†These authors contributed equally.
Academic Editor: Maurizio Pieroni
Background: The classic electrocardiogram (ECG) criteria have been
applied to left ventricular hypertrophy (LVH) screening but have low sensitivity.
Recently, the newly proposed Peguero-Lo Presti criterion has been proven to be
more sensitive in detecting LVH in patients with hypertension than several
current ECG criteria. The diagnostic value of the Peguero-Lo Presti criterion in
hypertrophic cardiomyopathy (HCM) patients has not been fully evaluated. This
study aims to test whether the new Peguero-Lo Presti criterion can improve the
diagnostic performance in patients with HCM. Methods: This study
included HCM patients and sex-and age-matched healthy control subjects. The
diagnostic performance of the Peguero-Lo Presti criterion was evaluated along
with the Sokolow-Lyon criterion, Cornell criterion, and total 12-lead voltage
criterion. Results: Overall, 63 HCM patients and 63 controls were
enrolled. The diagnostic accuracy, sensitivity and specificity of Peguero-Lo
Presti criterion were 74.6%, 73.0% and 76.2%, respectively. The Peguero-Lo
Presti criterion had the highest sensitivity, while the Cornell criterion and
Sokolow-Lyon criterion had the highest specificity (96.8%). The area under the
curve (AUC) showed that the Peguero-Lo Presti criterion was 0.809 (95% CI,
0.730–0.874; p
Left ventricular hypertrophy (LVH) is considered to be a major predictor of cardiovascular events [1, 2]. It has been reported that regardless of whether a patient is suffering from hypertension, LVH diagnosed by electrocardiogram (ECG) is strongly associated with cardiovascular morbidity and mortality [3, 4, 5]. Therefore, it is necessary to diagnose LVH as soon as possible for further examination and treatment. Echocardiography is considered as a central cardiac imaging modality for LVH diagnosis and monitoring [6]. However, ECG is also an important screening method for LVH detection because of its ease of use, wide availability and proven independent clinical prognostic impact. Some ECG criteria for LVH detection have been proposed. However, these ECG criteria have many limitations in clinical use because the electrocardiographic indicators of LVH are relatively insensitive. The sensitivity of these criteria also varies with the various etiologies of LVH [7]. Although many classical ECG voltage criteria are also used to screen hypertrophic cardiomyopathy (HCM), the overall reliability of these criteria is low [8]. Therefore, new criteria need to be developed to reduce the rate of false-negative screening.
Peguero et al. [9] proposed a novel ECG voltage criterion (Peguero-Lo Presti criterion) for identifying LVH with better sensitivity than several classical ECG voltage criteria in a group of patients with hypertension. Since then, the new criterion has been tested in several studies [10, 11, 12] for LVH detection in patients with hypertension but it has not been fully evaluated in the population of HCM. Therefore, the aim of this study is to test whether the Peguero-Lo Presti criterion can improve the diagnostic accuracy of HCM.
This study included HCM patients who were hospitalized in our hospital from
February 2019 to June 2021. In the same period, we selected sex-and age-matched
healthy subjects as the controls. These healthy subjects were selected from a
database who underwent regular physical examinations. Transthoracic
echocardiography was used to diagnose HCM according to the ESC guidelines [6].
HCM was defined by a wall thickness
Data collection was performed using standardized questionnaires. Height and
weight were measured by trained technicians while the patient was barefoot and
wearing light clothing. Dyslipidemia was defined as triglyceride
A standard 12-lead ECG (1 mV/10 mm and 25 mm/s) was performed for each subject
at rest by trained technicians on the same day as the echocardiography. ECG
interpretations were independently assessed by two experienced cardiologists who
had more than 15 years of work experience and did not know the echocardiographic
data. ECG measurements were performed manually with calipers. Inconsistent ECG
interpretation results were reconciled through consensus. The newly proposed
Peguero-Lo Presti criterion was obtained by adding SD (the amplitude of the
deepest S wave in any lead) to the S amplitude in V4 (SD + SV4). The cutoff
values were
The normality of the distribution of continuous variables was tested by the
Kolmogorov-Smirnov test. Continuous variables were presented as median
(interquartile range) or mean
Using a two-sided z-test at a significance level of 0.05, it was estimated that a sample of 17 patients with HCM and 17 controls achieved 92% power to detect a difference of 0.3 between AUC under the null hypothesis of 0.5 and AUC under the alternative hypothesis of 0.8.
A total of 63 HCM (35 men; mean age 60.1
| Parameter | HCM (N = 63) | Controls (N = 63) | p | |
| Age, yrs | 60.1 |
59.1 |
0.653 | |
| Body mass index, kg/m |
24.6 |
24.8 |
0.695 | |
| Body surface area, m |
1.7 |
1.7 |
0.758 | |
| Systolic blood pressure, mmHg | 124.4 |
127.9 |
0.101 | |
| Diastolic blood pressure, mmHg | 78.7 |
82.1 |
0.087 | |
| Diabetes mellitus, n (%) | 13 (20.6) | - | - | |
| Dyslipidemia, n (%) | 10 (15.9) | - | - | |
| Atrial fibrillation, n (%) | 9 (14.3) | - | - | |
| Coronary artery disease, n (%) | 19 (30.2) | - | - | |
| Stroke, n (%) | 10 (15.9) | - | - | |
| Laboratory tests | ||||
| Fasting blood glucose, mmol/L | 5.4 |
5.5 |
0.620 | |
| Hemoglobin, % | 6.0 |
6.1 |
0.521 | |
| Serum urea, mmol/L | 6.4 |
5.4 |
0.002 | |
| Creatinine, µmol/L | 69.4 |
62.4 |
0.033 | |
| Uric acid, µmol/L | 348.6 |
333.1 |
0.424 | |
| ECG criteria | ||||
| Cornell criterion (RaVL + SV3), mV | 2.4 |
1.4 |
||
| Sokolow-Lyon criterion (SV1 + RV5/RV6), mV | 4.1 |
2.3 |
||
| Total 12-lead voltage criterion (R wave to the nadir of the Q/S wave), mV | 22.5 |
14.9 |
||
| Peguero-Lo Presti criterion (SD + SV4), mV | 3.6 |
2.1 |
||
| Data are presented as the mean | ||||
Electrocardiographic analysis revealed that the HCM group had higher values than
the control group using the Cornell criterion (2.4
The diagnostic accuracy, sensitivity and specificity of the Peguero-Lo Presti criterion were 74.6%, 73.0% and 76.2%, respectively. Furthermore, the Peguero-Lo Presti criterion did not show a lack of agreement with the reference standard. Among the four ECG criteria, the Peguero-Lo Presti criterion had the highest sensitivity, while the Cornell criterion and Sokolow-Lyon criterion had the highest specificity (96.8%). The Sokolow-Lyon criterion and total 12-lead voltage criterion had the highest accuracy (78.6%) (Table 2).
| ECG criteria | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | McNemar Test* |
| Cornell criterion | 71.4 (62.7–79.1) | 46.0 (33.4–59.1) | 96.8 (89.0–99.6) | 93.6 (78.3–98.3) | 64.2 (58.7–69.3) | |
| Sokolow-Lyon criterion | 78.6 (70.4–85.4) | 60.3(47.2–72.4) | 96.8 (89.0–99.6) | 95.0 (82.7–98.7) | 70.9 (64.2–76.9) | |
| Total 12-lead voltage criterion | 78.6 (70.4–85.4) | 71.4(58.6–82.1) | 85.7 (74.6–93.3) | 83.3 (72.8–90.3) | 75.0 (66.7–81.8) | 0.122 |
| Peguero-Lo Presti criterion | 74.6 (66.1–81.9) | 73.0(60.4–83.4) | 76.2 (63.8–86.0) | 75.4 (65.8–83.0) | 73.9 (64.8–81.3) | 0.860 |
| *A p value | ||||||
The ROC curve was also performed. The AUC for the Peguero-Lo Presti criterion
was 0.809 (95% CI, 0.730–0.874; p
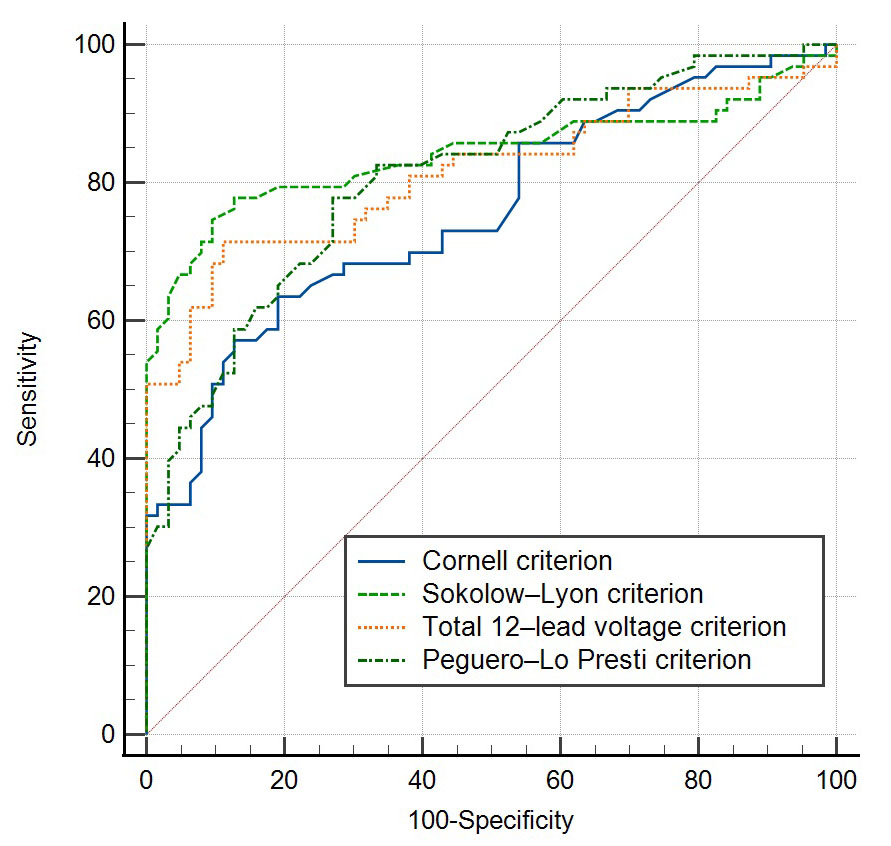 Fig. 1.
Fig. 1.ROC curve of the four ECG criteria in patients with HCM.
| ECG criteria | AUC | 95% CI | p* |
| Cornell criterion | 0.758 | 0.673–0.830 | |
| Sokolow-Lyon criterion | 0.841 | 0.766–0.900 | |
| Total 12-lead voltage criterion | 0.814 | 0.735–0.878 | |
| Peguero-Lo Presti criterion | 0.809 | 0.730–0.874 | |
| *The null hypothesis is that the AUC is 0.5. ECG, electrocardiogram; AUC, area under the ROC curve; CI, confidence interval. | |||
Compared with men, the Peguero-Lo Presti criterion for women had higher
diagnostic accuracy (71.4% for men; 82.1% for women), sensitivity (68.6% for
men; 78.6% for women), specificity (74.3% for men; 85.7% for women), PPV
(72.7% for men; 84.6% for women), and NPV (70.3% for men; 80.0% for women)
(Table 4). The AUCs for men and women were 0.765 (p
| Sex | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | McNemar Test* |
| Men | 71.4 (59.4–81.6) | 68.6 (50.7–83.2) | 74.3 (56.7–87.5) | 72.7 (59.3–83.0) | 70.3 (58.3–80.0) | 0.824 |
| Women | 82.1 (69.6–91.1) | 78.6 (59.1–91.7) | 85.7 (67.3–96.0) | 84.6 (68.5–93.3) | 80.0 (66.0–89.2) | 0.754 |
| *A p value | ||||||
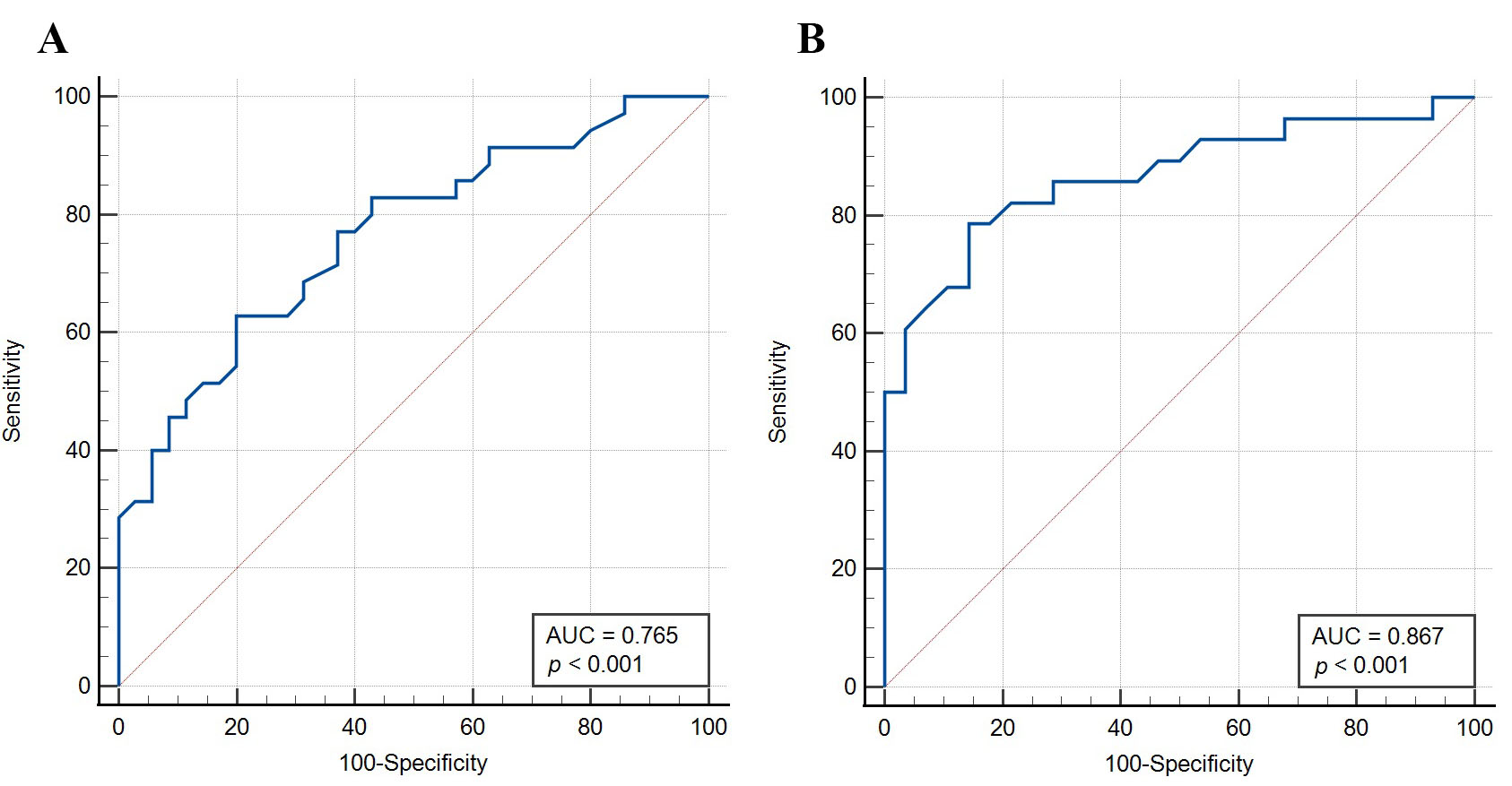 Fig. 2.
Fig. 2.ROC curve of the Peguero-Lo Presti criterion in men (A) and women (B) with HCM.
LVH is an important manifestation of preclinical cardiovascular disease, which can significantly predict cardiovascular events [17]. It has been reported that ECG-based diagnostic criteria are better than cardiovascular magnetic resonance imaging in predicting cardiovascular events [18]. Many ECG criteria for LVH have been proposed and used clinically, and those most commonly used are Sokolow-Lyon criterion and Cornell criterion [19]. However, these criteria have the characteristics of high specificity and low sensitivity. For example, the Sokolow-Lyon criterion has a median sensitivity of 21% (4%–52%) and specificity of 89% (53%–100%) [20]. The specificity of the Cornell criterion is approximately 90%, while the sensitivity is only 20%–40% [9, 21]. The performance of ECG for LVH detection is affected by several factors. In general, ECG evaluates the presence of LVH by detecting electrical voltage changes caused by an increased left ventricular mass. However, the electrical voltage is also affected by the myocardial interstitium (such as fibrosis and other material deposition), cardiac conduction abnormalities, left ventricular geometry, pulmonary diseases, and the distance between the heart and the electrodes [22]. Other factors affecting the results include sex and race [23]. Therefore, it is particularly urgent to propose a new ECG criterion with higher sensitivity for use in the clinic.
Peguero et al. [9] recently proposed a novel ECG voltage criterion (Peguero-Lo Presti criterion) for LVH detection. The Peguero-Lo Presti criterion was obtained by adding SD (the amplitude of the deepest S wave in any lead) to the S amplitude in V4 (SD + SV4) [9]. They found that the new criterion improved the sensitivity of LVH detection in patients with hypertension while maintaining sufficient specificity. The results also showed that the Peguero-Lo Presti criterion had higher diagnostic accuracy than the Sokolow-Lyon criterion and Cornell criterion. Since then, the new criterion has been validated in several studies [10, 11, 12] for LVH detection in patients with hypertension.
The Peguero-Lo Presti criterion has not been fully evaluated in patients with
HCM. Tiron et al. [24] found that compared with Sokolow-Lyon criterion
and Cornell criterion, Peguero-Lo Presti criterion was the only criterion related
to both left ventricular mass index and maximum thickness in HCM patients. In
this study, the Peguero-Lo Presti criterion was used to screen HCM and was
compared with other commonly used ECG criteria. Sensitivity and specificity are
classical parameters to characterize a diagnostic test. Sensitivity refers to the
percentage of patients correctly classified in the diseased category and the
specificity refers to the percentage of patients correctly classified in the
non-diseased category. For screening tests, sensitivity would be favored over
specificity, while for confirmatory tests, specificity would be favored over
sensitivity [25]. As a screening test, the results of this study showed that the
Peguero-Lo Presti criterion had the highest sensitivity (73.0%), followed by the
total 12-lead voltage criterion (71.4%). The Cornell criterion and Sokolow-Lyon
criterion were relatively insensitive (46.0% and 60.3%, respectively). However,
compared with other ECG criteria, the specificity of the Peguero-Lo Presti
criterion was relatively low (76.2%). ROC curve is a graph of sensitivity
(Y-axis) versus false-positive rate (1 – specificity) (X-axis), which can be
used to summarize the overall accuracy of the diagnostic test. AUC calculated
according to the ROC curve is a common index to measure the accuracy of a
diagnostic test. The value of AUC can be between 0.5 and 1.0. Ideally, AUC of 1.0
represents a completely accurate test, while the AUC along the diagonal line in
the graph is 0.5 that is no better than flipping a coin [25]. The ROC curve
demonstrated that the Peguero-Lo Presti criterion had an AUC of 0.809, indicating
its good overall performance. Therefore, the overall diagnostic accuracy of the
Peguero-Lo Presti criterion is reliable for HCM. Recently, Gamrat et al.
[26] applied the Peguero-Lo Presti criterion to detect LVH in patients with
severe aortic stenosis. The results showed that the Peguero-Lo Presti criterion
had improved sensitivity (55% vs. 9%–34%) and decreased specificity (72% vs.
78%–100%) for the detection of LVH compared with 8 single traditional ECG
criteria. Compared with the traditional ECG-LVH criteria, the agreement between
Peguero-Lo Presti criterion and echocardiographic LVH in patients with severe
aortic stenosis was slightly better [26]. Matusik et al. [27] concluded
that the Peguero-Lo Presti criterion and Cornell criterion were sex-specific and
could provide the highest level of diagnostic accuracy. When screening for LVH in
patients with cardiovascular diseases, routine use of Peguero-Lo Presti criterion
should be considered [27]. Besides, Matusik et al. [28] tested a novel
screening tool (CAR
We also compared the diagnostic performance of the Peguero-Lo Presti criterion
between men and women. Compared with men, the Peguero-Lo Presti criterion for
women had higher diagnostic accuracy (71.4% for men; 82.1% for women),
sensitivity (68.6% for men; 78.6% for women), and specificity (74.3% for men;
85.7% for women). To improve the diagnostic accuracy of the Peguero-Lo Presti
criterion in patients with HCM, we also calculated the optimal cutoff value of
the ROC curve based on the proposed sensitivity and specificity to be determined
by the maximum Youden index. The optimal cutoff value for men was
The Peguero-Lo Presti criterion has been proven to be sensitive (62%) while maintaining high specificity (90%) in the detection of LVH in patients with hypertension [9]. However, several studies have reported different results. The applicability of the Peguero-Lo Presti criterion is heterogeneous, especially in Asian populations, with relatively reduced specificity and AUC for LVH detection in patients with hypertension, which may be attributed to the different ECG characteristics of different races and the characteristics of specific study populations [29, 30]. Therefore, although the results of this study suggest that the novel criterion may be a suitable ECG screening tool for patients with HCM, a larger population and further adjustments may be needed, including consideration of extra cardiac factors such as race and sex [31].
We should recognize that there are some limitations of this study. First, the sample size of this study was relatively small, and this was a single-center study. Many large-scale studies are required to verify the accuracy of the Peguero-Lo Presti criterion in patients with HCM. Second, we only compared the Peguero-Lo Presti criterion with the Cornell criterion, Sokolow-Lyon criterion and total 12-lead voltage criterion. Further studies including more ECG diagnostic criteria are needed. Third, the diagnosis of HCM was evaluated by two-dimensional echocardiography in this study, whereas cardiovascular magnetic resonance imaging can provide more detailed information about the structure and function of the heart. Nonetheless, echocardiography is considered a central cardiac imaging modality for the diagnosis and monitoring of HCM with good reproducibility and is still the most commonly used method. We should also recognize that cardiovascular magnetic resonance imaging is difficult to apply widely due to its lack of availability and cost. Finally, our study excluded some patients with specific conditions, such as bundle branch block, so the diagnostic value of the Peguero-Lo Presti criterion for this population is unknown.
The newly proposed Peguero-Lo Presti criterion provides high sensitivity for ECG diagnosis in HCM patients and can be considered when applicable but it needs to be verified in a larger population.
YC, LL and XY contributed equally in the data collection, statistical analysis and manuscript drafting. XS, GC and JF participated in data collection and manuscript revision. HW was responsible for the study design, manuscript revision and consultation. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital (Approval No.: 2019-R012). All subjects gave their informed consent for inclusion before they participated in the study.
Not applicable.
This research was funded by Science and Technology Talent Support Program of Shaanxi Provincial People’s Hospital, grant number 2021JY-24.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
