Academic Editor: Ferdinando Carlo Sasso
Background: Geometrical alterations in the coronary resistance artery
network and the potential involvement of Tenascin C (TNC) extracellular matrix
protein were investigated in diabetic and control mice. Methods:
Diabetes was induced by streptozotocin (STZ) injections (n = 7–11 animals in
each group) in Tenascin C KO (TNC KO) mice and their Wild type (A/J) littermates.
After 16–18 weeks the heart was removed and the whole subsurface network of the
left coronary artery was prepared (down to branches of 40
Microvascular damage is one of the major severe consequences of diabetes. Diabetic microvascular pathology is characterized by uneven lumen diameter and increased wall thickness [1], local narrowing and dilation with microaneurysms prone to rupture [2, 3, 4], and tortuosity [2, 3, 5]. These conditions certainly hamper local hemodynamics. Histologically, loss of smooth muscle cells, accumulation of collagen and other connective tissue elements, basement membrane thickening, endothelial damage with impaired endothelial dependent dilation and increased permeability are characteristic of diabetes [6, 7]. Statistical-geometric analysis of the retinal microvasculature can be diagnostically important in diabetes [8], however, direct observation of coronary resistance artery geometry in the left ventricular tissue remains uninvestigated. Evidence suggests that a substantial part of the diabetic cardiomyopathy may be attributable to pathological alterations of the coronary resistance arteries. Clinically, reduced coronary flow reserve likely involves diabetic damage to the microvasculature [9, 10, 11]. Thickening of the basement membrane, thickening of the arteriolar media, perivascular fibrosis and microaneurysms [12, 13, 14] have been shown in coronary resistance arteries in diabetic patients and in animal models of diabetes. It is clinically well known that the angiographic macroscopic picture of such coronary angiograms mimics a “winter tree”, with no leaflets on the tree but only the large conductance vessels left. This is paralleled by a pronounced microangiopathy leading to the typical picture of diabetes-induced diffuse fibrosis and cardiomyopathy [12, 13, 14].
With pressure arteriography, Lynch et al. [15] did not find wall
thickening in the 50–150
The aim of the current study was to characterize whether streptozotocin-induced diabetes in mice yields significant geometrical remodeling of the coronary resistance artery system and the effects of TNC on the coronary resistance artery network.
Adult (8–10 weeks old) male TNC KO mice (KO, RBRC00007 A, Experimental Animal Division, Tsukuba, Japan) and their wild-type littermates (Wt, A/J, #000646, The Jackson Laboratory, Sacramento, CA, USA) were used [34, 35, 36]. All animals received a standard laboratory care and were housed in air-conditioned rooms at 22 °C with a 12/12 h day/night cycle, including free access to water and standard mouse chow. The experimental protocol was approved by the regional Ethics Committee for Laboratory Animal Experiment conforming with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Streptozotocin (STZ; 50 mg/kg) was injected intraperitoneally into model group
mice for five consecutive days. All mice were weighted accurately prior to STZ
injection. STZ was weighted according to the body weight and dissolved in sterile
Dulbecco’s PBS (DPBS, Gibco, Life Technologies Ltd, Basel, Switzerland). Because STZ should be
degraded within 20–30 min, the STZ solution was prepared immediately before use
then injected within 5 min. The STZ solution was freshly prepared on daily base.
On experimental day 6, blood glucose was monitored via tail vein blood withdrawal
as described previously [37]. Mice were considered diabetic if blood glucose
levels show
After 16–18 weeks, blood pressure was measured in anesthetized animals (45
mg/kg pentobarbital, i.p.) in the right carotid artery, then the animals were
exsanguinated, the whole vascular system was perfused with heparinized
Krebs-Ringer solution and the heart was removed. The vascular network of the left
coronary artery which in mice is running under the ventricular surface was
carefully microprepared for easy visibility, left in situ, and the orifice was
cannulated. The whole network was perfused with warm, oxygenated Krebs-Ringer
solution at pressures of 70–100 mmHg using a servo-controlled pump (Living
Systems, Burlington, VT, USA). Networks not able to keep at that pressure were
discarded. Vessels down to 40
Transversal histological sections at the middle of the orifice-apex distance were prepared after formaldehyde (Formaldehyde solution 4%, Merck, Darmstadt, Germany) fixation and conventional hematoxylin-eosin staining was performed for left ventricular geometry assessment.
Values are expressed as means
Body weight and heart geometry are shown in Table 1. Body weight decreased after
STZ the injection in Wt and TNC KO mice compared to controls, respectively
(p
| A/J | TNC KO | Two-way ANOVA | |||
| Non-diabetic | Diabetic | Non-diabetic | Diabetic | ||
| Body weight (gramm) | 22.69 |
20.52 |
21.96 |
17.25 |
|
| Arterial blood pressure (mmHg) | 80.5 |
67.1 |
77.9 |
73.2 |
n.s. |
| Heart | |||||
| Orifice-apex long axis (mm) | 5.93 |
5.75 |
5.84 |
5.25 |
n.s. |
| Transversal (mm) | 4.86 |
4.42 |
4.91 |
4.19 |
|
| Left ventricular | |||||
| Hind wall thickness (µm) | 1785 |
1509 |
1924 |
1581 |
|
| Septum thickness (µm) | 1362 |
1224 |
1396 |
1292 |
|
| Right ventricular | |||||
| Wall thickness (µm) | 906 |
793 |
937 |
850 |
n.s. |
The numbers of animals in Wt non-diabetic, Wt diabetic, TNC-KO non-diabetic and TNC-KO diabetic groups were 11, 9, 10, and 7, respectively. | |||||
Successful network preparations were made of 11 Wt non-diabetic, 9 Wt diabetic, 10 TNC KO nondiabetic and 7 TNC KO diabetic mice. Fig. 1 shows a typical network from each group. Morphological features often observed in the diabetic heart, include trifurcations, sharp bends of a larger branch, and branches leading in the retrograde direction with a vectorial component toward the orifice (Fig. 1B). In contrast, TNC KO mice with diabetes did not show significant elevation of the number of any such deformities significantly (Fig. 1D). The respective vascular “abnormalities” are shown at a higher magnification in Fig. 2 and the data summarized in Table 2.
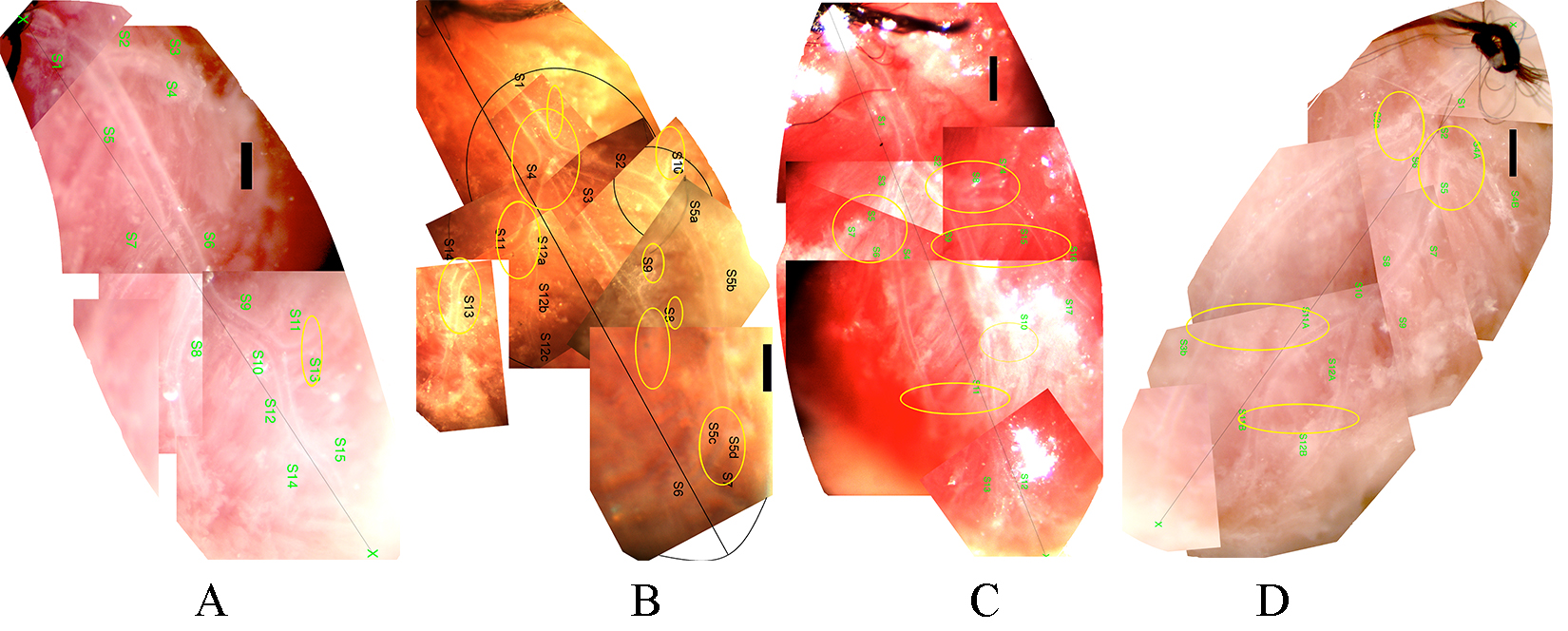 Fig. 1.
Fig. 1.Typical left coronary artery networks of mice, microprepared and
in situ perfused. (A) Wild type (A/J) non-diabetic. (B) Wild type (A/J)
diabetic. (C) Tenascin C KO non-diabetic. (D) Tenascin C KO diabetic mice. Bars,
500
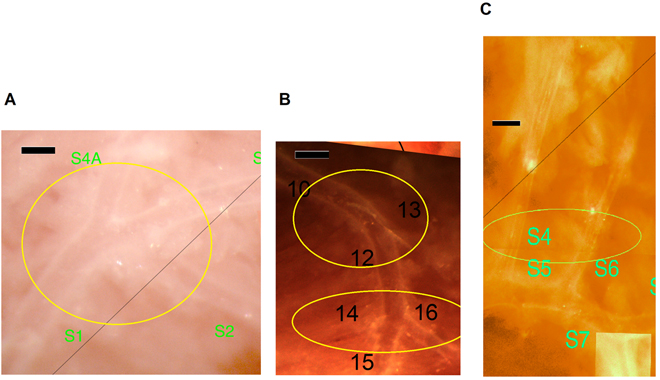 Fig. 2.
Fig. 2.Morphological abnormalities of the coronary arteriole network at
higher magnification. (A) Trifurcation (from a Wt diabetic mouse). (B)
Trifurcation and bending of a larger branch (from a Wt diabetic mouse). (C)
Larger branches running close to each other in parallel (from a TNC KO
non-diabetic mouse). Bars, 200
| Wt | TNC KO | |||
| Non-diabetic (n = 11) | Diabetic (n = 9) | Non-diabetic (n = 10) | Diabetic (n = 7) | |
| Trifurcations | 2 | 14 |
8 |
11 |
| Sharp bending | 7 | 25 |
11 | 13 |
| Retrograde branch | 0 | 9 |
0 | 3 |
| Parallel branches | 1 | 2 | 9 |
3 |
In all four groups, geometry of 187 bifurcations was analyzed. All four groups
adhered to the Murray-law. This law states that the cube of the lumen diameter of
the mother branch is equal with the sum of the cubes of lumen diameters of the
daughter branches. Pairwise comparisons for nondiabetic and diabetic groups as
well as for wild and TNC KO non-diabetic groups are shown in Fig. 3A–C.
Scattered line shows the sum of points where this law is valid. Scatter from this
line was not different for the four observed groups when compared with the F
probe. Fig. 3D shows an analysis of angles. The angle of the axis of the daughter
branch with the axis of the mother branch is plotted against the ratio of lumen
diameters. Fig. 3 also shows that while larger daughter branches
(D
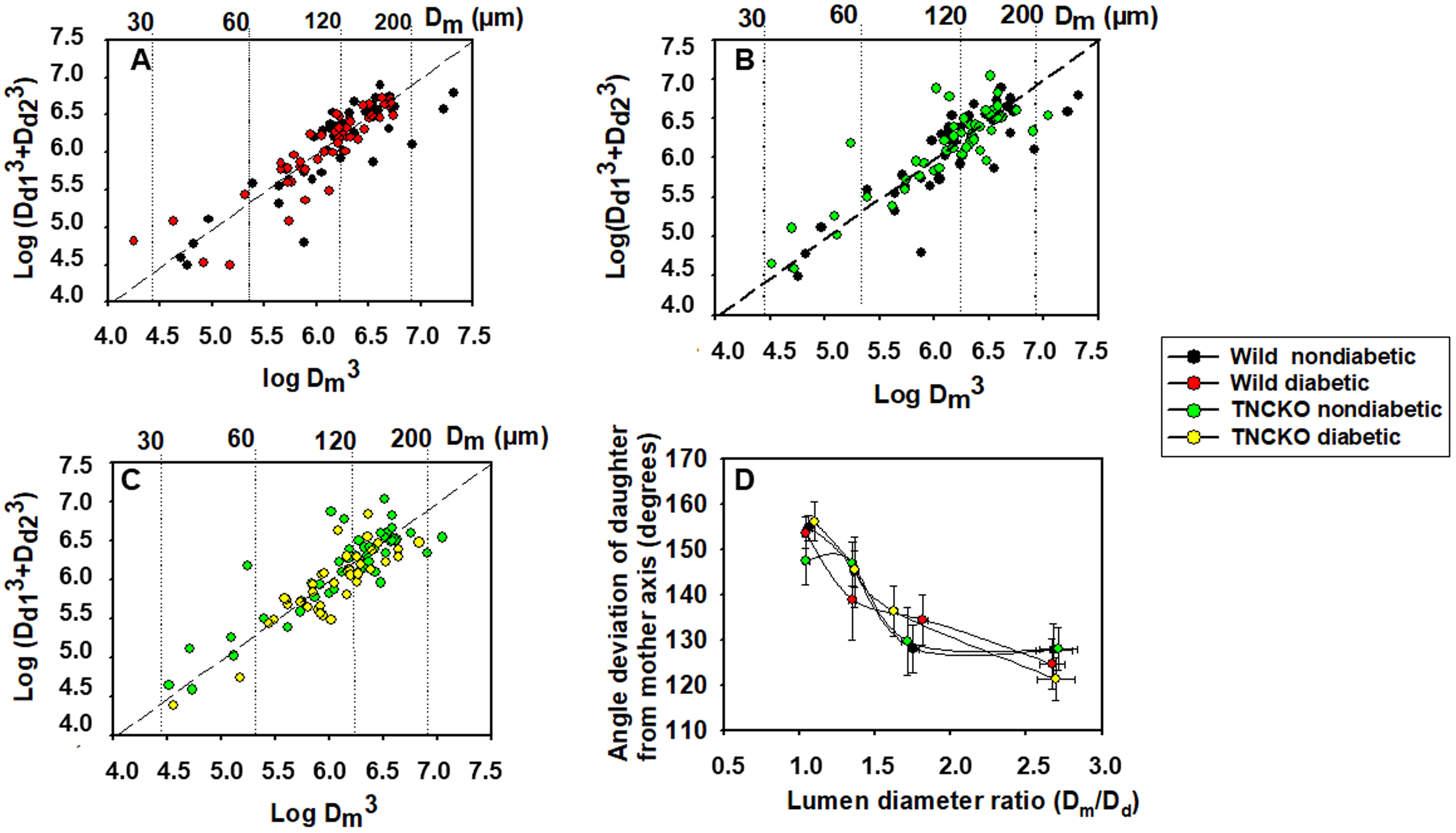 Fig. 3.
Fig. 3.Geometric analysis of bifurcations in coronary arteriole
networks. Sum of the cube of daughter branch diameter plotted against the cube
of the diameter of the mother branch. Logarithmic scales. The Murray–law states
that cube of the lumen diameter of the mother branch (D
A total of 531 resistance artery vascular segments were identified in the
microsurgical preparation subsurface left coronary artery network. Using the high
magnification synthetized pictures (collages, Fig. 1) each segment was divided in
50
There were substantial changes in wall thicknesses upon diabetes and
TNC deletion. Fig. 4 shows the wall thickness frequencies for different
outer diameter ranges in the four groups. The thickening of the wall of largest
vessels (
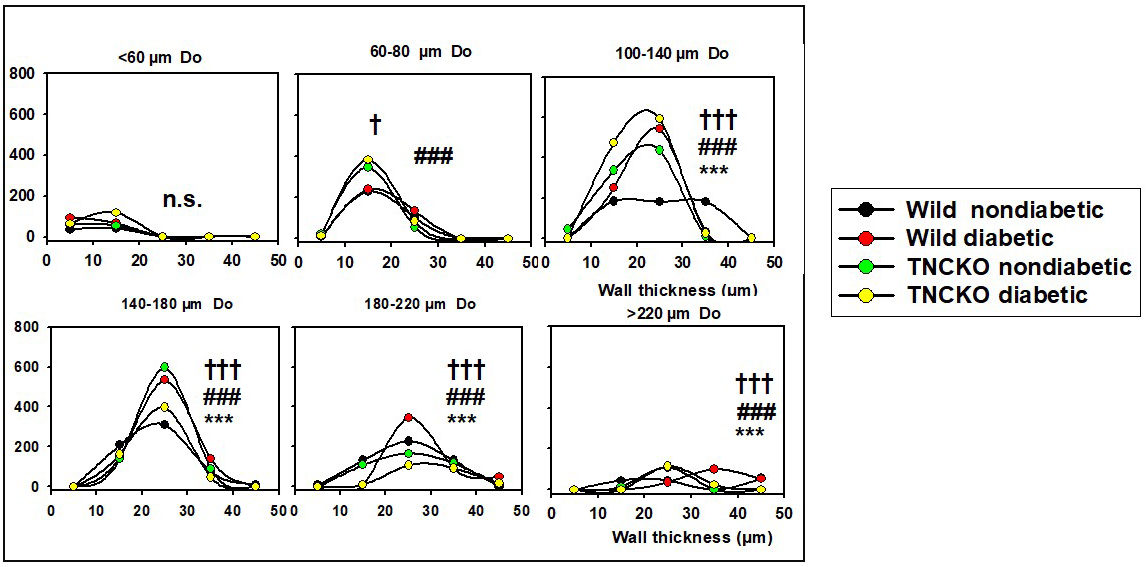 Fig. 4.
Fig. 4.Analysis of wall thicknesses. All network was theoretically
divided into 50
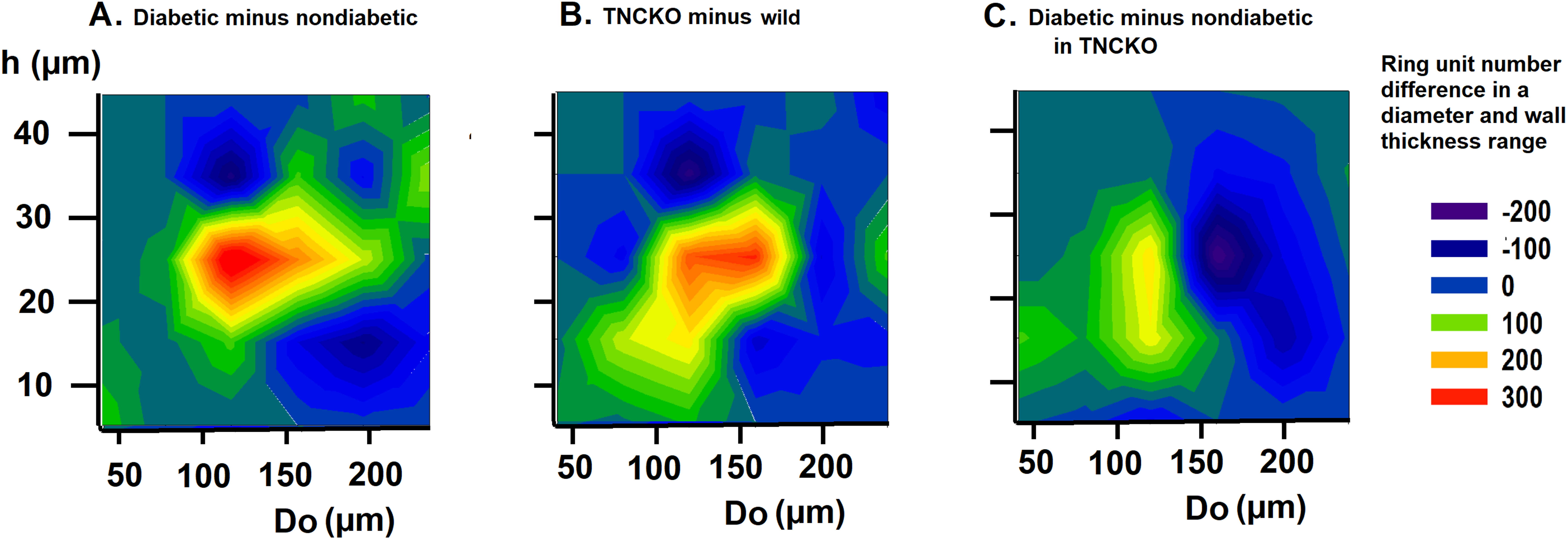 Fig. 5.
Fig. 5.Analysis of wall thicknesses. Differences of bidirectional histograms, showing the difference in the number of ring units for different outer diameter (Do) and wall thickness (h) ranges between diabetic and non-diabetic Wt mice (A), TNC-KO and Wt non-diabetic mice (B) and diabetic and non-diabetic TNC KO mice (C). Further explanation see in Legend of Fig. 4. Red spots mark more, deep blue spots less ring units compared. Note that ring number differences appear in certain diameter and wall thickness ranges.
We investigated in what regions of the network such alterations did take place
in the diabetic and TNCKO heart. Thus, outer diameter frequency histograms have
been constructed for different flow distance ranges. Fig. 6B shows the frequency
of vascular components in distances less than 1… 6 mm flow distance from
the orifice for the non-diabetic Wt, diabetic, Wt non-diabetic, TNC-KO
non-diabetic and diabetic TNC KO groups. Fig. 7 provides the difference of the
number of ring units in a certain flow distance and diameter range between two
groups (differences of two-dimensional histograms). Prominent is the elevation in
the number of 100–200
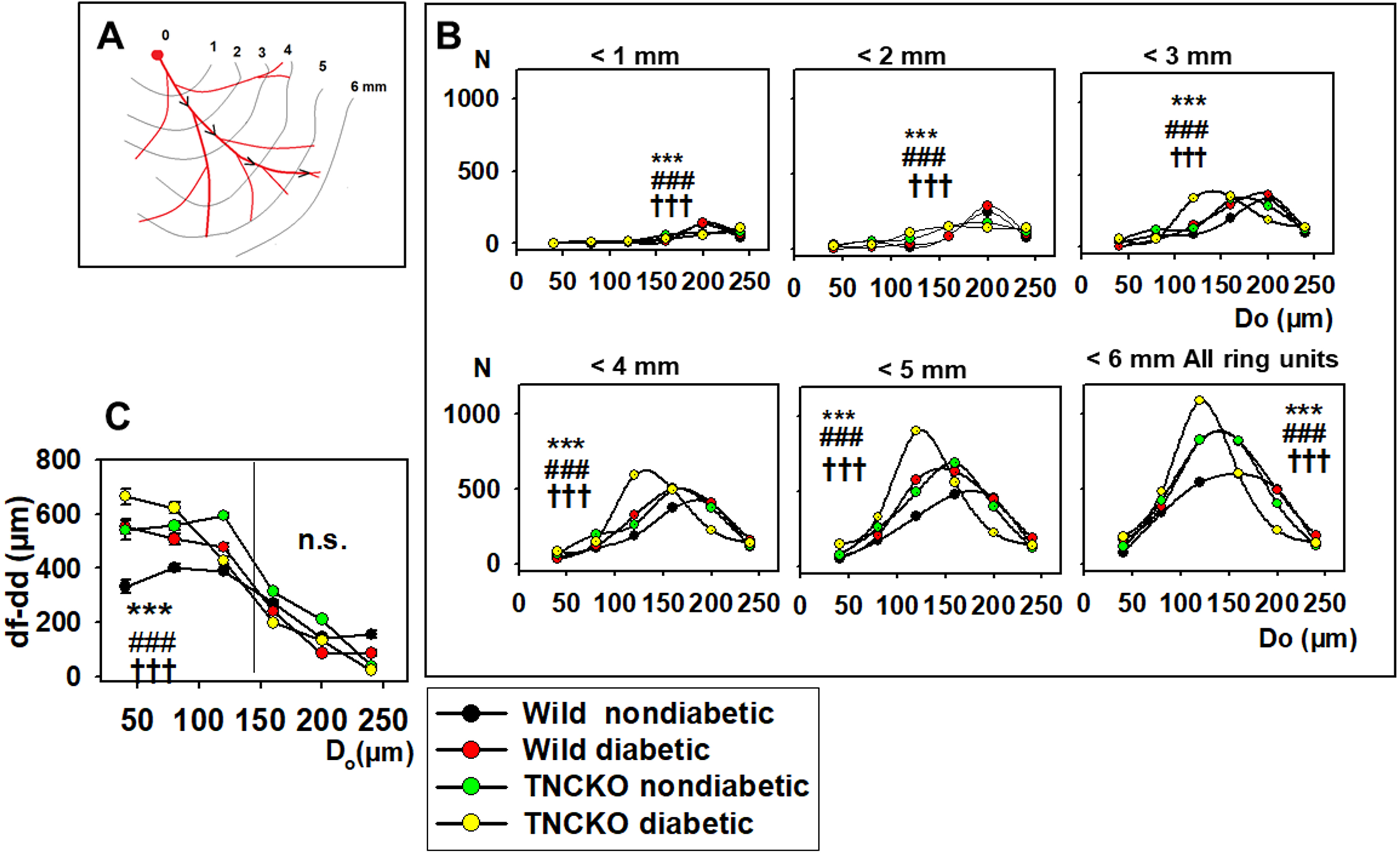 Fig. 6.
Fig. 6.Analysis of flow distances. (A) Flow distance is defined as a
distance that blood should flow from the orifice to the given ring unit. Data was
extracted from 9646 ring units of 37 animals, pooled, and normalized for 10
animals in each group. (B) Diameter distribution (histogram) of ring units with
flow distances (df) 1….6 mm from the orifice. Curves
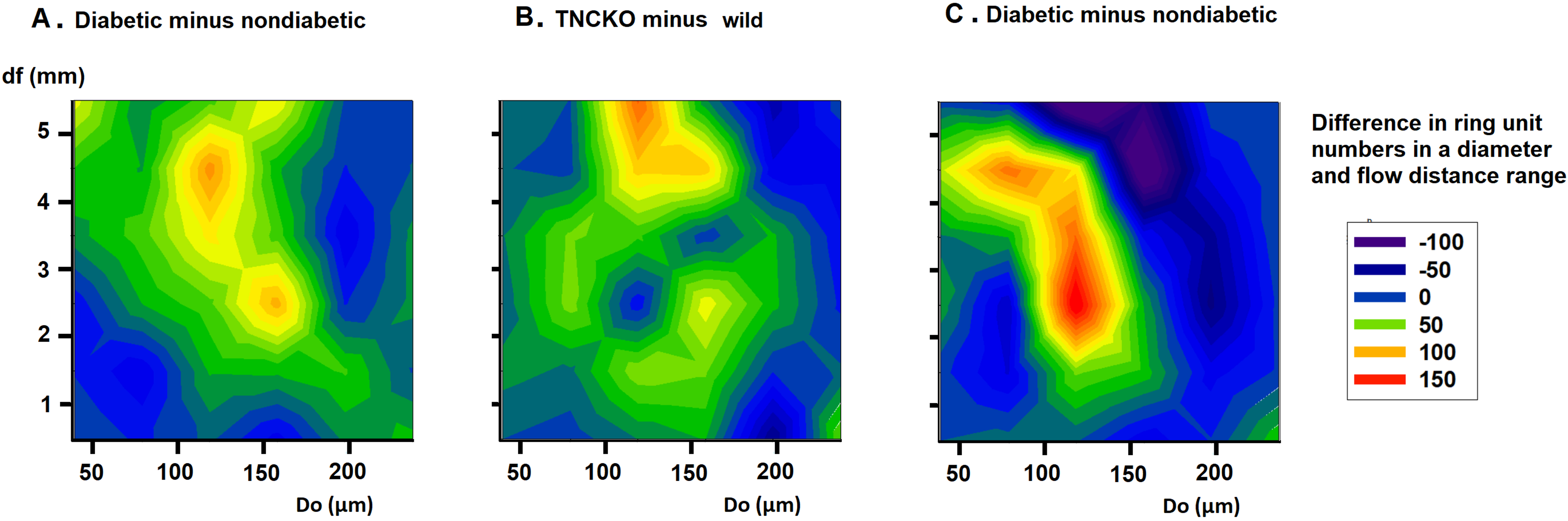 Fig. 7.
Fig. 7.Analysis of flow distances. Differences of bidirectional histograms, showing the difference of the number of ring units for different flow distance ranges between diabetic and non-diabetic Wt mice (A), TNC-KO and Wt non-diabetic mice (B) and diabetic and non-diabetic TNC KO mice (C). Further explanation see in Legend of Fig. 6. Red spots mark more, deep blue spots less ring units compared. Note that ring number differences appear in certain diameter and flow distance ranges.
Our results present new evidence for substantial alterations in the network
geometry of the left coronary artery tree in a mouse model of STZ-induced
diabetes. Here we also show that lack of extracellular matrix protein TNC effects
coronary network formation and prevents malformations of network geometry and
vessel wall thickness. Our data demonstrate for the first time that diabetes in
mice results in (1) morphological deformations in the network associated with
trifurcations instead of bifurcations, sharp bends of larger branches, and
retrograde branches, (2) thickening of the wall of the largest diameter branches
(
Literature search on on diabetic coronary resistance artery networks did not result in a comparable study. Of note, similar morphological deformations in the coronary resistance artery system, namely sharp bends of larger branches, trifurcations and parallel-running larger branches have been found previously in aged [17] and hypertensive [18] rats. Importantly, a common phenomenon of aging, hypertension, and diabetes is increased neuro-humoral activity including the renin-angiotensin-aldosterone system (RAAS). Activation of RAAS, particularly ACE 1 is a known regulator of vascular remodeling. Interestingly, a recent study highlights the interaction between ACE and TNC [28], which promotes adverse left ventricular remodeling.
Thickening of the coronary arteriolar wall is considered a characteristic
pathological diabetic change [13, 14, 39, 40]. Segmental observations of diabetic
coronary microvessels revealed inward hypertrophic remodeling in
db/db diabetic mice which could be dependent on the angiotensin
type 1 receptor [9] which is in concordance with our observations in the larger
(
TNC deletion was associated with early division followed by long, parallel
running medium-sized branches, the number 100–140
Vascular complications, particularly microvascular dysfunction are well-defined, substantial contributors to cardiac dysfunction in diabetes. Numerous studies indicate that metabolic dysfunction due to hyperglycemia promotes cardiac microvascular endothelial dysfunction [45]. Besides the effect of diabetes on cardiac microvascular endothelial dysfunction, the changes of microvascular geometry and network of the small resistance coronary arteries are similar to those observed in retinal vasculature [46]. Alternation of resistance artery geometry and increase of wall thickness certainly contribute to worsened cardiac perfusion and substantially to the progression of diabetic cardiomyopathy.
Considering the role of TNC in the formation of the coronary arteriolar network, we observed that this protein shapes the geometry of the coronary resistance artery network. Early division of the main branch, larger branches running parallel and close to each other were characteristic features in TNC KO animals. TNC is an embryonic protein, however its expression is resumed in cancerous tissue [47, 48], in chronic inflammatory processes [24] and even in proliferative diabetic retinopathy [49]. It interacts with several matrix proteins and inhibits cell adhesion through fibronectin, while also stimulates the expression of this cell adhesion protein [50]. TNC also contributes to fibrosis [24], and aggravates fibrotic remodeling in cardiac tissue after myocardial infarction [51]. Fibronectin-TNC aggregates are normally not present in the basement membranes but upon formation cause basement membrane thickening of retinal vessels in diabetic retinopathy [7]. Our present study revealed that lack of TNC KO resulted in a geometrically altered coronary resistance artery network that was characterized by early branching of the left anterior descending coronary artery. The emerging branches ran parallelly, in close proximity with each other, which resembles a “parallel fragmentation” of the network. Most surprisingly, STZ-induced diabetes did not induce further geometrical changes in TNC KO mice, suggesting that the re-expression of TNC in diabetes may constitute a key signaling molecule in the development of microvascular dysfunction in small coronary arteries.
Our data provide the first insight into the diabetic microvascular damage of coronary arterioles in a mouse model of STZ-induced diabetes. This revealed substantial changes in network geometry of coronary resistance arteries in diabetes and in mice lacking TNC expression. In diabetes, wall thickness of the largest branches increased (hypertrophic wall remodeling), the number of medium-sized vessels substantially increased (vascular neoformation), while wall thickness of smaller vessels decreased (dystrophic wall remodeling). In the present study, in situ perfusion, video-microscopic technique combined with the analysis of ring unit frequencies made it possible to demonstrate that these geometrical alterations appear in the characteristic diameter ranges of small arteries and arterioles; larger vessels closer to the orifice are affected in a different manner than smaller ones farther from it. This segmental specificity of diabetic microvascular pathology might be the most interesting observation. Surprisingly, the geometry of bifurcations showed no alterations, however large number of morphological malformations, trifurcations, sharp bends, and retrograde branches were found. Radial fragmentation of the network seems to be the main component of the pathology. We are convinced that such profound changes in network geometry contribute to the development of ventricular failure, they elevate the energy requirement of tissue oxygenation and disturb the adjustment of local flow patterns. Furthermore, TNC has an important role in forming coronary arteriole network geometry and certainly plays a causative role in the vascular wall thickening and remodeling, substantially contributes to microvascular dysfunction in diabetes.
The data that support the findings of the present study are available from the corresponding author upon request.
GyLN and AK coordinated the study and drafted and edited the manuscript. GyLN, AF, ZSA, IA, CD, PLSZ and MSZ performed the in vivo and ex vivo measurements. GyLN and AK summarized and visualized the data and performed statistics. GyLN, AF, ZSA, IA, CD, GTS, PLSZ, PP, MSZ, LH and BKP proofread, edited, and revised the manuscript. All authors read and approved the final version of the manuscript.
The experimental protocol was approved by the regional Ethics Committee for Laboratory Animal Experiment (66.009/0014-V/3b/2018) conforming with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
The authors thank Eszter Halász and Anders Soerheim (Medical student at the Semmelweis University, Budapest, Hungary) for the excellent technical assistance in preparative laboratory works. The authors thank the RIKEN Institute, Japan for providing TNC KO mice.
The work was supported by Hungarian National Grants OTKA TO 32019, OTKA K116954, by the “Aktion Österreich-Ungarn” grants Nos 98öu4, 104öu5; by a grant from the Dean of the Semmelweis University, Budapest, Hungary and Medizinisch-Wissenschaftlichen Fonds des Bürgermeisters der Bundeshauptstadt Wien (project-Number: 21001). Alexander Fees supported by the Fulbright Stipend.
The author declares no conflict of interest. Attila Kiss is serving as one of the Guest editors of this journal. We declare that Attila Kiss had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Ferdinando Carlo Sasso.
