†These authors contributed equally.
Academic Editor: Jerome L. Fleg
Background: The aim of the present study was to investigate whether
intra-aortic balloon pump (IABP) support was associated with better outcomes
after rotational atherectomy (RA) in patients with multivessel disease and low
left ventricular ejection fraction (LVEF). Methods: Between January 2015
and December 2021, 596 consecutive patients with severely calcified coronary
lesions who underwent elective RA were retrospectively enrolled. Of these, a
total of 156 patients were included in this study based on the propensity score
matching and divided into two groups according to elective IABP insertion (IABP
group, n = 80) or no insertion (non-IABP group, n = 76) before the RA procedure.
The primary endpoints were procedural success and major adverse cardiovascular
events (MACE) before discharge. The secondary endpoints were mortality and
readmission due to heart failure (HF) during 90-day and 180-day follow-up.
Results: 77 of patients (96.3%) in the IABP group and 72 of patients
(94.7%) in the non-IABP group got procedural success (p = 0.714),
separately. We had not observed significant differences in periprocedural
complications except for less frequent hypotension in the IABP group (p
Moderate or severe calcified coronary lesions occurred in approximately 20% to 38% of cases in patients who underwent percutaneous coronary intervention (PCI) [1, 2]. Although Rotational atherectomy (RA) is recommended to process heavily calcified lesions by American Heart Association 2011 guidelines for PCI [3]. Worse cardiovascular outcomes including significant mortality rates after RA are noted in patients with multivessel disease and impaired left ventricular (LV) function [4]. These patients have poor reserve to withstand the consequences of ischemia resulting from RA procedures. Hypotension, heart failure, and even cardiogenic shock (CS) may often occur in these patients.
The role of intra-aortic balloon pump (IABP) in augmenting coronary blood flow, decreasing myocardial oxygen demand, and maintaining hemodynamic stability is established. Additionally, IABP was uniquely effective in the treatment of cardiogenic shock complicating acute myocardial infarction (AMI). Nevertheless, the strategy of routine IABP placement before PCI (prophylactic IABP) in high-risk and complex coronary lesions is still controversial [5, 6], and its influence on the in-hospital and short-term outcomes following RA has not been well evaluated.
Therefore, the current study was carried out to assess the potential usefulness of IABP support to improve clinical outcomes after RA in patients with multivessel disease and reduced left ventricular ejection fraction (LVEF).
Between January 2015 and December 2021, 579 consecutive patients who received RA
therapy for severely calcified coronary lesions were retrospectively screened in
our institution. Inclusion criteria were as following: (1) The length of
calcified lesions
 Fig. 1.
Fig. 1.Study flow chart. Abbreviation: RA, rotational atherectomy; LVEF, left ventricular ejection fraction; IABP, intra-aortic balloon pump.
All RA procedures were performed by three senior experienced interventional
cardiologists with the Rotablator system (Boston Scientific Corporation, Natick,
MA, USA). The arterial access site was chosen based on peripheral vascular
conditions and procedural requirements. Initial RA burr size was either 1.25 mm,
1.5 mm, or rarely 1.75 mm according to senior operators’ selection, then the burr
was advanced proximally to the lesion, and moved forward with a slow pecking
motion. The initial burr speed was set within the range from 140,000 to 180,000
rpm with the duration of each run less than 30 s, and a decrease in rotational
speed
Severely calcified lesions were either visually assessed by coronary
angiography, defined as radiopacities noted without cardiac motion before
contrast injection, or Intra-vascular ultrasound (IVUS) indicated superficial
calcium involving more than 3 quadrants. Planned RA was defined as RA performed
directly before balloon predilation, while bailout RA was RA performed after
failure to balloon predilation or stents deliver to target lesions. Slow flow/no
re-flow was defined as less than Thrombolysis in Myocardial Infarction (TIMI) III
flow grade in the absence of dissection or thrombus immediately after RA. A final
residual stenosis
All patients were closely followed at 90-day and 180-day intervals after
discharge. Follow-up information was obtained by clinicians through outpatient
clinic visits, phone interviews, and hospital medical records. The primary
endpoints of the present study included procedural success, and in-hospital major
adverse cardiovascular events (MACE). MACE consisted of cardiac death, heart
failure, target vessel revascularization (TVR), and stent thrombosis (ST). Unless
a non-cardiac origin was surely documented, death was considered to be cardiac in
origin. Deterioration in signs and symptoms of in patients with previous chronic
heart failure (CHF) or new-onset heart failure (HF) requiring urgent therapy was
considered as in-hospital HF. Diagnostic criteria was based on an intravenous
administration of diuretic drugs, vasodilators, or inotropic drugs, and including
at least one of the followings: cardiac pulmonary edema or pulmonary vascular
congestion on chest radiograph; rales
The SPSS 26.0 system (IBM, Armonk, NY, USA) was utilized for statistical
calculations. A logistic model was used to calculate the probability of receiving
a IABP support before RA procedure (the propensity score). Baseline
characteristics including age, male, hypertension, diabetes mellitus (DM), atrial
fibrillation (AF), history of HF, pre-MI, pre-PCI, chronic kidney disease (CKD),
LVEF, NT-proBNP, systolic blood pressure (SBP) before RA, target vessel (LAD,
LCX, or RCA), and diseased vessels (two or three) were set as covariates. Based
on the propensity score in a 1:1 (IABP:Non-IABP) fashion, the nearest neighbor
matching was performed with a maximum caliper of 0.2. Categorical variables were
reported as value (percentage) and Chi-squared or Fisher’ exact test was
utilized. If the continuous variables were normally distributed determined by the
Wilk-Shapiro test, they were reported as a mean
Baseline demographics, comorbidities, and results of laboratory test were
presented in Table 1. More frequent history of prior MI (26.3% vs. 7.9%,
p = 0.002), more often CHF (42.5% vs. 5.3%, p
| Variables | All (n = 156) | Non-IABP group (n = 76) | IABP group (n = 80) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 72.3 |
72.8 |
71.9 |
0.544 | |
| Male, n (%) | 94 (60.3) | 49 (64.5) | 45 (56.3) | 0.294 | |
| Hypertension, n (%) | 120 (76.9) | 62 (81.6) | 58 (72.5) | 0.179 | |
| Diabetes mellitus, n (%) | 60 (38.5) | 33 (43.4) | 27 (33.8) | 0.215 | |
| Atrial fibrillation, n (%) | 16 (10.3) | 6 (7.9) | 10 (12.5) | 0.343 | |
| Smoking, n (%) | 56 (35.9) | 28 (36.8) | 28 (35.0) | 0.811 | |
| Heart failure, n (%) | 38 (24.4) | 4 (5.3) | 34 (42.5) | ||
| LVEF (%) | 33.8 |
34.0 |
33.6 |
0.067 | |
| CKD, n (%) | 7 (4.5) | 3 (3.9) | 4 (5.0) | 1.000 | |
| Dialysis, n (%) | 2 (1.3) | 1 (1.3) | 1 (1.3) | 1.000 | |
| Pre-MI, n (%) | 27 (17.3) | 6 (7.9) | 21 (26.3) | 0.002 | |
| Pre-PCI, n (%) | 62 (39.7) | 28 (36.8) | 34 (42.5) | 0.470 | |
| Stroke, n (%) | 52 (33.3) | 25 (32.9) | 27 (33.8) | 0.910 | |
| Medication, n (%) | |||||
| ACEI/ARB | 78 (50.0) | 41 (53.9) | 37 (46.3) | 0.337 | |
| CCB | 45 (28.8) | 31 (40.8) | 14 (17.5) | 0.001 | |
| Nitrates | 76 (48.7) | 44 (57.9) | 32 (40.0) | 0.025 | |
| 91 (58.3) | 43 (56.6) | 48 (60.0) | 0.665 | ||
| Statins | 154 (98.7) | 76 (100.0) | 78 (97.5) | 0.497 | |
| Aspirin | 156 (100.0) | 76 (100.0) | 80 (100.0) | - | |
| Clopidogrel | 78 (50.0) | 43 (56.6) | 35 (43.8) | 0.109 | |
| Ticagrelor | 78 (50.0) | 33 (43.4) | 45 (56.3) | 0.109 | |
| TC (mmol/L) | 3.8 |
3.7 |
3.9 |
0.081 | |
| TG (mmol/L) | 1.4 |
1.4 |
1.4 |
0.803 | |
| LDL-C (mmol/L) | 1.9 |
1.8 |
2.1 |
0.036 | |
| HDL-C (mmol/L) | 1.1 |
1.0 |
1.1 |
0.719 | |
| Creatinine (umol/L) | 75 (61.0–90.0) | 73.0 (59.0–90.0) | 76.0 (63.0–94.5) | 0.215 | |
| NT-proBNP (pg/mL) | 568.5 (118.0–1453.1) | 284.0 (75.8–904.5) | 1024.0 (201.3–2684.3) | ||
| SBP before RA (mmHg) | 137.4 |
138.9 |
135.9 |
0.401 | |
| DBP before RA (mmHg) | 71.2 |
71.5 |
70.9 |
0.783 | |
| HR before RA (bpm) | 76.6 |
74.9 |
78.3 |
0.133 | |
| Admission to procedure (days) | 3.1 |
3.0 |
3.1 |
0.112 | |
| LVEF, left ventricular ejection fraction; CKD, chronic kidney disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; TC, total cholesterol; TG, triglyceride; LDL-C, low density lipoprotein-cholesterol; HDL-C, high density lipoprotein-cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rates; IABP, Intra-aortic balloon pump. | |||||
Table 2 showed angiographic and procedural
characteristics. The incidence of procedural success was similar (94.7% vs.
96.3%, p = 0.714) in the two groups. Of note, higher post-procedural
SBP was observed in the IABP group (112.7
| Variables | All (n = 156) | Non-IABP group (n = 76) | IABP group (n = 80) | p-value | |
|---|---|---|---|---|---|
| Target vessel, n (%) | |||||
| LAD | 136 (87.2) | 65 (85.5) | 71 (88.8) | 0.547 | |
| LCX | 5 (3.2) | 3 (3.9) | 2 (2.5) | 0.676 | |
| RCA | 15 (9.6) | 8 (10.5) | 7 (8.8) | 0.707 | |
| Diseased vessels, n (%) | 0.245 | ||||
| Two | 31 (19.9) | 18 (23.7) | 13 (16.2) | ||
| Three | 125 (80.1) | 58 (76.3) | 67 (83.8) | ||
| Reference diameter (mm) | 2.86 |
2.87 |
2.85 |
0.724 | |
| MLD (mm) | 0.49 |
0.54 |
0.45 |
0.078 | |
| Stenosis, % | 82.3 |
80.9 |
83.7 |
0.175 | |
| Lesion length (mm) | 36.3 |
36.3 |
36.4 |
0.933 | |
| Angulation |
86 (55.1) | 45 (59.2) | 41 (51.2) | 0.318 | |
| Primary RA, n (%) | 101 (64.7) | 50 (65.8) | 51 (63.7) | 0.790 | |
| Burr number, n (%) | 0.395 | ||||
| 1 | 145 (92.9) | 72 (94.7) | 73 (91.3) | ||
| 2 | 11 (7.1) | 4 (5.3) | 7 (8.8) | ||
| Final burr size, n (%) | |||||
| 1.25 mm | 39 (25.0) | 16 (21.1) | 23 (28.7) | 0.267 | |
| 1.5 mm | 108 (69.2) | 56 (73.7) | 52 (65.0) | 0.240 | |
| 1.75 mm | 9 (5.8) | 4 (5.3) | 5 (6.3) | 1.000 | |
| Total run time (s) | 42.0 (30, 65.5) | 38.5 (27.2, 63) | 45.0 (33.5, 66.8) | 0.110 | |
| Mean rotational speed ( |
15.2 |
15.2 |
15.1 |
0.958 | |
| Rotablations times | 3.9 |
3.7 |
4.0 |
0.338 | |
| IVUS guided, n (%) | 20 (12.8) | 9 (11.8) | 11 (13.8) | 0.722 | |
| SBP in RA (mmHg) | 103.1 |
94.3 |
112.7 |
||
| DBP in RA (mmHg) | 66.2 |
65.8 |
66.5 |
0.764 | |
| HR in RA (bpm) | 68.8 |
67.6 |
70.1 |
0.320 | |
| Vasopressor usage n (%) | 16 (10.3) | 9 (11.8) | 7 (8.8) | 0.525 | |
| Procedural success n (%) | 149 (95.5) | 72 (94.7) | 77 (96.3) | 0.714 | |
| LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; MLD, minimal luminal diameter; IVUS, intravascular ultrasound; RA, rotational atherectomy; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rates. | |||||
Table 3 summarized outcomes of in-hospital, 90-day and 180-day follow-up.
Clinical follow-up was accomplished in all cases. Hypotension was less frequently
observed in the IABP group (13.8% vs. 53.9%, p
| Variables | All (n = 156) | Non-IABP group (n = 76) | IABP group (n = 80) | p-value | |
|---|---|---|---|---|---|
| Periprocedural complications | |||||
| Slow flow/no re-flow | 63 (40.4) | 35 (46.1) | 28 (35.0) | 0.160 | |
| Hypotension | 52 (33.3) | 41 (53.9) | 11 (13.8) | ||
| Bradycardia | 29 (18.6) | 14 (18.4) | 15 (18.8) | 0.958 | |
| Complete AV block | 1 (0.6) | 1 (1.3) | 0 (0) | 0.487 | |
| Sinus Arrest | 0 (0) | 0 (0) | 0 (0) | - | |
| Dissection | 24 (15.4) | 14 (18.4) | 10 (12.5) | 0.306 | |
| Perforation | 6 (3.8) | 3 (3.9) | 3 (3.8) | 1.000 | |
| Burr entrapment | 0 (0) | 0 (0) | 0 (0) | - | |
| Coronary spasm | 52 (33.3) | 28 (36.8) | 24 (30.0) | 0.365 | |
| In-hospital outcomes | |||||
| MACE | 26 (16.7) | 20 (26.3) | 6 (7.5) | 0.002 | |
| Heart failure | 23 (14.7) | 18 (23.7) | 5 (6.3) | 0.002 | |
| ST | 0 (0) | 0 (0) | 0 (0) | - | |
| TLR | 4 (2.6) | 2 (2.6) | 2 (2.5) | 1.000 | |
| Death | 4 (2.6) | 3 (3.9) | 1 (1.3) | 1.000 | |
| Periprocedural myonecrosis | 48 (30.8) | 26 (34.2) | 22 (27.5) | 0.364 | |
| Admission days | 6.3 |
7.1 |
5.6 |
||
| Outcomes within 90-day follow up | |||||
| Readmission | 37 (23.7) | 28 (36.8) | 9 (11.3) | ||
| All-cause mortality | 21 (13.5) | 13 (17.1) | 8 (10.0) | 0.194 | |
| Outcomes within 180-day follow up | |||||
| Readmission | 43 (27.6) | 29 (38.2) | 14 (17.5) | 0.004 | |
| All-cause mortality | 22 (14.1) | 14 (18.4) | 8 (10.0) | 0.131 | |
| AV, atrioventricular; MACE, major adverse cardiovascular events; ST, stent-thrombosis; TLR, target lesion revascularization. | |||||
Compared to the non-IABP group, in-hospital MACE was less frequently observed in the IABP group (7.5% vs. 26.3%, p = 0.002), mainly driven by in-hospital HF (6.3% vs. 23.7%, p = 0.002), as shown in Table 3. Compared to the non-IABP group, the incidence of periprocedural myonecrosis tended to be lower (27.5% vs. 34.2%, p = 0.364). No significant difference as for cardiac death and TVR were observed between the two groups, and stent thrombosis was observed in neither group.
The Kaplan-Meier analysis showed a significantly lower incidence of readmission due to HF in the IABP group during the 90-day follow-up (log-rank test: p = 0.002, HR = 0.32, 95% CI: 0.17–0.61, Fig. 2A).
 Fig. 2.
Fig. 2.Kaplan-Meier curves estimate incidence of readmission due to HF for patients undergoing elective RA with and without IABP support. (A) Kaplan-Meier curves of cumulative incidence of readmission due to HF within 90-day follow-up. (B) Kaplan-Meier curves of cumulative incidence of readmission due to HF within 180-day follow-up. Abbreviations: IABP, intra-aortic balloon pump; HF, heart failure; RA, rotational atherectomy; HR, hazard ratio.
In addition, the incidence was also significantly lower (log-rank test: p = 0.013, HR = 0.48, 95% CI: 0.27–0.88, Fig. 2B) during the 180-day follow-up.
Furthermore, Kaplan-Meier analysis showed that the cumulative survival rates within 90-day follow up were not different between the two groups (p = 0.274, Fig. 3).
 Fig. 3.
Fig. 3.Kaplan-Meier curves for cumulative survival rates within 90-day follow-up. Abbreviations: HR, hazard ratio; IABP, intra-aortic balloon pump.
Fig. 4 summarized the incidence of in-hospital HF, readmission due to HF at 90-day and 180-day intervals.
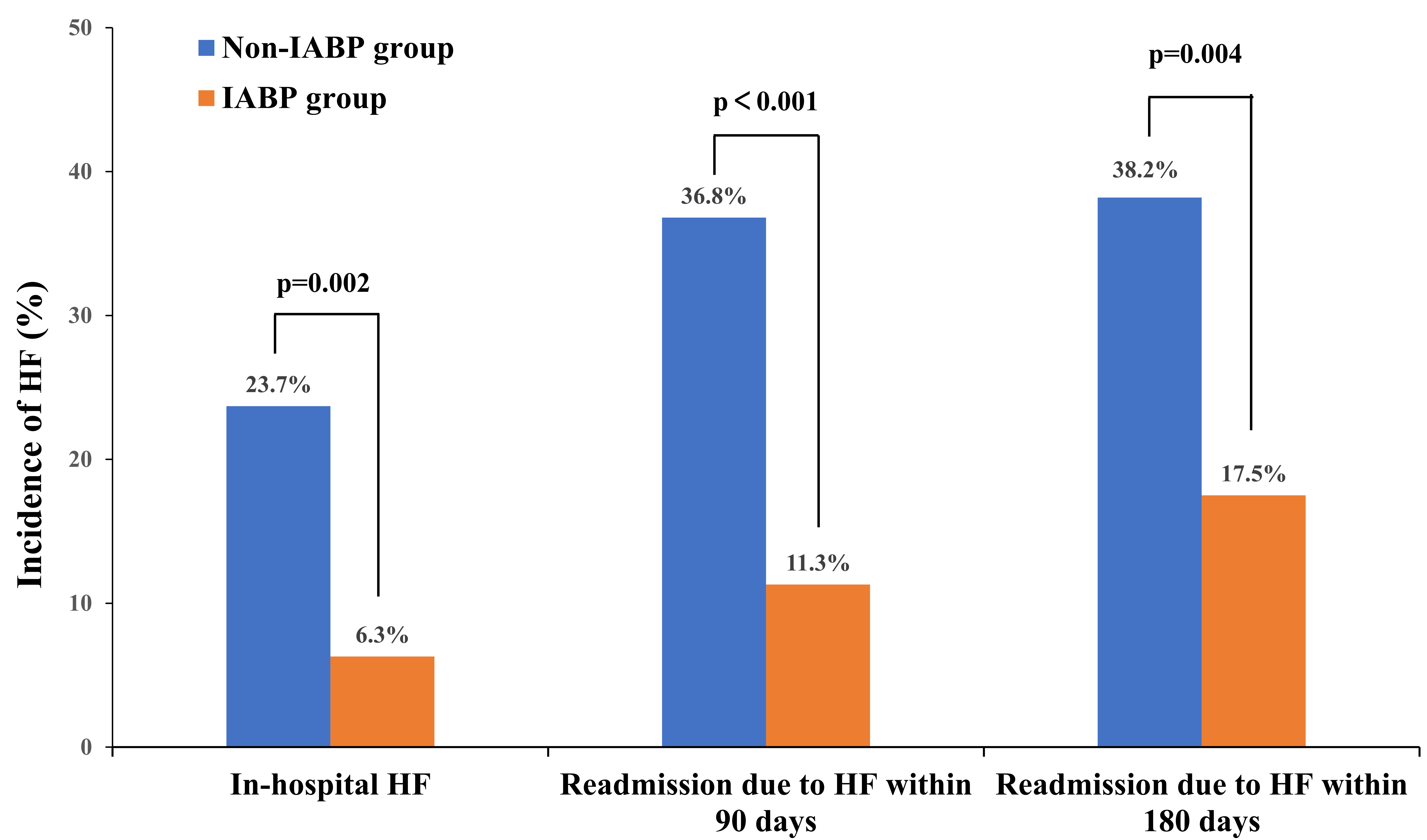 Fig. 4.
Fig. 4.Incidence of in-hospital HF, readmission due to HF at 90-day and 180-day intervals. Abbreviations: IABP, intra-aortic balloon pump; HF, heart failure.
Cox multivariate analysis was performed to investigate influential factors of readmission due to HF during the 90-day and 180-day follow-up. The analysis determined that IABP support (HR = 0.34, 95% CI: 0.15–0.76, p = 0.008), in-hospital HF (HR = 3.28, 95% CI: 1.29–8.36, p = 0.013), and periprocedural myonecrosis (HR = 4.26, 95% CI: 1.60–11.35, p = 0.004) were independently associated with readmission due to HF within 90-day follow up (Table 4).
| Variables | Univariate cox regression analyses | Multivariate cox regression analyses | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| IABP implantation | 0.25 (0.12–0.54) | 0.34 (0.15–0.76) | 0.008 | |
| Primary RA | 0.52 (0.27–0.99) | 0.048 | 0.72 (0.37–1.39) | 0.325 |
| In-hospital heart failure | 13.2 (6.75–25.76) | 3.28 (1.29–8.36) | 0.013 | |
| Periprocedural myonecrosis | 8.42 (4.06–17.45) | 4.26 (1.60–11.35) | 0.004 | |
| HR, Hazard ratio; CI, confidence interval; HF, heart failure; IABP, intra-aortic balloon pump; RA, rotational atherectomy. | ||||
Additionally, IABP implantation (HR = 0.47, 95% CI: 0.24–0.92, p = 0.028), in-hospital HF (HR = 3.50, 95% CI: 1.43–8.58, p = 0.006), and periprocedural myonecrosis (HR = 3.20, 95% CI: 1.34–7.67, p = 0.009) were independent predictors of readmission due to HF during 180-day follow up (Table 5).
| Variables | Univariate cox regression analyses | Multivariate cox regression analyses | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| IABP implantation | 0.37 (0.20–0.71) | 0.003 | 0.47 (0.24–0.92) | 0.028 |
| Primary RA | 0.47 (0.26–0.85) | 0.012 | 0.61 (0.33–1.13) | 0.113 |
| In-hospital heart failure | 11.25 (6.00–21.09) | 3.50 (1.43–8.58) | 0.006 | |
| Periprocedural myonecrosis | 6.14 (3.26–11.54) | 3.20 (1.34–7.67) | 0.009 | |
| HR, Hazard ratio; CI, confidence interval; HF, heart failure; IABP, intra-aortic balloon pump; RA, rotational atherectomy. | ||||
In recent years, interventional cardiologists are paying more attention to the
revascularization of complex and high-risk coronary diseases. A universally
agreed definition of high-risk PCI is still on debate, they may present with
severely calcified, multivessel coronary disease and reduced ejection fraction
(LVEF
Theoretically, IABP serves to rise myocardial perfusion by augmenting the coronary pressure gradient from the aorta to the epicardial coronary circulation and reducing the afterload of LV by active deflation immediately before the onset of LV systole [11, 12]. However, the role of IABP support in improving clinical outcomes of RA for complex and high-risk coronary interventions is still controversial [13, 14]. The present study investigated the impact of IABP support on in-hospital, 90-day, and 180-day outcomes after RA in patients with multivessel disease and reduced LVEF.
In the present study, all subjects were presented with high-risk and complex lesions, the IABP group had more patients with a history of pre-MI and chronic heart failure, the NT-pro BNP level was also higher, reflecting a worse cardiac function, which was the reason why more prophylactic IABP was used in these patients.
Although RA could be successfully performed in patients with impaired LV function without hemodynamic support according to Hoyle L
et al. [14], more bailout hemodynamic support, according to the subgroup
analysis, was required in the patients with impaired LV systolic function.
Moreover, microvascular embolization by a large amount of debris can cause
microvascular dysfunction and adversely affect the cardiac function during the RA
procedure. Nevertheless, the compensation mechanism cannot be established in time
and subsequently, hemodynamic compromise may occur. Therefore, patients in the
present study were at high risk of hemodynamic instability since they were all
presented with impaired LV systolic function (LVEF
Patients receiving prophylactic IABP implantation showed better in-hospital outcomes in this study. The rates of in-hospital MACE were significantly lower in the IABP group (7.5% vs. 26.3%, p = 0.002), and most of the MACEs were both driven by in-hospital heart failure in the two groups. There are two possible explanations for why IABP support positively affects the in-hospital prognosis in these high-risk patients. Firstly, IABP counterpulsation plays a vital role in maintaining cardiac output by reduction of the afterload (with reduced oxygen consumption and myocardial ischemia), as confirmed by a lower incidence of post-procedure hypotension in the IABP group, which may augment coronary perfusion afterwards and contribute to a decrease in ischemia [15]. Secondly, previous studies revealed that slow-flow/no-reflow during RA is mainly associated with the distal embolization of microparticulate debris [16, 17]. Since coronary blood flow occurs predominantly in diastole, IABP gives rise to the coronary pressure and increases coronary blood flow, which may hence microparticulate debris clarity and subsequently decrease the incidence of slow flow/no reflow. The present study showed a slightly lower incidence of slow flow/no reflow in the IABP group, which may decrease the risk of worsen LV function and subsequent in-hospital heart failure.
For patients who receive PCI, a low LVEF is reported to be an independent
predictor of adverse cardiac events [18]. Although RA can be safely and
effectively performed in patients with low LVEF with similar procedural success
rates and in-hospital mortality [14], the long-term rate of MACEs was
significantly higher, and low LVEF was still an independent predictor of
long-term MACEs, mainly driven by HF requiring rehospitalization [19]. In our
study, all patients were presented with poor LV function (LVEF
The presence of heart failure with decreased LVEF was reported as an independent predictor of mortality following RA and PCI [20, 21]. In the present study, IABP implantation before RA procedure showed a benefit of an absolute 7.1% difference in mortality during 90-day follow- up, but this difference was not statistically significant. Divaka et al. [6] compared the all-cause mortality after RA with IABP versus without IABP support at 6 months and found no significant difference (4.6% vs. 7.4%, p = 0.320), which was consistent with our findings.
This study was a retrospective and observational analysis of data from single center with a limited sample size. There is no doubt that regularly taking medicine is of great importance for patients with CAD and HF. However, the findings from post-operational visit including regular and rational use of medicines were not available for this study, hence, it is difficult to determine the exclusive contributions of IABP to the endpoints. Nevertheless, IABP may play a vital role in maintaining hemodynamic stability during PCI with RA, especially in patients with severely calcified lesions accompanied by multivessel disease and reduced LVEF. We found that IABP was associated with reduced RA-related complications such as slow flow/no re-flow and periprocedural myonecrosis, which may partially improve short-term outcomes. In the future, prospective randomized controlled trails in a large group were needed to furtherly confirm the findings.
The present study suggests the important role of IABP support in improving the outcomes of patients after RA if multivessel disease and low LVEF are anticipated. Prophylactic IABP implantation was related to a lower incidence of in-hospital MACE, and readmission due to HF within 90-day and 180-day follow-up without significant impact on the procedural success and all-cause mortality.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conceptualization, HH, LKM, and JWW; Funding acquisition, LKM; Data collecting, HH, ZQG and JWW; Statistical analysis and writing-original draft, ZQG; Writing-review & editing, ZQG, HH and LKM.
The Institutional Review Board of the first affiliated hospital of USTC approved the data collection of the study (2019KY165) and all patients provided written informed consent to undergo PCI with RA before the procedure.
We thank all the participants of the study and all the peer reviewers for their opinions and suggestions.
This study was supported by research grants from the National Natural Science Foundation of China (No. 81870192 and No. 82170263).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
