Academic Editor: Fabian Sanchis-Gomar
Objective: Myocardial infarction (MI) carries a strong risk of death and the development of major adverse cardiovascular events (MACE). A number of biomarkers have been proposed for risk stratification among patients with MI. The aim of this study was to determine whether elevated galectin-3 and midregional-pro atrial natriuretic peptide (MR-proANP) levels can be used as predictors of MACE in patients with acute myocardial infarction (AMI). Methods: Plasma levels of galectin-3 and MR-proANP were collected from 96 patients following their first AMI hospitalised in our clinic over the course of a year. Samples were taken on admission, and on the first and fifth day of hospitalization. During hospitalization, all patients were followed up for the occurrence of early major adverse cardiac events (MACE), defined as sudden cardiac arrest, new onset atrial fibrillation and need to use pressor amines. All patients were also followed up twelve months after AMI for the occurrence of late MACE defined as cardiac death, reinfarction and need for unscheduled PCI. Results: Patients who experienced early MACE had significantly higher galectin-3 and MR-proANP levels assessed on admission (p = 0.007, p = 0.003). ROC curve analysis found also galectin-3 concentration assessed on admission to be a strong predictor of late MACE (AUC = 0.75, p = 0.0061). MRproANP does not appear to have any value in predicting late MACE. Conclusions: A high concentration of galectin-3 and MR-proANP observed on admission in patients with acute myocardial infarction has significant prognostic value: it may identify patients at high risk of early adverse cardiac events after AMI. In contrast to MR-proANP, a high concentration of galectin-3 observed on admission may also identify patients at high risk of late MACE.
According to the fourth universal definition, myocardial infarction (MI) is an indication of myocardial necrosis in a clinical setting consistent with acute myocardial ischaemia [1]. Coronary artery disease (CAD) is the most common disease of the circulatory system in developed countries, with myocardial infarction and sudden cardiac death being the most common causes of death. MI affects men more often than woman, especially in the 40–55 year age group; however no such difference is apparent among seniors.
Myocardial infarction is typically diagnosed based on clinical, electrocardiographic and laboratory criteria. The typical biomarkers used in standard laboratory test panels include Troponin T (TnT) and Creatine Kinase Myocardial Band (CK-MB mass).
Despite the era of invasive treatment, MI carries a strong risk of major cardiovascular events and is often fatal. Therefore, in recent years, a number of emerging biomarkers have been proposed to facilitate risk stratification of patients with MI. These include myeloperoxydase (MPO) [2], human fatty acidbinding protein (h-FABP) [3], mid-regional pro-adrenomedullin (MR-proADM) [4], galectin-3 or midregional-pro atrial natriuretic peptide (MR-proANP).
Galectin-3 is a member of the lectin family. It is encoded by a single gene, LGALS3, located on chromosome 14, locus q21-q22 [5, 6]. The protein plays an important role in the regulation of many physiological and pathophysiological pathways, and is known to trigger inflammation in many conditions, such as diabetes and atherosclerosis [7]. In addition, galectin-3 expression is known to be associated with heart failure, inflammation, fibrogenesis and ventricular remodelling [8].
Pro-atrial natriuretic peptide (proANP) is a natriuretic peptide synthesized and secreted by cardiac muscle cells in the walls of the atria. It is known to play a role in natriuresis, diuresis and vasodilatation. It also counteracts the mechanisms that aggravate heart failure by stimulating prostaglandin synthesis, thus inhibiting the renin-angiotensin-aldosterone system, and reducing the secretion of antidiuretic hormone. MR-proANP has been found to demonstrate greater analytical stability and a longer half-life than ANP or its precursor fragments, and may hence be a more suitable marker [9, 10].
The aim of this study was to determine whether elevated galectin-3 and MR-proANP levels are predictors of MACE in patients with acute myocardial infarction (AMI).
This prospective clinical study included 96 patients admitted to the Department
of Interventional Cardiology of Medical University of Lodz, Poland. All had
received a first diagnosis of acute myocardial infarction within one
year. The inclusion criteria comprised STEMI or NSTEMI and invasive
treatment of MI (primary PCI), while the exclusion criteria included age below 18
years or over 80 years, prior MI, the presence of chronic kidney disease (eGFR
The study was approved by the local ethics committee (Medical University of Lodz). All patients gave their informed consent to participate in this study.
All patients qualified for the study underwent coronary angiography with PCI. All received standard pharmacological therapy used in myocardial infarction.
The following data was collected from all patients: sex, age and histories of
hypertension, coronary artery disease, hypercholesterolemia (according to ESC
guidelines), heart failure, diabetes mellitus (according to WHO diagnosis
criteria), chronic kidney disease (eGFR 31–59 mL/min/1.73 m
During hospitalization, all patients were followed up for the occurrence of early major adverse cardiac events (MACE), defined as sudden cardiac arrest, new onset atrial fibrillation and need to use pressor amines. Patients were also followed up twelve months after AMI for the occurrence of late MACE, defined as cardiac death, reinfarction and need for unscheduled PCI.
Three sets of venous blood samples were collected during hospitalization: on admission to hospital, and on day one and day five of hospitalisation. The blood samples were centrifuged and then frozen and for storage at –20 °C until assay.
Galectin-3 assay was performed by sandwich ELISA, using a commercial kit by BioVendor (Laboratorni medicina a.s), with a sensitivity of 0.29 ng/mL. Although the assay detects both natural and recombinant Galectin-3; however no cross reactivity or interference was detected.
The MR-proANP level in the blood sample was determined by automated immunofluorescent assay (BRAHMS MR-proANP KRYPTOR) with a sensitivity of 10 pmol/L. The assay detects human MR-proANP; however, no significant cross-reactivity or interference was observed between human MR-proANP and analogues.
Categorical variables were summarized as frequencies with percentage values. The Shapiro-Wilk test was used to assess the normality of continuous variables. Any non-normal distributions were subjected to non-parametric tests and presented as medians with interquartile range.
Correlations were assessed using Spearman’s rank correlation coefficient. Continuous variables were compared using the Mann-Whitney U-test, and categorical variables with the chi-squared test with Yates’s correction for continuity. To assess the suitability of selected biomarkers in predicting MACE, receiver operator characteristic curves were constructed. Univariate logistic analysis and stepwise multiple regression were used to determine the predictive value of biomarkers in predicting MACE.
Statistical analysis was performed using STATISTICA 13.3 (StatSoft Inc, Tulsa,
OK, USA). A p-value
The study included 96 patients, most of whom were men (67%). Eighty-six percent of patients were in Killip class I. STEMI of the inferior wall was diagnosed in 53% of patients. A diagnosis of NSTEMI was noted among 23% of patients. As stated in coronary angiography, the majority of patients were diagnosed with two-vessel disease. The participants were experiencing hyperlipidemia, hypertension or diabetes. Other study group features are presented in Table 1.
| Patients characteristics | N = 96 |
| Age (years) | 65 (58–71) |
| Men | 64 (67%) |
| Heart rate | 79 (65–90) |
| Systolic blood pressure [mmHg] | 140 (124–160) |
| Type of MI | |
| STEMI inferior wall | 23 (24%) |
| STEMI anterior wall | 51 (53%) |
| NSTEMI | 22 (23%) |
| Killip class | |
| I | 83 (86%) |
| II–IV | 13 (14%) |
| BMI | 27 (24–30) |
| eGFR (mL/min/1.73 m |
84 (65–97) |
| Diabetes | 22 (23%) |
| Heart Failure | 9 (9%) |
| Hypertension | 63 (66%) |
| Hyperlipidemia | 35 (36%) |
| Family history of CAD | 10 (10%) |
| Current or former smoker | 53 (55%) |
| Results of coronarography | |
| One-vessel disease | 35 (36%) |
| Two-vessel disease | 36 (38%) |
| Three-vessel disease | 25 (26%) |
| Concomitant therapy | |
| Aspirin | 94 (98%) |
| Ticagrelor | 43 (45%) |
| Clopidogrel | 53 (55%) |
| GP IIb/IIIa blocker | 60 (63%) |
| Statins | 94 (98%) |
| Beta-blockers | 86 (90%) |
| ARB | 0 (0%) |
| ACEI | 87 (91%) |
| Diuretics | 34 (35%) |
Mean galectin-3 plasma concentrations were higher on admission than on the first and fifth day of hospitalization: 12.56 ng/mL (9.9–20.7) on admission, 10.86 ng/mL (8.17–16.5) on day one of hospitalization, and 9.01 ng/mL (7.06–13.32) on day five.
Weak correlations were observed between heart rate on admission and the concentrations of galectin-3 on day one (p = 0.022, R = 0.23) and day five of hospitalization (p = 0.010; R = 0.26).
Neither sex, age, blood pressure, Killip class on admission, history of
hypertension, hyperlipidemia, renal failure, lung disease, new-onset atrial
fibrillation or obesity had any effect on galectin-3 levels, neither on
admission, nor on day one or day five of hospitalization. However, patients with
diabetes demonstrated significantly higher values of galectin-3 at all three time
points (p = 0.020, p = 0.013, p
Patients with impaired systolic LV function had higher values of galectin-3 than
patients with preserved systolic LV function (p
MR-proANP plasma concentration was 151.2 pmol/L (99.9–282.7) on admission, 99.03 pmol/L (81.6–174.7) on day one of hospitalization and 104.6 pmol/L (78.6–164.8) on day five.
Neither heart failure, prior myocardial infarction, history of hypertension, diabetes, obesity, heart rate or sex had any effect on MR-proANP levels at any of the three measurement points.
Patients with normal systolic LV function had lower values of MR-proANP than
patients with impaired systolic LV function (p = 0.005, p
During hospitalization, eighteen individuals developed early MACE. The univariate logistic regression analysis found that among the tested parameters (including heart rate, glucose and creatinine levels, NT-proBNP, MR-proANP, galectin-3, CRP among others), only galectin-3, MR-proANP, NT-proBNP and CRP assessed on admission turned out to be significant predictors of early MACE (p = 0.0103, p = 0.0028, p = 0.0254, p = 0.0112 respectively; Table 2).
| Parameter | OR | 95% CI | p value |
| Univariable regression | |||
| CRP [mg/L] | 1.016 | 1.004–1.028 | 0.0112 |
| NT-proBNP on admission [pg/mL] | 1.000 | 1.000–1.000 | 0.0254 |
| Galectin-3 on admission [ng/mL] | 1.044 | 1.010–1.078 | 0.0103 |
| MR-proANP on admission [pmol/L] | 1.005 | 1.002–1.008 | 0.0028 |
| Multivariable regression | |||
| CRP [mg/L] | 1.018 | 1.004–1.031 | 0.011 |
| Galectin-3 on admission [ng/mL] | 1.054 | 1.015–1.095 | 0.007 |
| MR-proANP on admission [pmol/L] | 1.005 | 1.002–1.009 | 0.003 |
ROC curve analysis found the concentration of CRP, galectin-3 and MR-proANP assessed on admission to be strong predictors of MACE during hospitalization (AUC = 0.688, p = 0.0064; AUC = 0.689, p = 0.0072; AUC = 0.742, p = 0.0000 respectively) (Fig. 1).
 Fig. 1.
Fig. 1.ROC curves - variables tested: the concentrations of significant predictors of early MACE.
Stepwise forward logistic regression analysis found CRP, galectin-3 and MR-proANP assessed on admission to be significant predictors of early MACE (p = 0.011, p = 0.007, p = 0.003; Table 2).
A follow-up conducted 12 months after the MI found nine individuals to have developed late MACE. Three patients had experienced reinfarction, four had undergone unscheduled coronary angioplasty and four had died.
No significant differences in the concentration of galectin-3 on day one of
hospitalization were found between patients who experienced late MACE and
uneventful survivors (p = 0.56). ROC curve analysis found galectin-3
concentration assessed on admission to be a strong predictor of MACE 12 months
after discharge (AUC = 0.75, p = 0.0061) (Fig. 2); in this case, the
galectin-3 cut-off value was 23.183 ng/mL (95% CI 2.664–54.059). In addition,
ROC curve analysis for each major adverse cardiac event revealed that galectin-3
concentration collected on admission may be also used as a strong predictor of
death (AUC = 0.854, p
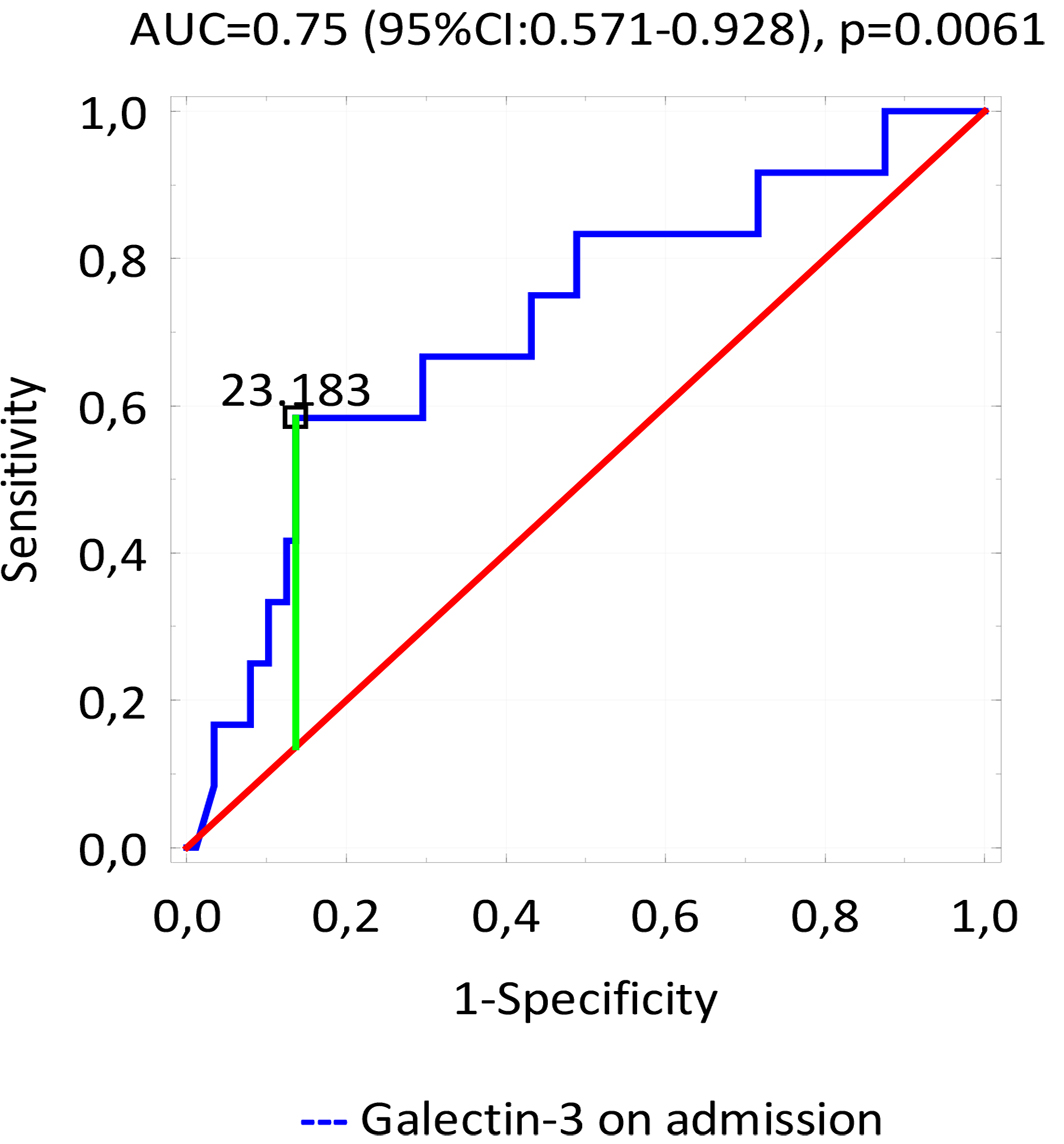 Fig. 2.
Fig. 2.ROC curve - variable tested: the concentrations of significant predictors of late MACE.
In univariate logistic regression analysis, among the many tested factors (including age, sex, obesity, new-onset AF, diabetes, ejection fraction, NT-proBNP and MR-proANP), only galectin3 collected on admission was found to be significant predictor of late MACE (p = 0.0297; Table 3); this was confirmed by stepwise forward logistic regression analysis (p = 0.0297; Table 3).
| Parameter | OR | 95% CI | p value |
| Univariable regression | |||
| Galectin 3 on admission | 1.044 | 1.004–1.085 | 0.0297 |
| Multivariable regression | |||
| Galectin 3 on admision | 1.044 | 1.044–1.085 | 0.0297 |
No significant differences in MR-proANP concentration at any time point were observed between patients who developed late MACE and those who did not (p = 0.83, p = 0.76, p = 0.7). MRproANP was not found to be a significant factor predicting late MACE, either by univariate or stepwise forward logistic regression analysis.
Early and late MACE were also analysed together. Stepwise forward logistic regression analysis found CRP, galectin-3 assessed on admission and NT-proBNP assessed on day five to be significant predictors of both early and late MACE (p = 0.034, p = 0.007, p = 0.043; Table 4).
| Parameter | OR | 95% CI | p value |
| Multivariable regression | |||
| CRP [mg/L] | 1.014 | 1.001–1.027 | 0.034 |
| Galectin-3 on admission [ng/mL] | 1.047 | 1.012–1.083 | 0.007 |
| NT-proBNP on the fifth day [pg/mL] | 1.000 | 1.000–1.000 | 0.043 |
Our present study yielded two key findings. Firstly, high concentrations of galectin-3 and MR-proANP assessed on admission may be significant factors predicting early MACE in patients with AMI. In addition, in contrast to MR-proANP, a high concentration of galectin-3 on admission may also be a predictor of late MACE.
It has been suggested that MR-proANP could be used to predict cardiovascular death [11], and that it might be a predictor of all-cause mortality and MACE in patients with symptomatic CAD [12]. High plasma concentrations of MR-proANP have been found to effectively identify patients with higher risk of death/AMI at 360 days after episode of chest pain [13, 14]. However, in the present study, no relationship was found between MR-proANP level and the occurrence of MACE 12 months after AMI.
Numerous studies indicate a relationship between MR-proANP and chronic heart failure (HF). Indeed, increased levels of MR-proANP have been found to be associated with an increased risk of death in patients with HF [15, 16]. In addition, others confirm a relationship between MR-proANP level and acute HF [17, 18]. Patients with dyspnea also demonstrated higher levels of MR-proANP, and the addition of this biomarker to the standard laboratory test panel improved the diagnostic accuracy for acute HF. Furthermore, MR-proANP may be an indicator of impaired left ventricular function [19].
Our present findings confirm that patients with normal systolic LV function had lower values of MR-proANP than those with impaired systolic LV function. In addition, no relationship was found between galectin-3 level on day one of hospitalization and the occurrence of both early and late MACE after acute myocardial infarction. However, the presence of high plasma concentrations of galectin-3 measured on admission was a significant predictor of death after AMI.
Tsai et al. [20] found the concentration of galectin-3 assessed six
hours after PCI to be a strong predictor of 30-day mortality among patients with
STEMI undergoing primary PCI (p
Similarly to our present findings, Lisowska et al. [21] found galectin-3 to be a predictor of cardiovascular death after AMI in a group of 233 patients. In this case, the concentration of galectin-3 was assessed within 24 hours of admission. Patients with significantly higher mean concentrations of galectin-3 were more likely to die during the follow-up period (mean 2.8 years) (20.0 ng/mL vs 8.0 ng/mL; p = 0.0005). However, many more exclusion criteria were used than the present study, such as severe congestive heart failure and unstable haemodynamic state. Another study based on 1013 patients found galectin-3 to be a strong predicting factor of cardiovascular death among patients with stable CAD and AMI who underwent coronary angiography (HR 1.87: 95% CI 1.04–3.33; p = 0.036) [22].
Several studies have found galectin-3 concentration to be an independent predictor of mortality after MI. Tymińska et al. [23] found galectin-3 level to predict cardiovascular death in patients with first-time STEMI (p = 0.01); however, unlike our present study, galectin-3 concentration was measured only after 72 to 96 hours after hospital admission. Galectin-3 has been found to peak 12 hours after acute inflammatory stimulation [24]; this has been confirmed in a previous study, but with a mean follow-up of 5.4 years [25]. Our findings indicate that the concentration of galectin-3 assessed on admission was also a predictor of reinfarction; this is in line with Szadkowska et al. [26], who also report that galectin-3 may be a predictor of reinfarction early after first MI.
Our present findings suggest that patients with normal systolic LV function had
lower values of galectin-3 than those with impaired systolic LV function. A
previous study of 1342 patients with myocardial infarction found those with
higher galectin-3 levels to have a higher risk of developing heart failure after
MI [25]. High galectin-3 levels have also been found to be positively correlated
with advanced congestive heart failure, but negatively correlated with LVEF (R=
–0.253; p
Our present findings confirm those of previous studies indicating that patients with diabetes tended to have higher levels of galectin-3 [30, 31, 32, 33]. Interestingly, galectin-3 levels have also been found to be associated with all-cause mortality and incident cardiovascular events in type 2 diabetes [34]. In addition, a relationship was observed between galectin-3 level and GFR in our group of patients; furthermore, higher levels of galectin-3 have previously been found to be associated with incident chronic kidney disease over a 10-year follow-up [35, 36] and with the progression of chronic kidney disease [37].
Firstly, the present study is based on a relatively small group of patients with a relatively limited number of events. As such, further studies are needed to confirm our findings. Secondly, the study group was quite heterogeneous, including both STEMI and NSTEMI patients. Additionally, most patients were in Killip class I; however, we did not exclude any patients in a worse haemodynamic state. The study also excluded patients with previous MI or with conservative treatment of MI; as such, our findings do not apply to these groups.
A high concentration of galectin-3 and MR-proANP observed on admission in patients with acute myocardial infarction has significant prognostic value: it may identify patients at high risk of early adverse cardiac events after AMI. In contrast to MR-proANP, a high concentration of galectin-3 observed on admission found also to be a significant factor predicting late MACE in patients with AMI. Although no single universal cardiac biomarker appears to exist for predicting MACE in patients with acute myocardial infarction, galectin-3 seems to be an effective predictor of both early and late MACE.
MI, myocardial infarction; AMI, acute myocardial infarction; STEMI, ST elevation myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; MACE, major adverse cardiovascular events; ANP, atrial natriuretic peptide; MR-proANP, midregional pro-atrial natriuretic peptide; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; MPO, myeloperoxydase; H-FABP, human fatty acid-binding protein; MR-proADM, mid-regional pro-adrenomedullin; CRP, C-reactive protein; GFR, glomerular filtration rate; PCI, percutaneus coronary intervention; LV, left ventricular; EF, ejection fraction; AF, atrial fibrillation; CAD, cardiovascular disease.
Conceptualization—KI, MK and MZ; methodology—KI, MK and MZ; formal analysis—MK and MZ; investigation—KI and MK; data curation—KI; writing - original draft preparation—KI and MK; writing - review and editing—KI, MK and MZ; visualization—MK; supervision—MZ; project administration—KI and MZ; funding acquisition—KI and MZ.
The study was approved by the local ethics committee (Medical University of Lodz, Number: RNN/257/15/KE). All patients gave their informed consent to participate in this study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
