†These authors contributed equally.
Academic Editors: Carmela Rita Balistreri and Jawahar L. Mehta
Background: This study aimed to explore the levels of circulating
inflammatory factors CRP, IL-6, IL-10 and TNF-
Abdominal aortic aneurysm (AAA) is a kind of vascular degenerative disease, which occurs mostly in middle-aged and elderly people. Apart from the factors such as smoking, age, hypertension and dyslipidemia, genetic background is also an important risk factor for AAA [1]. Typically, AAA degeneration of the abdominal aorta is a manifestation of a systemic process characterized by inflammation, apoptosis of smooth muscle cells, and destruction of elastin and collagen in the media and adventitia. AAA rupture is associated with a great mortality rate, and selective surgical repair is an effective and relatively safe intervention measure [2]. However, endovascular repair (EVAR) within the aneurysm has been currently evaluated as an alternative to open surgical repair [3]. At present, AAA shows highest incidence rate among aneurysms and exhibits a high incidence rate in cardiovascular diseases (CVDs) in the general population with increased patient suffering and the risks of rupture and death [4].
AAA has the characteristics of tissue structural destruction because of chronic inflammation with unknown causes [5].
Factors such as CRP, IL-6, IL-10 and TNF-
C-reactive protein (CRP), the acute-phase protein, can also
serve as the early marker for inflammatory disease or infection. Previous studies
have investigated the associations between AAAs and circulating CRP levels with
specific genetic polymorphisms [11]. For instance, the studies by Stephen
et al. [12] showed that CRP modulated inflammation, and its expression
increased among AAA cases, making CRP one of the prominent inflammatory factors
aggravating AAA. Interleukin (ILs) are the lymphokines that interact with
leukocytes or immune cells. Among them, IL-6, one of the pro-inflammatory
factors, is suggested to show increased circulating levels in AAA cases, which
may be associated with aorta diameter. Common genetic variations in the IL-6 gene
promoter may affect the circulating IL-6 levels [13]. IL-10 can reduce
pro-inflammatory cytokine production through macrophages, neutrophils and T
cells. Its expression increases among AAA cases. Based on this IL-10 has turned
out to be one of the susceptibility factors for AAA [14]. Tumor necrosis
factor-alpha (TNF-
At present, polymorphisms of several pro-inflammatory cytokines are identified as AAA-related. However, there is modest systemic and quantitative analysis on the relationship between polymorphism of inflammatory factors with AAA susceptibility. The present work carried out an integrative meta-analysis of the relevant published articles to investigate the relationship between the polymorphisms of inflammatory factors and AAA susceptibility. As there are inconsistent results from the published articles, the present meta-analysis was performed to clarify the relation of inflammatory factor SNPs with AAA susceptibility.
The present work was registered at PROSPERO (https://www.crd.york.ac.uk/PROSPERO; registration No., CRD42021259433) on 11th August, 2021. This work was carried out following the criteria of Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [16] and guidelines of the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) [17] (http://www.prisma-statement.org/).
Electronic databases PubMed and Web of Science (WOS) were systemically searched on May 5th, 2021. Two researchers (Z. Z. and H. W.) were independently assigned the task of reviewing the study related to the inflammatory mediators associated with human AAA. The review study was based on the Web of Science Core Collection database (via Web of Science [v.5.35]; 1926 to May, 2021) and MEDLINE database (via PubMed; 1966 to May, 2021).
The search strategies were summarized as follows, (“polymorphism” or “genetic
polymorphism” or “single nucleotide polymorphism” or “SNP” or “genetic
variants” or “gene mutation”) and (“abdominal aortic aneurysm” or “aortic
aneurysm, abdominal” or “AAA”) and (“high sensitivity C-reactive protein” or
“acute phase proteins” or “C Reactive Protein” or “CRP”) or
(“interleukin-6” or “IL-6”) or (“interleukin-10” or “IL-10”) or
(“TNF-alpha” or “TNF-
Studies were selected according to the framework of PICOS (Population, Intervention/Exposure, Control, Outcomes, and Study design):
(1) Participants/population. Participants with the diagnosis of AAA by the local AAA screening project were enrolled.
(2) Intervention(s), exposure(s): (i) Serum samples were acquired in the whole
blood from AAA cases and healthy subjects to measure the CRP, TNF-
(3) Control groups. Participants from the screening project who were verified to have no AAA by ultrasound or medical imaging examination (e.g., CT) were selected in the control group.
(4) Outcome(s). Standard Mean Differences (SMDs) were used to
calculate the correlations of circulating CRP, IL-6, IL-10, and TNF-
(5) Study design: This study enrolled case-control or cohort studies that explored the relations of one or more SNPs of inflammatory mediator genes with the AAA risk.
Exclusion criteria:
(1) Studies that involved animal models;
(2) Studies that investigated participants suffering from concurrent connective tissue diseases (like Ehlers Danlos syndrome or Marfan’s syndrome);
(3) Duplicated studies;
(4) Reviews;
(5) Studies that mentioned non-inflammatory mediators.
Two reviewers (Z. Z. and H. Z.) selected the studies that were in line with the
eligibility criteria for systematic review. Titles and abstracts of all the
recruited articles were read by these two researchers, and differences in opinion
between them were resolved by a third researcher (Y. H.). The
Newcastle-Ottawa Scale (NOS) [18] was utilized to evaluate the quality of the
enrolled studies. After NOS guideline modification, studies with the NOS score
Data were independently collected from the eligible studies by two researchers
(Z. Z., and H. W.) by adopting the unified report form. Any disagreement between
them was settled by a third author (Y. H). The collected data included the
contents of circulating inflammatory mediators and their gene polymorphisms was
also assessed. The study was grouped based on population characteristics, study
design, author, year of publication, country, ethnicity, numbers of cases and
controls, genotyping method, gender and genotype frequencies of each group. In
addition, this study standardized SNP annotations by adopting the reference
sequence (rs) numbers. dbSNP resource (http://www.ncbi.nlm.nih.gov/snp) was
employed to assign the missing rs numbers by the NCBI server if available.
Information on the polymorphisms of CRP, IL-6, IL-10, and TNF-
This study applied I
SMDs were used to calculate the correlations between circulating CRP, IL-6,
IL-10, and TNF-a levels and the AAA susceptibility. p-values were
calculated by Mantel-Haenszel statistical approach under the allele, homozygous,
heterozygous, dominant and recessive models. Unadjusted ORs were calculated based
on original data on genotype frequencies, and were pooled for meta-analysis by
using the random effects model. Allele and genotype comparisons study separately
assessed the risks of variant vs. wild-type (WT) alleles, heterozygote vs. WT
homozygotes, and variant vs. WT homozygotes. Subsequently, our study individually
evaluated the risks of recessive and dominant effects for variant allele
(heterozygote + variant homozygote vs. WT homozygote and variant homozygote vs.
WT homozygote + heterozygote). Due to the small number of rare homozygotes,
overestimated CIs were obtained by adopting the recessive model of analysis
compared with the dominant model [20]. p
The study inclusion and exclusion flow chart of this systemic review and meta-analysis is presented in Fig. 1, which is in line with the PRISMA group. According to the search strategies, 121 relevant documents were preliminarily obtained after removing 444 duplicates. Post screening, 88 articles met the standard, and a total of 41 citations were determined. After full-text screening, 20 studies were found that reported the associations between CRP level/SNPs and AAA susceptibility, 17 mentioned the relations of IL-6 level/SNPs with AAA susceptibility, 7 reported the relationship between TNF-a level/SNPs and AAA susceptibility, and 10 mentioned the associations of IL-10 level/SNPs and AAA susceptibility.
 Fig. 1.
Fig. 1.Flow chart demonstrating study inclusion and exclusion criteria.
Overall, 6 SNPs in four genes representing inflammatory mediators were examined
(Table 1, Ref. [11, 12, 13, 14, 15, 21, 22, 23, 24, 25]). Table 1 summarizes the
previously reported associations between SNPs in CRP, IL-6, IL-10, and
TNF-
| Author | Year | Group | Number | Study | Country | Inflammatory | Detection method | Gene | Genotype | Genotyping method | Age (years) | Smoking (n) | Male/Females (n) | Hypertension (n) | BMI (kg⁄m |
Diabetes (n) | Dyslipidemia |
| Badger et al. [12] | 2009 | Case | 248 | Case-control | UK | CRP | rs3091244 | TT/AA/TA = 33; CT/CA = 108; CC = 107 | 174 | ||||||||
| Control | 400 | TT/AA/TA = 46; CT/CA = 182; CC = 172 | 228 | ||||||||||||||
| Smallwood et al. [13] | 2008 | Case | 677 | Case-control | AUS | IL-6 | rs1800795 | GG = 222; GC = 300; CC = 104 | TaqMan | 73.3 | 579 | 27.2 | 67 | ||||
| Control | 656 | GG = 224; GC = 302; CC = 124 | 72.3 | 420 | 26.6 | 38 | |||||||||||
| Bown et al. [23] | 2007 | Case | 389 | Case-control | UK | IL-10 | ELISA | rs1800896 | AA = 104; GA = 201; GG = 84 | 460 | 355/34 | 282 | 31 | ||||
| Control | 404 | AA = 81; GA = 205; GG = 118 | 323 | 395/9 | 179 | 37 | |||||||||||
| Duellman et al. [24] | 2014 | Case | 141 | Case-control | USA | IL-10 | rs1800896 | AA = 42; GA = 60; GG = 39 | |||||||||
| Control | 168 | AA = 48; GA = 77; GG = 43 | 82 | 74/94 | 94 | 29.3 |
83 | ||||||||||
| Wang et al. [25] | 2015 | Case | 425 | Case-control | China | IL-10 | rs1800896 | AA = 64; GA = 161; GG = 156 | PCR-RFLP | 273 | 315/66 | 256 | |||||
| Control | 381 | AA = 46; GA = 151; GG = 184 | 209 | 315/66 | 166 | ||||||||||||
| Saratzis et al. [11] | 2013 | Greece Case | 351 | Case-control | Greece | CRP | Nephelometry | rs3091244 | TT/AA/TA = 70; CT/CA = 165; CC = 116 | 69 |
257 | 322/29 | 271 | 77 | |||
| Greece Control | 391 | TT/AA/TA = 35; CT/CA = 129; CC = 227 | 73 |
311 | 327/64 | 300 | 78 | ||||||||||
| UK Case | 371 | UK | CRP | TT/AA/TA = 82; CT/CA = 193; CC = 96 | 72 |
326 | 347/24 | 187 | 58 | ||||||||
| UK Control | 362 | TT/AA/TA = 54; CT/CA = 167; CC = 141 | 71 |
302 | 345/17 | 178 | 52 | ||||||||||
| Shangwei et al. [22] | 2017 | Case | 155 | Case-control | China | CRP | rs1800947 | GG = 0; GC = 8; CC = 143 | 69.2 |
132 | 138/17 | 108 | 118 | ||||
| GG = 0; GC = 16; CC = 286 | 69.5 |
167 | 276/34 | 143 | 138 | ||||||||||||
| Control | 310 | rs1205 | TT+TC = 127; CC = 23 | 69.2 |
132 | 138/17 | 108 | 118 | |||||||||
| TT+TC = 249; CC = 53 | 69.5 |
167 | 276/34 | 143 | 138 | ||||||||||||
| Jabłońska et al. [15] | 2020 | Case | 104 | Case-control | Austria | IL-6 | ELISA | rs1800795 | GG = 24; GC = 52; CC = 28 | PCR-RFLP | 70.5 |
61 | 27.54 |
||||
| GG = 43; GC = 46; CC = 23 | 69.7 |
11 | 27.41 |
||||||||||||||
| Control | 112 | TNF- |
rs1800629 | AA = 12; GA = 47; GG = 45 | 70.5 |
61 | 27.54 |
||||||||||
| AA = 10; GA = 32; GG = 70 | 69.7 |
11 | 27.41 |
||||||||||||||
| Bown et al. [14] | 2003 | Case | 100 | Case-control | UK | IL-6 | rs1800795 | GG = 33; GC = 48; CC = 19 | PCR-RFLP, SSP | 84 | 55 | 6 | 20 | ||||
| GG = 28; GC = 57; CC = 15 | 73 | 36 | 15 | 14 | |||||||||||||
| TNF- |
rs1800629 | AA = 6; GA = 30; GG = 64 | 84 | 55 | 6 | 20 | |||||||||||
| Control | 100 | AA = 5; GA = 32; GG = 63 | 73 | 36 | 15 | 14 | |||||||||||
| IL-10 | rs1800896 | AA = 34; GA = 49; GG = 17 | 84 | 55 | 6 | 20 | |||||||||||
| AA = 23; GA = 48; GG = 29 | 73 | 36 | 15 | 14 | |||||||||||||
| Qin et al. [21] | 2018 | Case | 155 | Case-control | China | CRP | rs1800947 | GG = 0; GC = 8; CC = 143 | Sequenom’s Mass-ARRAY | 69.2 |
132 | 138/17 | 108 | 24.4 |
76 | ||
| GG = 1; GC = 8; CC = 144 | 69.6 |
69 | 138/17 | 64 | 25.3 |
34 | |||||||||||
| Community Control | 155 | GG = 0; GC = 8; CC = 143 | 69.2 |
132 | 138/17 | 108 | 24.4 |
76 | |||||||||
| GG = 1; GC = 8; CC = 142 | 69.5 |
98 | 138/17 | 79 | 24.2 |
27 | |||||||||||
| rs1205 | TT = 51; TC = 76; CC = 23 | 69.2 |
132 | 138/17 | 108 | 24.4 |
76 | ||||||||||
| Hospital Control | 155 | TT = 48; TC = 73; CC = 31 | 69.6 |
69 | 138/17 | 64 | 25.3 |
34 | |||||||||
| TT = 51; TC = 76; CC = 23 | 69.2 |
132 | 138/17 | 108 | 24.4 |
76 | |||||||||||
| TT = 48; TC = 80; CC = 22 | 69.5 |
98 | 138/17 | 79 | 24.2 |
27 |
| Author | Year | Group | Number | Study | Country | Inflammatory | Detection method | Age (years) | Smoking (n) | Male/Females (n) | Hypertension (n) | BMI (kg⁄m |
Diabetes (n) | Dyslipidemia (n) |
| Palazzuoli et al. [35] | 2008 | Case | 98 | Case-control | Italy | CRP | 74 |
59 | 76/22 | 29 |
||||
| Control | 82 | 74 |
31 | 50/32 | 28 |
|||||||||
| Cersit et al. [26] | 2021 | Case | 150 | Case-control | Turkey | CRP | 66.8 |
38 | 117/33 | 58 | 27.1 |
57 | 61 | |
| Control | 100 | 64.7 |
23 | 75/25 | 30 | 25.5 |
32 | 36 | ||||||
| Dawson et al. [52] | 2007 | Case | 25 | Case-control | UK | CRP | 73 | 27/0 | 4 | |||||
| Control | 12 | 50 | 3/12 | 0 | ||||||||||
| Golledge et al. [28] | 2007 | Case | 318 | Case-control | AUS | CRP | ELISA | |||||||
| Control | 634 | |||||||||||||
| Wanhainen et al. [40] | 2005 | Case | 35 | Case-control | Sweden | CRP | 3 | |||||||
| Control | 140 | 17 | ||||||||||||
| Parry et al. [37] | 2010 | Case | 75 | Case-control | UK | CRP and IL-6 | ELISA | 72 | 26.95 | 13 | ||||
| Control | 90 | 72 | 27.32 | 2 | ||||||||||
| Golledge et al. [27] | 2010 | Case | 312 | Case-control | AUS | CRP | 71.8 | 268 | 158 | 21 | 150 | |||
| Control | 1046 | 72.8 | 650 | 429 | 78 | 366 | ||||||||
| Pan et al. [36] | 2011 | Case | 45 | Case-control | China | CRP | 76 | 31 | 39/6 | 21 | 23.7 |
7 | ||
| Control | 49 | 74 | 24 | 41/8 | 14 | 24.8 |
2 | |||||||
| Lindqvist et al. [34] | 2012 | Nonruptured AAA Case | 78 | Case-control | Sweden | CRP and IL-6 | ELISA | 71 | 34 | 62/16 | ||||
| Control | 36 | 72 | 15 | 30/6 | ||||||||||
| Ruptured AAA Case | 41 | 73 | 17 | 33/8 | ||||||||||
| Ramos-Mozo et al. [38] | 2012 | Case | 30 | Case-control | Spain | CRP | ELISA | 69 |
17 | 30/0 | 15 | 3 | 9 | |
| Control | 30 | 67 |
12 | 29/1 | 19 | 5 | 16 | |||||||
| Kadoglou et al. [32] | 2012 | Case | 108 | Case-control | Greece | CRP, IL-6, and IL-10 | 72 |
41 | 28.98 |
21 | ||||
| Control | 42 | 69 |
6 | 29.36 |
12 | |||||||||
| Hellenthal et al. [30] | 2012 | Small AAA Case | 59 | Case-control | Netherlands | CRP | ELISA | 70.1 |
55 | 45/14 | 40 | 4 | ||
| Control | 69 | 71.6 |
30 | 59/10 | 24 | 24 | ||||||||
| Medium AAA Case | 64 | 71.7 |
58 | 55/9 | 40 | 8 | ||||||||
| Control | 69 | 71.6 |
30 | 59/10 | 24 | 24 | ||||||||
| Large AAA Case | 95 | 72.7 |
90 | 89/6 | 52 | 7 | ||||||||
| Control | 69 | 71.6 |
30 | 59/10 | 24 | 24 | ||||||||
| Treska et al. [46] | 2002 | Case | 32 | Case-control | AUS | IL-6 and TNF- |
||||||||
| Control | 14 | |||||||||||||
| Qin et al. [21] | 2013 | Case | 31 | Case-control | China | CRP | ELISA | 63.45 |
19 | 25/6 | 22 | 26.26 |
||
| Control | 32 | 58.88 |
7 | 15/17 | 1 | 24.55 |
||||||||
| Jones et al. [31] | 2016 | Case | 442 | Case-control | New Zealand | CRP and IL-10 | 75.0 |
334/108 | 262 | 50 | ||||
| Control | 970 | 68.5 |
741/229 | 307 | 66 | |||||||||
| Sohrabi et al. [39] | 2014 | Case | 86 | Case-control | UK | CRP | 73 | 67/19 | 48 | 27 | 11 | |||
| Control | 158 | 71 | 114/44 | 73 | 27 | 22 | ||||||||
| Wong et al. [41] | 2013 | Case | 311 | Case-control | AUS | CRP | 77.7 | 292 | 2971 | 27 | 67 | 255 | ||
| Control | 3922 | 76.5 | 2549 | 247 | 26.5 | 596 | 2773 | |||||||
| Golledge et al. [29] | 2007 | Case | 233 | Case-control | AUS | CRP | ELISA | 76.0 |
188 | 233/0 | 113 | 18 | 149 | |
| Control | 233 | 74.6 |
145 | 233/0 | 81 | 12 | 122 | |||||||
| Wallinder et al. [49] | 2009 | Small AAA Case | 38 | Case-control | Sweden | IL-6 and IL-10 | 70 | 16 | 27/11 | |||||
| Control | 41 | 72 | 18 | 33/8 | ||||||||||
| EAAA Case | 40 | 71 | 19 | 35/5 | ||||||||||
| Control | 41 | 72 | 18 | 33/8 | ||||||||||
| Fowkes et al. [44] | 2006 | Case | 89 | Case-control | UK | IL-6 | ELISA | 73.5 |
79 | 64/25 | 25.0 |
|||
| Control | 98 | 73.5 |
67 | 70/28 | 26.3 |
|||||||||
| Juvonen et al. [45] | 1997 | Case | 50 | Case-control | Finland | IL-6 | Radioimmunoassay RIA | 40/10 | ||||||
| Control | 38 | 17/21 | ||||||||||||
| Ahnström et al. [42] | 2010 | Case | 343 | Case-control | Sweden | IL-6 | 74 |
119 | 271/72 | 25.4 |
41 | |||
| Control | 214 | 68 |
26 | 99/115 | 27.1 |
12 | ||||||||
| Lindberg et al. [10] | 2016 | Case | 116 | Case-control | Sweden | IL-6 and TNF- |
ELISA | |||||||
| Control | 239 | |||||||||||||
| Buffa et al. [43] | 2019 | Case | 60 | Case-control | Italy | IL-6 | ELISA | 70 | 42 | 40/20 | 38 | 25.9 | 10 | 12 |
| Control | 80 | 72 | 5 | 35/45 | 2 | 25.8 | 0 | 2 | ||||||
| Liao et al. [33] | 2015 | Small AAA Case | 385 | Case-control | Denmark | CRP and IL-10 | ELISA | |||||||
| Control | 200 | |||||||||||||
| Large AAA Case | 91 | |||||||||||||
| Control | 200 | |||||||||||||
| Aria et al. [50] | 2018 | Case | 5 | Case-control | Iran | IL-10 | ||||||||
| Control | 5 | |||||||||||||
| Windsor et al. [51] | 2017 | Case | 20 | Case-control | AUS | IL-10 | ELISA | 74 | 13 | 14 | 27 | 1 | 16 | |
| Control | 20 | 71 | 11 | 5 | 26 | 0 | 6 | |||||||
| Treska et al. [47] | 2007 | Ruptured AAA Case | 54 | Case-control | Czech | IL-6 and TNF- |
||||||||
| Control | 15 | |||||||||||||
| Asymptomatic ruptured AAA Case | 41 | |||||||||||||
| Treska et al. [48] | 2011 | Case | 345 | Case-control | Unknown | IL-6 and TNF- |
||||||||
| Control | 30 | |||||||||||||
| Badger et al. [12] | 2009 | Case | 248 | Case-control | UK | CRP | 174 | |||||||
| Control | 400 | 228 | ||||||||||||
| Bown et al. [23] | 2007 | Case | 389 | Case-control | UK | IL-10 | ELISA | 460 | 355/34 | 282 | 31 | |||
| Control | 404 | 323 | 395/9 | 179 | 37 |
Table 1 displays the basic study characteristics. All the recruited articles
were observational studies that were published from year 2000 to 2019 involving
9007 AAAs patients and 14,315 normal control individuals. Among them, 40 articles
reported the circulating inflammatory mediators enrolled in this meta-analysis (n
= 20 for CRP, n = 17 for IL-6, n = 8 for IL-10, and n = 9 for TNF-
Initially, the circulating CRP level was
used as a risk factor to explore whether the plasma CRP level affected the
occurrence of AAAs. According to our results, the SMD was 0.30 mg/L (95% CI:
0.17–0.43, p
 Fig. 2.
Fig. 2.The forest plot illustrating the SMD and 95% CI for the association between circulating CRP levels and abdominal aortic aneurysm. (A) Meta-analysis of plasma CRP levels. (B) Subgroup analysis of plasma CRP levels. (C) Sensitivity analysis of plasma CRP levels. (D) Egger test of plasma CRP levels.
Sensitivity analysis (Fig. 2C) was also carried out. It was found that each of the eliminated studies slightly affected the pooled results, with no obvious change in the impact of every single study, thus substantiating the result of our analysis.. Egger’s test was performed to identify the potential source of publication bias. As shown in Fig. 2D, p = 0.793 was obtained, indicating no significant evidence of publication bias.
It was found that the CRP allele rs3091244 (minor allele
frequency = 36.8%) was significantly associated with AAA and the recessive
models of inheritance (A/A+T/T +A/T vs A/C+T/C+C/C, I
 Fig. 3.
Fig. 3.The forest plot demonstrating the OR and 95% CI association between CRP rs3091244, rs1205, rs1800947 and abdominal aortic aneurysm. (A) rs3091244 dominant gene model. (B) rs3091244 recessive gene model. (C) rs3091244 homozygous model. (D) rs3091244 heterozygous model. (E) rs3091244 allele model. (F) rs1205 dominant gene model. (G) rs1800947 allele model. (H) rs1800947 heterozygous model.
p
Difference in the rs1800947 locus showed no significant
difference between the allele and the heterozygous models (p = 1.000).
The forest plot is exhibited in Fig. 3G,H. Under the allele model (G vs C),
the susceptibility to AAAs was not significantly related to the dominant model of
inheritance (OR = 1.15, 95% CI: 0.68–1.95, I
A total of 17 relevant studies were included in the IL-6 group. Interestingly,
the circulating IL-6 levels of AAA patients were dramatically elevated compared
to the control group (SMD = 0.34, 95% CI: 0.20–0.49, I
 Fig. 4.
Fig. 4.The forest plot comparing the SMD and 95% CI association between circulating interleukin-6 levels and abdominal aortic aneurysm, and the OR and 95% CI association between rs1800795 and abdominal aortic aneurysm. (A) Meta-analysis of plasma IL-6 levels. (B) rs1800795 dominant gene model. (C) rs1800795 recessive gene model. (D) rs1800795 homozygous model. (E) rs1800795 heterozygous model. (F) rs1800795 allele gene model.
Sensitivity analysis was also conducted in order to explore the potential heterogeneity source. As a result, the inclusion of each study had little effect on the pooled results. The Egger’s test was also performed, as shown in Supplementary File 3, and the p-value was 0.469, revealing low publication bias and reliable analysis results.
This study analyzed the relationship between the IL-6 locus
rs1800795 and the risk of AAA. The dominant gene model (G/G+G/C vs C/C, OR =
1.04, 95% CI: 0.81–1.33, I
It was assessed that individuals carrying the G mutant gene of rs1800795 might be less susceptible to AAA than those with C allele, with greater impact on the Australian and Austrian populations. The Egger’s test was also conducted to test the publication bias, as shown in Supplementary File 3. Small publication bias was detected, indicating that the results of this meta-analysis had a certain degree of credibility.
A total of 8 studies were included, and no
statistically significant increase or decrease in circulating IL-10 levels were
observed between AAA patients in comparison with controls (SMD = –0.01, 95% CI:
–0.09–0.06, I
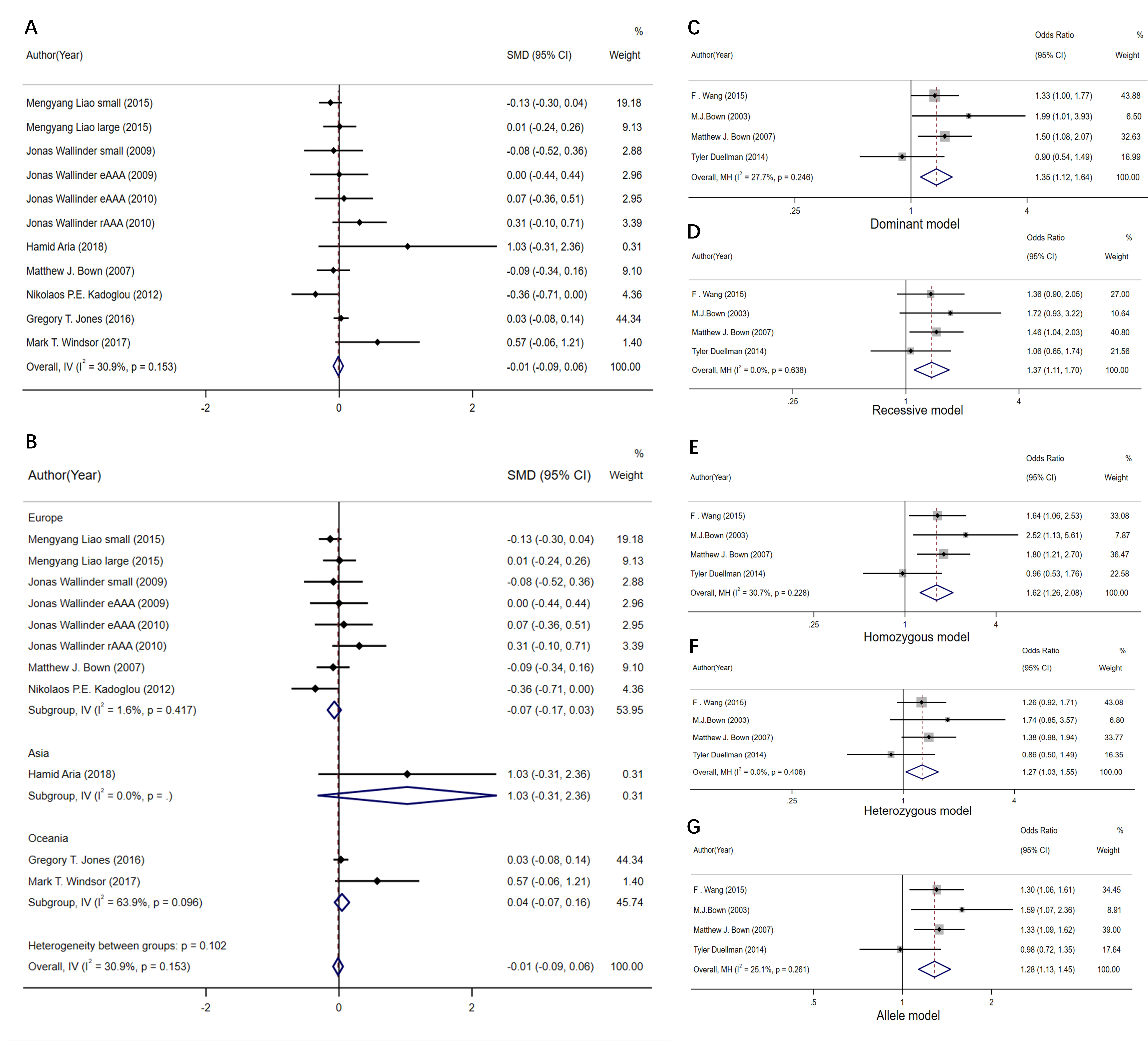 Fig. 5.
Fig. 5.The forest plot comparing the SMD and 95% CI association between plasma interleukin-10 levels and abdominal aortic aneurysm, and the OR and 95% CI association between rs1800896 and abdominal aortic aneurysm. (A) Meta-analysis of plasma IL-10 levels. (B) Subgroup analysis of plasma IL-10 levels. (C) rs1800896 dominant gene model. (D) rs1800896 recessive gene model. (E) rs1800896 homozygous model. (F) rs1800896 heterozygous model. (G) rs1800896 allele model.
At the same time, sensitivity analysis was performed on the IL-10 locus
rs1800896. Compared to healthy subjects, the dominant gene model of
IL-10/rs1800896 SNP (A/A+A/G vs G/G) was more prevalent among AAA patients (OR =
1.35, 95% CI: 1.12–1.64, I
AAA cases showed an increased heterozygous genotype rate for
IL-10 (rs1800896) SNP (A/A vs G/G) compared to normal
controls (OR = 1.62, 95% CI: 1.26–2.08, I
Based on the above results, it was inferred that the A mutant gene might have a higher susceptibility to AAA than the G allele, which also had a greater impact on the Chinese and British populations. Therefore, sensitivity analysis was further extended, and Egger’s test for publication bias was performed on this set of data under the allelic model. The analysis indicated a certain degree of credibility in our results, as shown in Supplementary File 4.
8 studies were included to investigate circulating TNF-
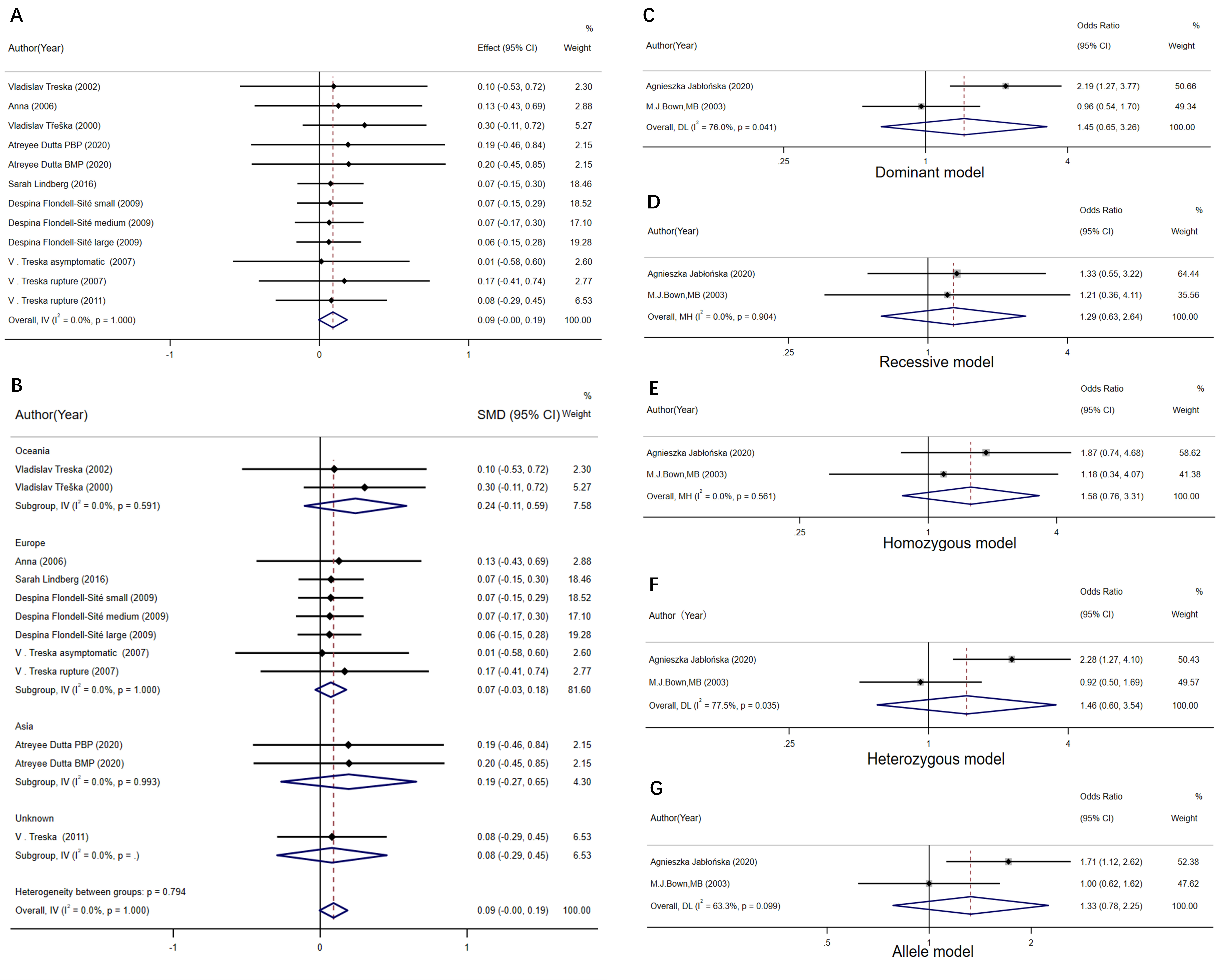 Fig. 6.
Fig. 6.The forest plot comparing the SMD and 95% CI association
between plasma TNF-
This study also analyzed the susceptibility of TNF-
Several articles identifying the AAA-related
genetic risk factors have been published [55, 56]. Numerous previous studies have
reported that the levels of various inflammatory factors or cytokines (ILs, TNF,
and NO) and their polymorphisms are closely associated with AAA onset. However,
the relationships between inflammatory factor gene SNPs and AAA susceptibility
remain unknown because inconsistent results have been obtained from studies
having small sample sizes. Currently, it is still not conclusive regarding the
significance of results in each published study. This work aimed to explore the
associations of inflammatory mediator levels and their gene polymorphisms with
the susceptibility to AAAs, and summarize the susceptibility factors of AAAs.
Firstly, this study examined the levels of four inflammatory factors in human
AAA. In this study, healthy subjects were enrolled in the control group, whereas
AAA patients were enrolled in the experimental group to detect their circulating
levels of CRP, IL-6, IL-10, and TNF-
In this study we initially analyzed the levels of four continuous variables
(CRP, IL-6, IL-10, and TNF-
This meta-analysis also indicated that the
IL-6 level had a significant effect on the occurrence of AAA. It was also found
that the IL-6 levels were associated with AAA susceptibility in the Asian,
European and Oceanian populations by subgroup analysis. IL-6 is the
pro-inflammatory factor that plays a critical role in triggering systemic
inflammatory response [62]. As revealed by our meta-analysis results, the
circulating IL-6 levels were dramatically elevated among AAA cases (p =
0.030); likewise, IL-6 levels were elevated among AAA patients, while IL-10
levels were not significantly changed, which was supported by results of IL-6
obtained from aortic tissues [63]. IL-10 is a strong anti-inflammatory factor,
and the imbalance between IL-6 and IL-10 may interpret the inflammatory
heterogeneities between AAA patients and normal subjects [64]. Recently, a
relevant study also reported that the circulating IL-10 and TNF-
In this study, six SNP loci (rs3091244, rs1800947, rs1205 for CRP; rs1800795 for IL-6; rs1800896 for IL-10; and rs1800629 for TNF) were analyzed.
For CRP, this study detected seven possible SNPs with key functions, including
rs3093058, rs3091244, rs1417938, rs1800947, rs3093066, rs1205,
and rs2808630. As revealed by haplotype analysis, tri-allelic rs3091244 (G
Simultaneously, the CRP gene locus rs1800947 was under the allele model (G vs C) and the heterozygous model (G/C vs C/C). There was no difference in the susceptibility to AAA between the GC genotype population and the CC genotype population, or between individuals carrying the G mutant gene and those carrying the C allele of rs1800947. There was no difference in the susceptibility to AAA, and the CRP locus rs1205 was under the dominant gene model (T/T+T/C vs C/C). In addition, difference in the susceptibility to AAA was not significant between the TT, TC and CC genotypes.
ILs are responsible for transmitting information, activating and modulating immunocytes, and mediating the growth/activation/differentiation of B and T cells. They also have key functions in inflammatory response. IL-6 is produced by aneurysm, and its expression is associated with aneurysmal surface area [52]. The circulating IL-6 levels are higher among AAA cases [52, 68]. Angiotensin II has a certain effect on AAAs by regulating the IL-6 pathway in mice.
The associations of IL-6 SNPs with AAA susceptibility have been analyzed among diverse populations [14, 69], however, no consistent results were obtained in these studies. In the present study, we found that individuals carrying the G mutant gene of rs1800795 (IL-6 gene locus) might be less susceptible to AAA than those with C allele (OR = 0.91). In addition to that, the homozygous or heterozygous recessive genotypes of IL-6/rs1800795 SNPs might not increase the AAA susceptibility. IL-6/rs1800795 SNPs were not related to AAA risk, as reported in several other studies [14, 15, 70]. Jones et al. [71] suggested that a mutation existed in more than one allele of -174G/C SNP, which might be related to cardiovascular mortality for patients with small aneurysms in the future.
IL-10 accounts for the potent anti-inflammatory factor, which inhibits the function of macrophages and indirectly affects T lymphocytes through regulating cell signals related to T cell antigen presentation. In this meta-analysis, individuals carrying the IL-10 locus rs1800896, the A mutant gene, might have a higher susceptibility to AAA than individuals with the G allele either in the recessive (A/A vs G/G+A/G) or the dominant (A/A+A/G vs G/G) gene model.
TNF was first discovered because of its anti-cancer activity. It is now
considered to coordinate the extremely complex response of the body towards
injury and infection. The TNF-
Certain limitations should be noted in the present meta-analysis. Firstly, this meta-analysis was not adjusted for correcting the commonly seen risk factors for AAA (such as smoking, age, and gender), because no consistent reports on these factors are available in any independent study. As a result, those P-values and ORs obtained might be the over-estimated true biological risks. Additionally, there were obvious inter-study differences in the control population screening approach. Among our enrolled articles, merely 4 selected the control subjects by ultrasound, while other articles selected the non-specific approaches like questionnaires or did not mention control selection at all. Therefore, certain articles might have false negative diagnoses. In this regard, the control populations might not actually represent the normal (AAA-free) individuals. Moreover, based on the statistical analysis, many articles (or many SNPs examined in the present meta-analysis) did not have adequate capacity in detecting the relations because having a small study size. Lastly, the above findings should be interpreted under the meta-analysis constraints. The cumulative results might be confounded by variable quality based on methodological, heterogeneity, and publication bias among the enrolled articles. Our analyses suggest that the above factors might not interpret the results in their entirety; nevertheless, our results should be interpreted with caution due to the relatively small number of articles enrolled in the present meta-analysis.
We report a systematic review and meta-analysis on the association of inflammation mediators level and gene polymorphisms and AAA. The meta-analysis demonstrated a significant difference between C-reactive protein (CRP) and IL-6 levels in patients with and without AAA. Our analyses suggest that individuals with A and T mutant genes at locus rs3091244 (CRP) might have a higher tendency of AAA susceptibility than those with C allele. In addition, individuals with a G mutant gene at locus rs1800795 (IL-6) might be less susceptible to AAA than those with C allele. Further investigation of this marker may improve our understanding of AAA pathogenesis and benefit targeted AAA screening programs.
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
HW and ZZ were in charge of study conception and design. HZ, ZZ, DJ and FY were responsible for data analysis and interpretation. YH, JC, XZ and ZZ wrote the manuscript. YH and KL was responsible for the final approval of the manuscript. HZ, PG, KY and YH were in charge of statistical analysis. YH and KL was responsible for overall responsibility.
Not applicable.
Not applicable.
This work was supported by the Fundamental Research Funds for the Central Universities (grant number: DUT19RC(3)076), the National Natural Science Foundation of China (grant number: 81600370), and the China Postdoctoral Science Foundation (grant number: 2018M640270).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
