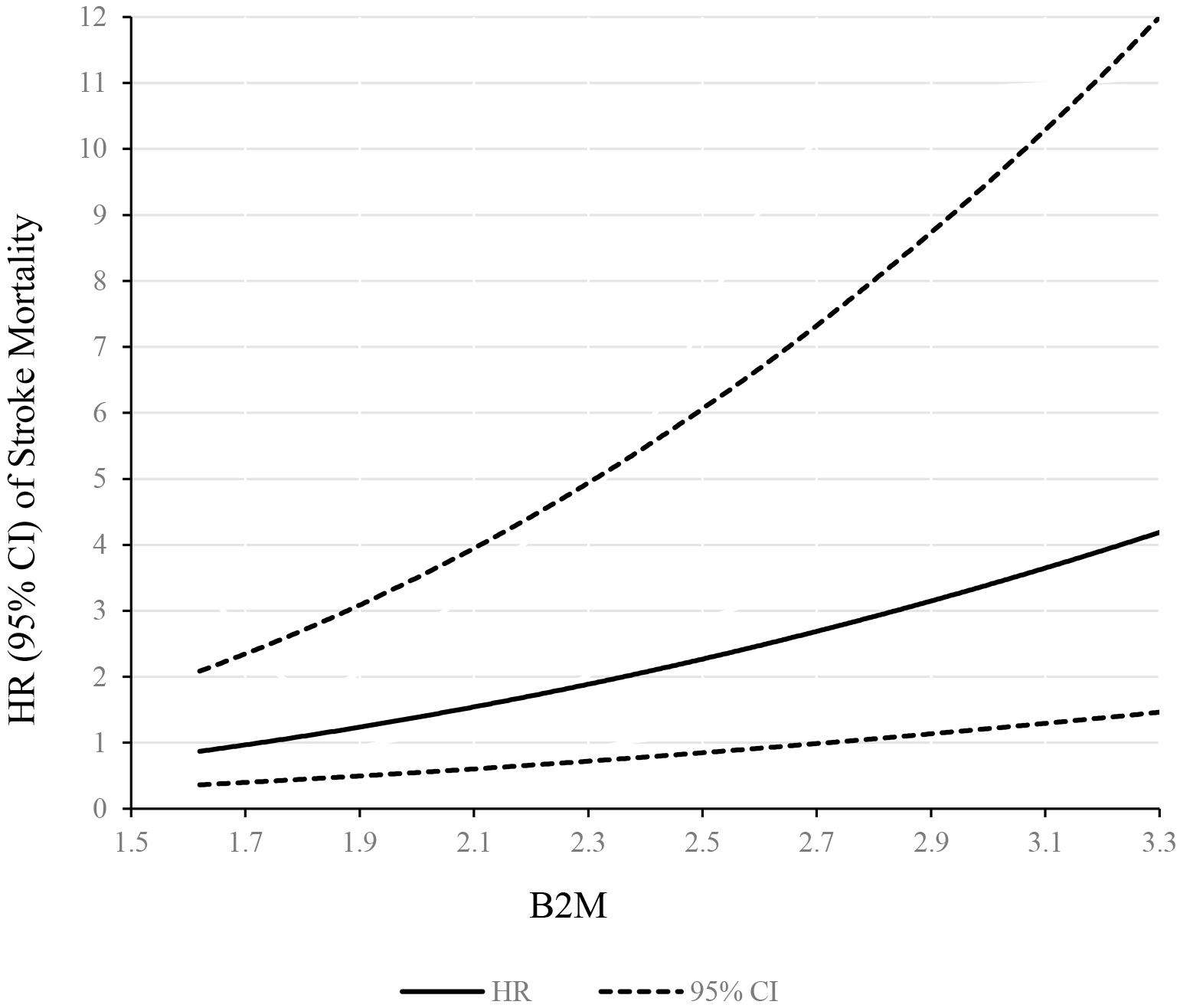 Fig. 2.
Fig. 2.
Dose–response relationship between 2M concentration and stroke
mortality. The solid line and dashed line represent the HRs and their 95%
confidence intervals.
As shown in Fig. 3, participants with the highest 2M relative to their lower
2M had much steeper declines in survival over more than 20 years of follow-up.
Among participants with the highest 2M at baseline, about 10% for stroke
patients had died after 25 years of follow-up. As for the lowest 2M, about only
5% for stroke patients had died.
 Fig. 3.
Fig. 3.
Adjusted survival curves for stroke and all-cause mortality by
quartile.
3.3 All-Cause Mortality
For all-cause mortality, the similar association was observed with 2M. In
unadjusted model, 2M was strongly associated with all-cause mortality (Q5 VS Q1;
HR 8.05, 95% CI 6.42–10.10, p for trend 0.001). 2M was positively
associated with higher all-cause mortality in the age-, sex-, race/ethnicity-,
and marital status-adjusted model 1 (Q5 VS Q1; HR 4.37, 95% CI 3.47–5.49; p for
trend 0.001). The positive association remained significant after further
adjusting for other sociodemographic characteristics and chronic disease (Q5 VS
Q1; HR 4.17, 95% CI 3.33–5.21 in the model 2; HR 3.95, 95% CI 3.05–5.12 in
the model 3). Also, a linear dose–response association was observed between 2M
levels and risk of all-cause mortality (Fig. 4). Mortality risk increased
monotonically with 2M modeled continuously, with no indication of a
threshold.
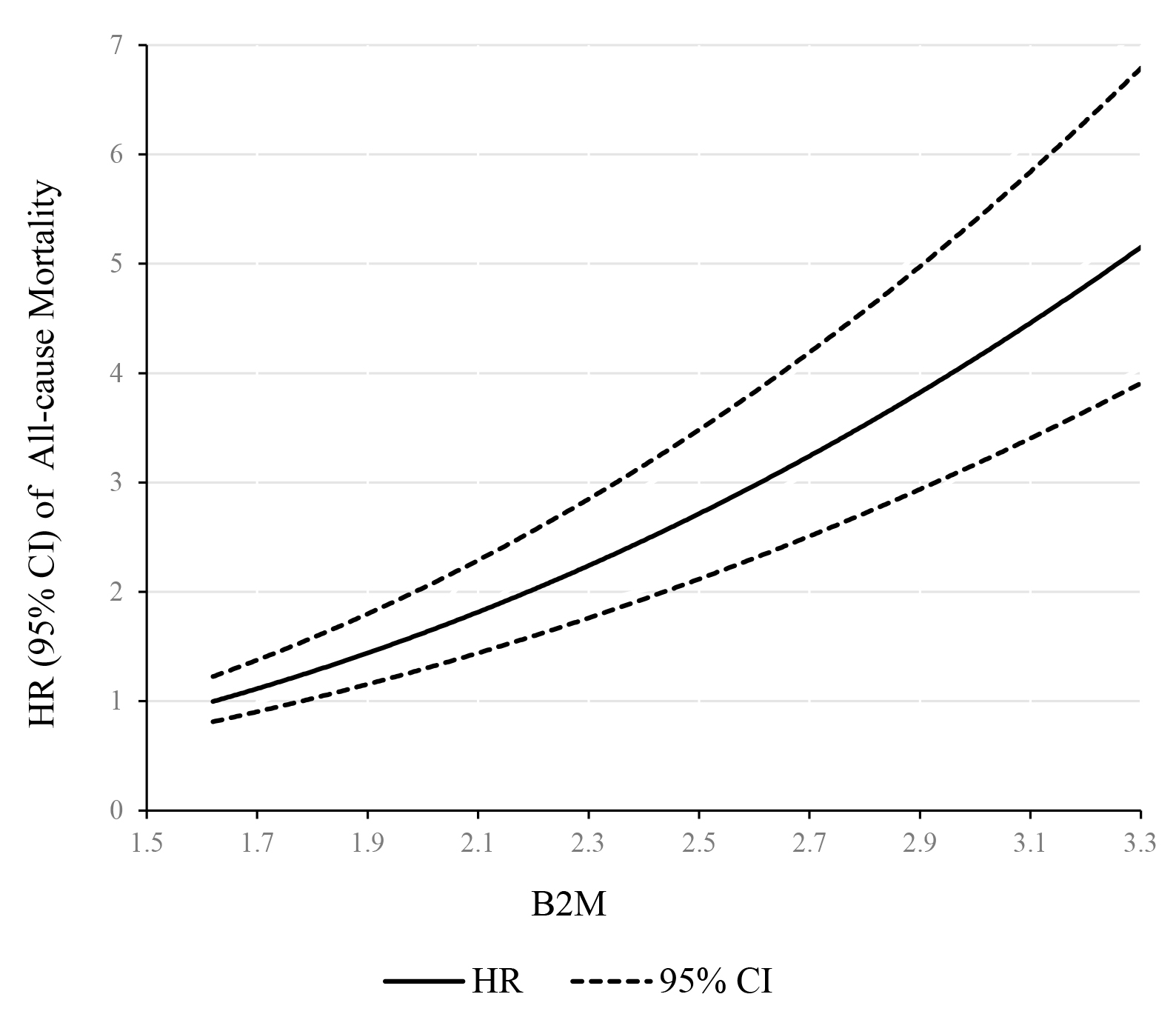 Fig. 4.
Fig. 4.
Dose–response relationship between 2M concentration and
all-cause mortality. The solid line and dashed line represent the HRs and their
95% confidence intervals.
As shown in Fig. 3, similar to stroke mortality, participants with the highest
2M relative to their lower 2M had much steeper declines in survival over more
than 20 years of follow-up. Among participants with the highest 2M at baseline,
about 90% for all-cause mortality had died after 25 years of follow-up. As for
the lowest 2M, about 70% for all-cause mortality had died.
3.4 Subgroup Analyses
In the subgroup analyses (Fig. 5), the positive association between
concentrations of 2M and stroke mortality was consistent among all participants
and all subgroups, and there was no significant difference by varying strata of
risk factors for stroke mortality (p 0.05). For all-cause
mortality, although the association between 2M and all-cause mortality persisted
in all the subgroups, the positive associations were stronger among age 60
years (p = 0.010), ratio of family income to poverty
1.58 (p = 0.036), current smokers (p =
0.001), non-hypertensive participants (p = 0.001).
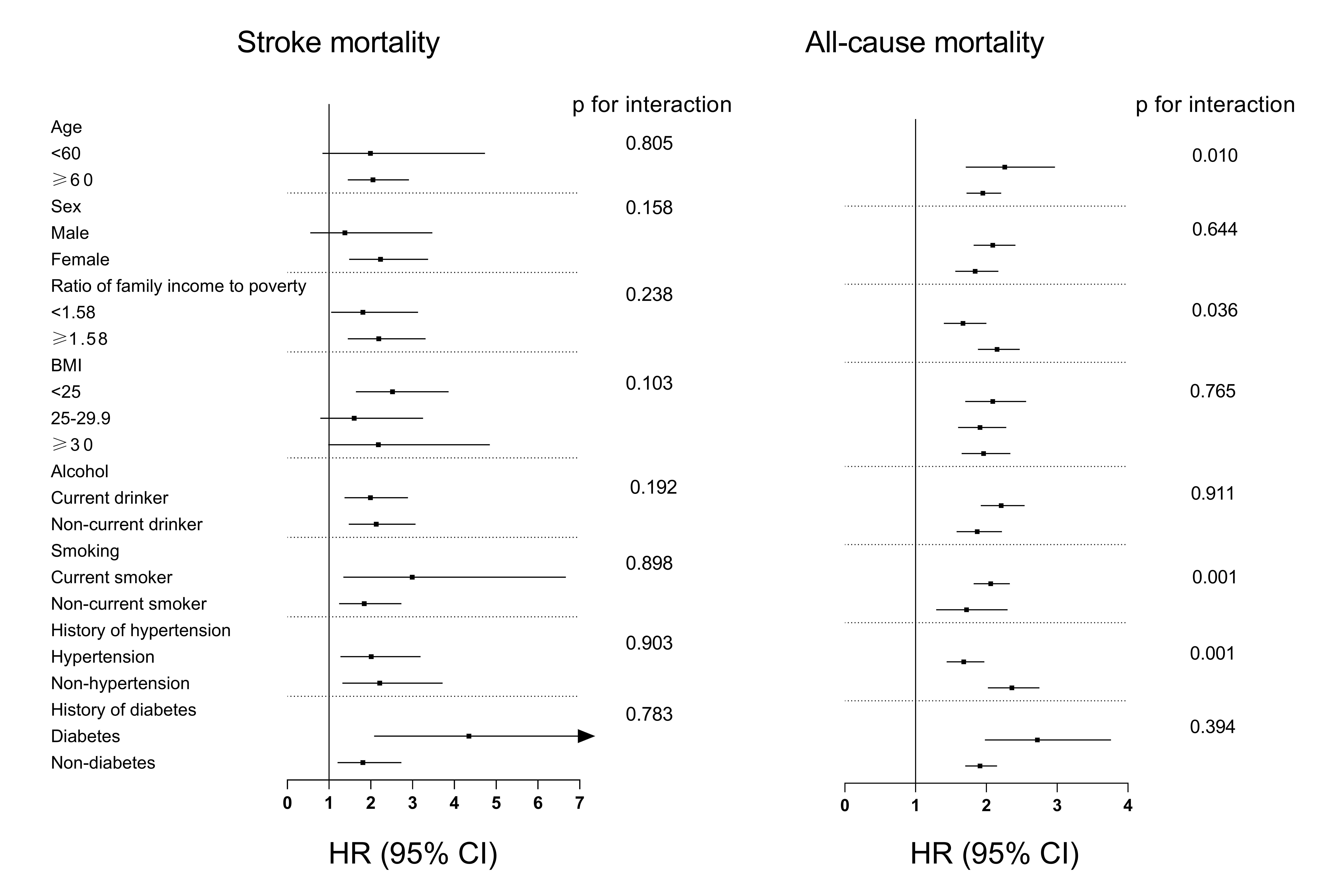 Fig. 5.
Fig. 5.
Subgroup analysis of the association of 2M with stroke and
all-cause mortality.
3.5 Sensitivity Analyses
First, we observed similar associations when we excluded deaths during the first
two years of follow-up (Supplementary Table 1). Second, there was no
significant difference in the association 2M with stroke and all-cause mortality
when we excluded participants with histories of chronic kidney disease, diabetes
or hypertension disease (Supplementary Table 2). Furthermore, we also
found unchanged associations when we excluded those with GFR 60 mL/min/1.73
m, the cutoff for abnormal renal function/chronic kidney disease
(Supplementary Table 3).
4. Discussion
This study comprehensively showed that serum 2M concentrations were associated
with stroke and all-cause mortality in 4917 U.S. adults aged 40 years or more
during a median follow-up of 19.4 years. Also, the association between serum 2M
concentrations and stroke mortality was independent of sociodemographic and
general stroke risk factors. The risk estimates for the positive association
between serum 2M concentrations and all-cause mortality varied the participants’
age, ratio of family income to poverty, smoking status, and history of
hypertension.
According to the previous literature, some studies had demonstrated the
association between 2M with CVD mortality [10] and stroke incidence [20, 21, 22];
nevertheless, the association between serum 2M and specific stroke mortality had
not been investigated. 2M may be a marker of subclinical renal dysfunction and
small vessel disease, and is associated with incident peripheral artery disease
and severity of peripheral artery disease [9, 10, 11, 12, 13, 14]. 2M levels was elevated with
systemic inflammation, due to systemic inflammation, and exacerbated by
in-hospital infections, can increase morbidity and mortality in stroke patients
[30, 31], suggesting that inflammation may explain some, but not all, of the
association between 2M and stroke risk. Furthermore, previous studies indicated
that higher 2M levels were associated with 1.3–2 times increased risk of stroke
incidence compared with lower 2M levels [22, 32]. In the Risk for Cardiovascular
Events Study of 1005 male and female, median follow-up of 3 years, 2M levels
(Q4: 2.59 VS Q1: 1.49 mg/L) was positively associated with an
increased risk of stroke, adjusted HR (95% CI) was 1.62 (1.16–2.67) [32]. For
the risk of ischemic stroke among 946 women in the Nurses’ Health Study [22],
women in the highest quartile (2.59 mg/L) of 2M had a statistically
significant increase in the odds of developing an ischemic stroke (OR 1.71, 95%
CI 1.14–2.56) compared to those in the lowest quartile (1.49 mg/L) after
adjustment for traditional stroke risk factors. In the Atherosclerosis Risk in
Community Study of 8622 men and women, with a median follow-up of 11.9 years
[20], 2M levels were associated with significantly increased risk of total
stroke in both those with and without chronic kidney disease. The Multivariable
HR (95% CI) was 1.16 (1.04–1.30) in those with chronic kidney disease and was
1.30 (1.13–1.49) in those without. This suggests that even among those with
“normal” kidney function as indicated by creatinine-based GFR, 2M levels may
predict the risk of stroke. However, in this study, we explored the association
between 2M and stroke mortality, and expand on previously prior findings from
stroke incidence to stroke mortality by demonstrating that baseline 2M levels
were associated with risk of stroke mortality, during a median follow-up of 19.4
years. The HR (95% CI) associated with Q5 compared to Q1 of serum 2M was 3.46
(1.34, 8.95).
This study indicated that higher 2M concentrations were positively associated
with all-cause mortality. Previous studies have also indicated that the 2M was
positively associated with all-cause mortality in participants with chronic
kidney disease , HR and 95% CI: 2.52 (1.89, 3.36) [9], chronic obstructive lung
disease, HR and 95% CI: 1.09 (1.05, 1.14) [10], diabetes, HR and 95% CI: 7.35
(1.01, 53.38) [33], cancer, HR and 95% CI: 1.25 (1.06–1.47) [34], hemodialysis
mortality, HR and 95% CI: 1.09 (1.05–1.14) [35], and CVD, RR and 95% CI: 2.29
(1.51–3.49) [36]. Circulating 2M is a potential biomarker that reflects the
oxidative stress, or dialysate contamination, that involved in mucosal immunity,
tumor monitoring, immunoglobulin and albumin homeostasis [37]. In addition, the
increase of 2M was also closely related to the inflammatory response and the
decrease of glomerular filtration rate (GFR) [9, 10, 38, 39]. In addition, other
studies also show that a wide range of inflammatory risk factors increase the
risk of all-cause mortality [40, 41]. The present study found significant
associations between higher 2M levels and increased risks of all-cause mortality
even after adjustment for inflammatory markers (e.g., serum creatinine,
c-reactive protein, and GFR), which is generally consistent with the results of
previous studies [8, 42].
In subgroup analyses, the association of 2M and stroke mortality was
independent of sociodemographic and general stroke risk factors (p 0.05). However, the association between 2M with all-cause
mortality varied age, ratio of family income to poverty, smoking status, and
history of hypertensive, and were stronger in participants with aged 60 years,
non-married, ratio of family income to poverty 1.58, current smokers, or
non-hypertension. A large number of studies have shown that age is closely
related to a variety of chronic diseases [43, 44, 45]. However, we found that stronger
association between 2M and all-cause mortality in participants aged 60 years
in this study. This relationship is not surprising because the study speculated
that age may hide the association between 2M and all-cause mortality in
participants aged 60 years, due to the additional effect of age on
all-cause mortality.
The importance of socio-economic status as predictor for all-cause mortality has
been emphasized by many studies [24, 46]. High ratio of family income to poverty
symbolized low family income. Previous research showed that health inequalities
from income inequality exist in low- and middle-income countries as well as in
high-income countries [43]. Income fluctuations may have a general impact on
health, and may be mediated by physiological changes, psychological changes or
health care, including worse mental status, overall quality of life, access to
health care, and mortality. Previous studies found that clinical or drug
treatment of chronic diseases effectively reduced the concentration of 2M
[40, 47, 48]. However, some low-income populations with chronic diseases may
abandon clinical or drug treatment and health care services to deal with
unexpected financial instability, resulting in high 2M and increased disease
risk or disease deterioration.
Smoking and hypertension are strong risk factors of mortality in the United
States [49, 50]. Our study found stronger significant association with 2M and
all-cause mortality in current smokers, which was consistent with Paweł
Wańkowicz’s current study [3]. However, the detailed impact mechanism is
still unclear and needs further exploration. To the contrary, we observed a
stronger association of 2M with all-cause mortality in non-hypertensive
participants than hypertensive participants. There are two possible reasonable
explanations for this result. First of all, patients with hypertension may suffer
from other chronic diseases before treatment, such as hyperlipidemia,
atherosclerosis, and diabetes. Therefore, effective prevention and treatment of
risk factors such as blood glucose, blood lipid and blood pressure for patients
with chronic diseases and general healthy people can reduce the disease burden
and mortality in the future [50]. Second, previous studies indicated that blood
pressure treatment was closely associated with serum 2M concentrations,
effectively reducing the concentration of 2M [47, 51, 52]. Therefore, we speculate
that the changes of function and physiological tissue structure in hypertensive
population may mask the association of 2M and all-cause mortality due to
additional blood pressure treatment.
In summary, the evidences provided in this study showed the importance of 2M to
overall health, especially cerebrovascular disease health, and supported that 2M
is an effective predictor of stroke and all-cause mortality. In recent years, as
a rare cause, blood elements are closely related to the occurrence of stroke, but
the association between 2M and stroke mortality is relatively lacking, so our
research will help to further explain the relationship between blood diseases and
stroke to some extent. Additionally, 2M may participate in the inflammatory
response in the humoral microenvironment and interact with other antigenic bodies
or immune molecules. Therefore, it is necessary to further explore the mechanism
of 2M and its therapeutic effect in clinical practice. Considering that the
pathophysiology, prognosis and clinical characteristics of acute small vessel
ischemic stroke are different from other types of cerebral infarction, and
lacunar infarction is the stroke subtype with the best functional prognosis. An
indispensable research in the future will be to evaluate the correlation between
2M and lacunar and non lacunar acute stroke.
5. Conclusions
High 2M levels were associated with an increased risk of stroke and all-cause
mortality in U.S. adults of aged 40 year in this study. Future studies
are needed to further elucidate the mechanisms by which 2M increases the risk of
stroke and all-cause mortality, to measure changes in 2M levels during
follow-up, and to determine whether 2M levels can be modified through
interventions.
6. Strengths and Limitations
The strengths of our study included the use of a multi-stage, complex
prospective cohort study based on population aged 40 years in the U.S.,
the combined large sample size, and the long follow-up period. In addition, when
the study excluded deaths during the first 2 years of follow-up, participants
with major chronic diseases (hypertension and diabetes mellitus) at baseline, and
GFR 60 mL/min/1.73 m, the associations have not changed substantially,
indicating the obvious robustness of our results, and the statistical type II
error minimized.
The study also needs to acknowledge several limitations. First, the serum 2M
concentration was measured only at baseline, and participants’ serum 2M may have
changed during follow-up due to changes in living habits and diet. Second,
residual confounding was likely, although a number of covariates were adjusted
(for example, aspirin use, hormone replacement therapy, and dietary pattern).
Third, mortality outcomes were determined through linkage to the National Death
Index with a probabilistic matching algorithm to determine the mortality status
that may result in some misclassification, which may exaggerate or narrow the
observed findings. Fourth, due to the limitation of death data, the study did not
analyze stroke subtypes, such as ischemic and hemorrhagic strokes.
Abbreviations
NHANES, the National Health and Nutrition Examination Survey; NCHS, the National
Center for Health Statistics; CVD, cardiovascular disease; 2M, serum beta-2
microglobulin; BMI, body mass index; FFQ, food frequency questionnaire; HEI-2010,
the Healthy Eating Index-2010; ICD-10, the International Classification of
Diseases, 10th revision; HR, hazard ratio; CI, 95% confidence interval; Q1,
lower quartile; Q2, second quartile; Q3, third quartile; Q4, fouth quartile.
Author Contributions
YNZ and XBZ contributed to the conception of the study. XBZ analyzed the data
and YNZ wrote the manuscript. KYL, WZM, SYL, JZ, MY, FZ, JHC, BX and ESE
contributed to editorial changes in the manuscript. YNZ and XBZ revised the final
manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
The study protocol was approved by Wuhan University of Science and Technology
Medical Ethics Review Form (No. 202195). All participants provided written informed consent.
Acknowledgment
We would like to thank the National Center for Health Statistics and each of the
survey teams and study participants who made this analysis possible. We also
thank our colleagues from Osaka University Center of Medical Data Science,
Advanced Clinical Epidemiology Investigator’s Research Project, for providing
their insight and expertise for our research.
Funding
This study was supported by the National Social Science Fund of China in 2015
(grant number: 15BSH057).
Conflict of Interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5.