Academic Editors: Fabrizio D’Ascenzo and Brian Tomlinson
Background: Cases of infective endocarditis (IE) with
The number of endocarditis episodes diagnosed worldwide in one year could be as high as 250,000 cases [1], and the mortality rate within one year exceeds 30%. Especially when large vegetations form, often with multiple severe complications, the situation is often life-threatening, requiring early diagnosis and timely intervention.
Vegetations of infective endocarditis can form on the native or prosthetic heart
valve. Vegetations
Twenty-two patients with IE complicated with large vegetation were included in the study. They were diagnosed by ultrasound, intraoperative observation, pathogen and postoperative biopsy results according to modified Duke’s criteria [9]. Data include age, gender, risk factor such as basic heart diseases, signs and symptoms (fever, dyspnea, cardiac murmur, other signs of embolism, etc.), laboratory indicators (white blood cells [WBC], hemoglobin, neutrophil percentage [NE%], lymphocyte percentage [LY%], C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], procalcitonin [PCT], N-terminal pro-brain natriuretic peptide [NT-proBNP], D-dimer, blood culture, drug sensitivity test results, etc.), transthoracic echocardiography (TTE), treatment, comorbidity, and outcomes. Twenty-two age- and sex-matched IE cases with 10–20 mm vegetations were used as a control group.
The main analysis method was comparative analysis shown in the tables and a
tornado diagram. The highest body temperature from the onset of the initial
symptom until surgery and blood test results at admission were recorded. Surgical
risk was evaluated using the established European System For Cardiac Operative
Risk Evaluation II (EuroSCORE II) [10]. The search strategy for all case reports
(2020 to 2022) of IE with
Data were processed with SPSS 26 (IBM, Armonk, NY, USA).
Enumeration data are shown as n (%), and
were compared with the chi-square tests between the two groups. Considering that
the sample size is relatively small due to the rarity of cases, all measurement
data are expressed in median (25th–75th percentiles [Q1–Q3]) and were analyzed
using nonparametric tests. Preoperative WBC count, NE%, CRP, ESR, prothrombin
time (PT), fibrinogen degradation product (FDP), activated partial thromboplastin
time (APTT), direct bilirubin, NT-proBNP, lactate dehydrogenase (LDH), creatine
kinase (CK), PCT, D-dimer were compared with the upper reference limit, while
hemoglobin, LY%, total protein, albumin were compared with the lower limit of
the reference value. The single-sample Wilcoxon test was conducted to compare
patients’ laboratory indicators with reference values. The Wilcoxon paired rank
test was used for preoperative and postoperative comparisons. Quantitative data
comparing the
Basic information and clinical characteristics of the 22 patients with
| Characteristics | 10–20 mm group | p value | ||
| Demographics | ||||
| Male sex | 10 (45.5) | 11 (50.0) | 0.763 | |
| Age (years) | 41(33–59) | 52 (31–52) | 0.324 | |
| 4 (18.1) | 6 (27.3) | - | ||
| 30–60 | 12 (54.5) | 13 (59.1) | - | |
| 6 (27.3) | 3 (13.6) | - | ||
| EuroSCORE II | 4 (3–5) | 3 (2–4) | 0.062 | |
| Risk factors | ||||
| Basic heart disease | 13 (59.0) | 11 (50.0) | 0.545 | |
| CHD | 6 (27.3) | 11 (50.0) | 0.122 | |
| Previous rheumatic heart disease | 3 (13.6) | 0 | - | |
| CDRIE | 2 (9.1) | 0 | - | |
| HCM | 1 | 0 | - | |
| DCM | 1 | 0 | - | |
| Recent skin infection | 2 (9.1) | 0 | - | |
| Diabetes | 2 (9.1) | 1 | - | |
| CKD5 | 1 | 1 | - | |
| Manifestations | ||||
| Fever | 18 (81.8) | 14 (63.6) | 0.176 | |
| Maximum body temperature (°C) | 38.9 (37.8–39.7) | 38.1 (37.2–39.1) | 0.252 | |
| Dyspnea or chest pain | 10 (45.5) | 6 (27.3) | 0.210 | |
| Cardiac murmur | 19 (86.4) | 21 (95.5) | 0.294 | |
| Arrhythmia | 9 (40.1) | 4 (18.2) | 0.099 | |
| Recent Complications | ||||
| Heart failure | 19 (86.4) | 10 (45.5) | 0.004* | |
| Embolism | 11 (50.0) | 6 (27.3) | 0.122 | |
| cerebral infarction | 6 (27.3) | 3 (13.6) | ||
| Spleen | 4 (18.2) | 3 (13.6) | ||
| limb vessel | 2 (9.1) | 0 | - | |
| Pulmonary embolism | 2 (9.1) | 0 | - | |
| Others | 4 (18.2) | 1 | ||
| Continuous variables are presented as median (Q1–Q3), counts as n (%). Abbreviations: EuroSCORE II, European System for Cardiac Operative Risk Evaluation II; CHD, congenital heart disease; CDRIE, cardiac device-related infective endocarditis; HCM, hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy; CKD5, chronic kidney disease stage 5. *p | ||||
In the
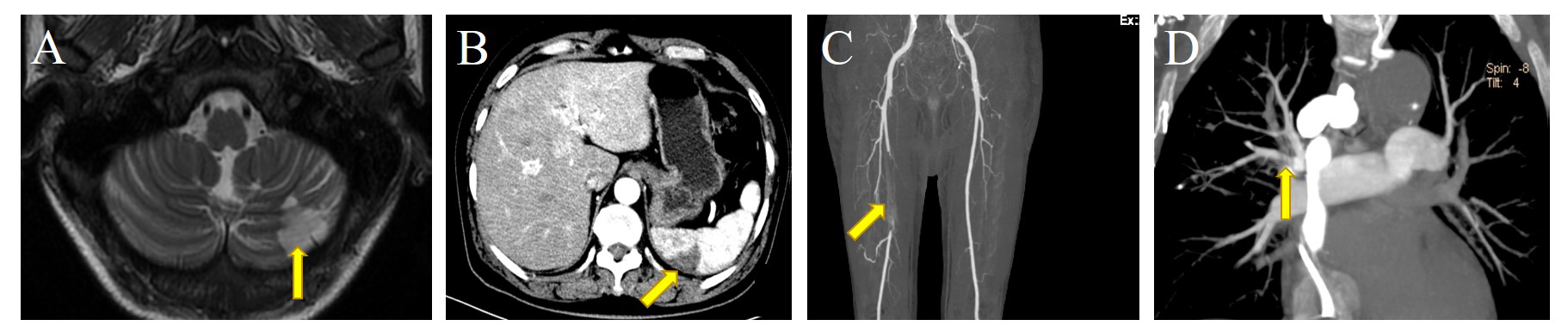 Fig. 1.
Fig. 1.Embolization of different sites in patients in this study. (A) Arrow: left cerebellar hemisphere infarction. (B) Arrow: spleen infarction, manifesting as subcapsular wedge-shaped reduced density of the spleen. (C) Arrow: the right femoral artery occlusion, with filling defect. (D) Arrow: pulmonary embolism.
In the
| Laboratory variables | Preoperative value | Reference value | p value (vs. reference) | 3rd post-operative day | p value (vs. postoperative value) |
| WBC count ( |
9.4 (7.4–13.4) | 3.5–9.5 | 0.615 | 13.2 (11.6–15.3) | 0.006† |
| Hemoglobin (g/L) | 89.5 (72.0–98.5) | 130.0–175.0 | 88.0 (76.8–102.8) | 0.851 | |
| Platelets ( |
191.0 (97.5–276.3) | 125.0–350.0 | NA | 209.0 (118.0–263.0) | 0.963 |
| NE (%) | 80.2 (69.6–87.5) | 40.0–75.0 | 85.9 (82.0–89.1) | 0.108 | |
| LY (%) | 15.5 (8.9–18.5) | 20.0–50.0 | 7.0 (5.2–11.6) | 0.029† | |
| CRP (mg/L) | 61.9 (32.3–83.4) | 76.7 (41.0–105.0) | 0.243 | ||
| ESR (mm/h) | 67.0 (34.8–82.5) | 72.3 (47.5–86.0) | 0.508 | ||
| APTT (s) | 31.8 (22.6–29.5) | 25.0–43.0 | NA | 35.8 (29.5–42.0) | 0.265 |
| Albumin (g/L) | 31.2 (27.9–35.1) | 40.0–55.0 | 31.2 (26.9–35.1) | 0.935 | |
| TBIL ( |
12.8 (9.5–17.9) | 1.7–17.1 | NA | 10.6 (7.6–19.6) | 0.639 |
| DBIL ( |
6.1 (4.2–10.7) | 0.783 | 5.8 (3.4–9.6) | 0.581 | |
| Creatinine ( |
111.5 (78.5–128.0) | NA | 68.6 (62.0–99.0) | 0.622 | |
| LDH (U/L) | 336.0 (217.7–403.5) | 120.0–250.0 | 0.013* | 391.0 (280.3–461.6) | 0.203 |
| Total protein (g/L) | 74.7 (62.4–74.7) | 65.0–85.0 | 0.733 | 65.1 (54.2–70.0) | 0.935 |
| PT (s) | 13.8 (12.9–14.9) | 10.0–16.0 | NA | 14.7 (13.8–21.9) | 0.026† |
| Urea (mmol/L) | 4.4 (2.8–9.1) | 2.6–7.5 | 0.465 | 5.7 (4.3–9.0) | 0.445 |
| NT-proBNP (pg/mL) | 1686 (966.1–3535.0) | 3458 (1168.0–10979.0) | 0.012† | ||
| ALT (U/L) | 23.6 (15.3–36.5) | 7.0–40.0 | NA | 19.7 (13.6–35.2) | 0.494 |
| AST (U/L) | 24.6 (18.0–39.6) | 13.0–35.0 | 0.277 | 29.6 (20.7–53.1) | 0.117 |
| CK (U/L) | 28.9 (26.7–59.0) | 40.0–200.0 | 0.845 | 66.5 (27.4–269.0) | 0.034† |
| CK-MB (U/L) | 11.4 (7.2–16.9) | NA | 14.9 (10.3–24.8) | 0.569 | |
| PCT (ng/mL) | 1.5 (0.2–2.4) | 2.3 (1.2–5.4) | 0.017† | ||
| D-dimer (mg/L) | 0.7 (0.4–1.4) | 0.024* | 1.1 (0.7–3.0) | 0.019† | |
| cTn I (ng/mL) | 0.1 (0.0–0.2) | 0.043* | 1.5 (0.4–2.2) | 0.005† | |
| Variables are presented as median (Q1–Q3). NA (not applicable): that the
laboratory index was basically normal, which is significantly lower than the high
reference value and significantly higher than the low reference value. Abbreviations: WBC, white blood cells; NE%, neutrophils percentage; LY%, lymphocytes percentage; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; APTT, activated partial thromboplastin time; TBIL, total bilirubin; DBIL, direct bilirubin; LDH, lactate dehydrogenase; PT, prothrombin time; NT-proBNP, N-terminal pro-brain natriuretic peptide; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CK-MB, creatine kinase MB; PCT, procalcitonin; cTn I, cardial troponin I. *p | |||||
All 22 patients got blood cultures more than two times. Fifteen patients were
positive in blood culture. We found Streptococcus in six cases,
Staphylococcus aureus in three cases, and one case each of
Abiotrophia defectiva, Actinomyces nasicola, Klebsiella
pneumonia, Granulicatella adiacens, and Aspergillus.
Interestingly, in the EuroSCORE II
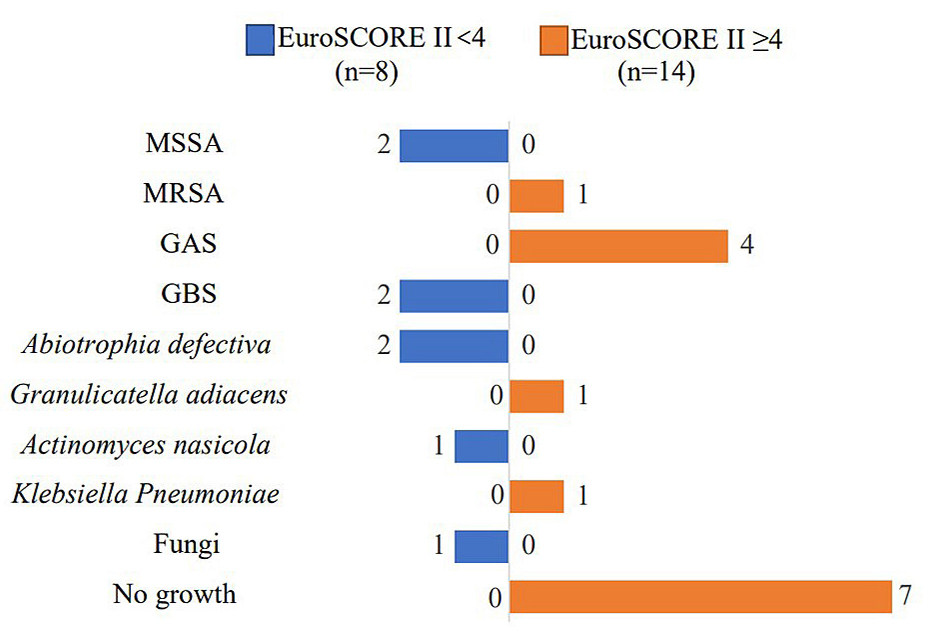 Fig. 2.
Fig. 2.Acquisition of infective endocarditis and causative organisms
(N= 22). Blue: EuroSCORE II
| Antibiotics | Sensitive pathogen | 10–20 mm group | ||||
| Staphylococcus aureus (n = 3) | Streptococcus (n = 6) | Abiotrophia defectiva (n = 2) and Actinomyces nasicola (n = 1) | Total (N = 12) | Streptococcus (n = 10) | Total (N = 12) | |
| Ampicillin | 0 | 6 | 3 | 9 | 10 | 12 |
| Oxacillin | 2 | 0 | 0 | 2 | 0 | 0 |
| Vancomycin | 3 | 0 | 3 | 6 | 10 | 11 |
| Gentamicin | 3 | 0 | 3 | 6 | 0 | 1 |
| Ceftriaxone | 0 | 3 | 3 | 5 | 10 | 12 |
| Rifampicin | 3 | 2 | 0 | 5 | 0 | 0 |
| Meropenem | 0 | 3 | 1 | 5 | 10 | 12 |
The ultrasonic diagnosis was consistent with the pathological diagnosis in
81.8% (18/22) patients. One case was misdiagnosed as thrombosis and three cases
were misdiagnosed as atrial myxomas. The average length of the vegetations was
36.9
| The traits of vegetations | 10–20 mm group | The lesion of vegetations | 10–20 mm group | ||
| Maximum diameter (mm) | 34.5 (30.0–39.8) | 12.5 (11.0–15.0) | Mitral valve | 8 (36.4) | 10 (45.5) |
| Multiple vegetations | 12 (54.5) | 16 (72.7) | Aortic valve | 4 (16.7) | 8 (36.4) |
| Crumby structure | 16 (72.7) | 13 (59.1) | Tricuspid valve | 5 (22.7) | 1 (4.5) |
| Strip structure | 6 (27.3) | 9 (40.9) | Mitral and aortic valve | 4 (16.7) | 2 (9.1) |
| Pulmonary hypertension | 11 (50.0) | 4 (18.2) | Tricuspid and aortic valve | 1 (4.5) | 0 (0.0) |
| Severe valvular regurgitation | 15 (68.2) | 11 (50.0) | EF value (%) | 53.0 (49.0–57.0) | 59.0 (55.8–64.0) |
| Variables are presented as count (%) or median (Q1–Q3). | |||||
All 22 patients received antimicrobials and surgical treatment. Patient age, sex, intensive care unit length of stay (ICU-LOS), locations of vegetations, antimicrobial therapy, pathogen, EuroScore II, surgical treatments and major complications are summarized in Table 5. All 22 patients received initial empiric antibiotic therapy, and then physicians individually adjusted the antibiotics regimen according to the etiological evidence. Fourteen patients (63.6%) were treated with two or more antibiotics. Ten patients (45.5%) received cephalosporins-based antibiotic-treatment, often combined with Piperacillin-Tazobactam (TZP), VAN, and AMP. The most commonly used antibiotic was cephalosporin (in 14 cases), followed by penicillin (in 5 cases), and VAN (in 3 cases).
| Age/Sex | ICU-LOS (days) | Major involved area | Antimicrobial Therapy (duration before surgery) | Pathogen | Euro Score II | Surgery | Major complications | |
| Native valve involvement | ||||||||
| 1 | 29/M | Mitral annulus | OXA (1 d) | MSSA | 0 | MVP + TVP | Septic shock | |
| 2 | 54/M | PML | CRO (5 d) | S. sanguinis | 5 | MVR | - | |
| 3 | 54/F | 3.0 | AML, PML | CRO/TZP (5 d) | S. agalactiae | 11 | MVR + TVP | - |
| 4 | 25/F | PML | CRO (6 d) | Abiotrophia defectiva | 0 | MVR | - | |
| 5 | 17/F | 10.0 | PML | CRO/TZP (1 d) | MSSA | 3 | MVR | ICH |
| 6 | 62/F | 1.9 | PML | CRO (2 d) | No growth | 11 | MVR | Cardiac arrest |
| 7 | 37/F | AV | OXA/CRO/TZP (8 d) | S. intermedius | 4 | DVR | ICH | |
| 8 | 52/M | AV | CRO (1 d) | MRSA | 3 | DVR | SP | |
| 9 | 50/F | 3.0 | AV | CRO (1 d) | Actinomyces naeslundii | 2 | AVR | - |
| 10 | 54/M | BAV | CXM (3 d) | No growth | 4 | AVR + DAA operation | - | |
| 11 | 52/M | 2.0 | MV + AV | AMP/GEN (3 d) | Abiotrophia defectiva | 4 | AVR | Septic shock |
| 12 | 75/F | 1.8 | MV + AV | CRO/TZP (8 d) | Granulicatella adiacens | 8 | Bentall + MVR | Hemoperi-cardium, SP |
| 13 | 45/F | 3.5 | MV + AV | CRO/VAN (4 d) | No growth | 4 | AVR + PFO closure | - |
| 14 | 53/M | ATVL | AMP/CZO (3 d) | No growth | 4 | TVR | - | |
| 15 | 64/F | 4.8 | ATVL | IMP/TZP (19 d) | S. sanguinis | 5 | CRT-D extraction | PE, DIC |
| 16 | 34/M | TSL | AMP/CRO (8 d) | S. oralis | 5 | TVR + aortic aneurysm repair | PE | |
| 17 | 36/F | 1.7 | ATVL | VOR/MFX (5 d) | Aspergillus flavus | 0 | TVR | Multiple atrial thrombus |
| 18 | 66/M | 6.8 | MV + ATVL | CFP/VAN/IMP (14 d) | Klebsiella Pneumoniae | 9 | TVR + AVR | - |
| 19 | 37/M | 6.9 | AML | VAN/CRO (1 d) | No growth | 6 | MVP + TVP | - |
| Prosthetic valve involvement | ||||||||
| 20 | 62/F | AML | CFP (2 d) | No growth | 6 | MVR | - | |
| 21 | 66/F | 5.4 | PML | CXM/VAN (14 d) | No growth | 5 | DVR + TVR | Pericardial thrombus, ICH |
| 22 | 29/F | ATVL | VAN (6 d) | S. agalactiae | 3 | TVR | - | |
| Abbreviations: ICU-LOS, intensive care unit length of stay; AML, anterior mitral leaflet; PML, posterior mitral leaflet; AV, aortic valve; BAV, bicuspid aortic valve; MV, mitral valve; ATVL, anterior tricuspid valve leaflet; OXA, oxacillin; CRO, ceftriaxone; TZP, piperacillin-tazobactam; CXM, cefuroxime; AMP, ampicillin; GEN, gentamicin; VAN, vancomycin; CZO, cefazolin; IMP, imipenem; VOR, voriconazole; MFX, moxifloxacin; CFP, cefoperazone; MRSA, methicillin-resistant staphylococcus aureus; MSSA, methicillin-sensitive staphylococcus aureus; MVP, mitral valve repair; TVP, tricuspid valve repair; DVR, double valve replacement; AVR, aortic valve replacement; DAA, double aortic arch; PFO, patent foramen ovale; CRT-D, cardiac resynchronization-defibrillator device; TVR, tricuspid valve replacement; ICH, intracerebral hemorrhage; SP, severe pneumonia; PE, pulmonary embolism; DIC, disseminated intravascular coagulation. | ||||||||
Most (86.3%, 19 cases) patients had at least one valve replaced, and most (72.7%, 16 cases) underwent surgery within one week of admission. Intraoperative observation found leaky or faulty valves in 16 cases, including 5 cases of valve rupture, 1 case of paravalvular abscess, and 1 case of valve perforation. Except for one patient who only underwent cardiac resynchronization-defibrillator device (CRT-D) extraction, all patients received valve replacement or repair and vegetectomy surgery, with vegetations collected for pathological biopsy. Patients were transferred to cardiac surgery intensive care unit (ICU) after surgery, with a median of length of stay (LOS) of 1.75 days (range: 0.42–6.9 days), and a median hospital stay of 16.5 days (range: 8–48 days). Severe postoperative adverse events after surgery were as follows: ICH in three cases (bleeding sites were subarachnoid space, frontal lobe and cerebellum (Fig. 3)), and septic shock in two cases. Other adverse events included severe pneumonia (SP), pulmonary embolism (PE), and disseminated intravascular coagulation (DIC). Case 6 lost consciousness twice, and suffered from cardiac arrest. A temporary pacemaker was installed because of post-sinus arrest. She underwent surgery again on the tenth day after surgery due to sternal dehiscence. Case 12 developed postoperative hypovolemic shock and electromechanical separation. On the 3rd day after operation, thoracotomy was performed again to reveal hemopericardium and to stop the bleeding. Case 21 developed local thrombosis in the right atrium after the operation, leading to restricted right atrial filling (Fig. 3). As his blood indicators improved, he was discharged with oral warfarin 1.25 mg daily. All 22 patients survived without IE recurrence during six-month follow-up. Only one patient developed heart failure requiring hospitalization for his previous dilated cardiomyopathy. In patients with 10–20 mm vegetations, 14 patients (63.6%) received a single antibiotic (6 cases of second-generation cephalosporin, 4 cases of ceftriaxone (CRO), and 4 cases of TZP), and patients with enterococci received penicillin + levox/cef-benzacillin. At least one valve was replaced in 86.3% (19 cases), and 90.1% (20 cases) underwent surgery within one week of admission. The average intensive care unit length of stay (ICU-LOS) was 21.0 (15.5–45.3) hours. One patient developed postoperative hemorrhage in the right temporal lobe with a hematoma volume of 40 mL, and died one week after discharge.
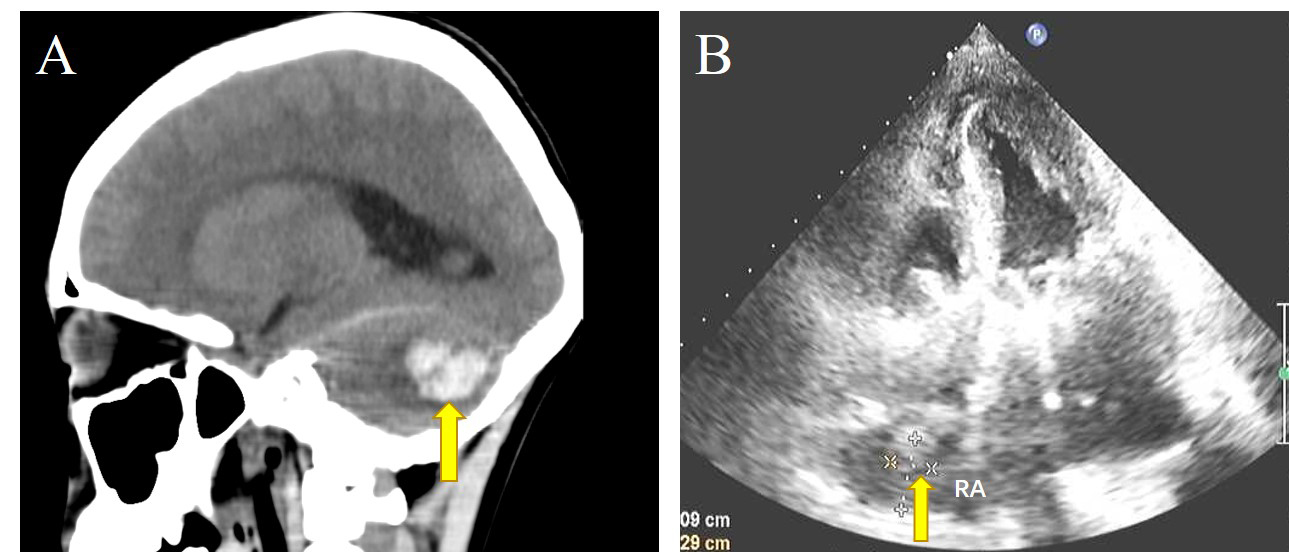 Fig. 3.
Fig. 3.Postoperative adverse events. (A) The patchy high-density foci in the left cerebellar hemisphere of Case. 21, and the plain computed tomography (CT) value was about 72 Hounsfield units. Arrow: left cerebellar hemorrhage. (B) Arrow: local thrombus in the pericardium under the roof of the right atrium.
Diagnosis and management of IE remains challenging because of its clinical
diversity and changing epidemiology [11, 12]. The disease pattern and prognosis
of IE vary greatly due to pathogenic microorganisms, basic heart disease,
implantation of prosthetic valves and cardiac devices, etc. Therefore, even risk
factor classification in international guidelines is inconsistent with clinical
practice [13, 14, 15]. In addition, there are only infrequent occurrence and sparse
reports of IE with very large vegetations, so the original clinical evaluation
can confound its diagnosis. Patients with atypical symptoms visit multiple
departments. Less-experienced physicians may consider their diagnosis as chronic
infection, rheumatic disease, neurological disease, autoimmune disease, malignant
tumor, etc. [16, 17, 18]. Although TTE is a mainstay in the diagnostic toolkit for IE
[19], sometimes it is difficult to differentiate one mass from another. Our
findings suggested that, in IE patients with atypical clinical presentation, the
mass in the left atrium is readily misdiagnosed as myxoma. In this study, Case 1
showed that the pedunculated vegetation seemed to be attached to the mitral valve
annulus (Fig. 4). A similarly confusable state also existed in Case 2 with the
vegetation located in the posterior mitral valve and in Case 3 with the pedicle
of vegetation attached to the junction of the anterior and posterior mitral
valves. These were originally considered as left atrial myxoma and were planned
to undergo tumor enucleation. During the operation, the surgical plan was changed
to vegetectomy and mitral valve replacement. Finally, the postoperative biopsy
confirmed the diagnosis of IE. A mass in the right atrium might be wrongly
diagnosed as thrombosis. The TTE of Case 16 showed tricuspid septal fixed
irregular hypoechoic mass, and the TTE of Case 15 showed fixed fuzzy echoes of
the right atrium, surrounded by electrodes incarcerated in the tricuspid valve
orifice (Fig. 4). The TTE diagnosis of Case 15 and Case 16 were high possibility
of thrombosis, but postoperative pathology confirmed IE (Fig. 5). The blood test
results of 22 patients in this study are consistent with the authoritative review
[20], which points out that an IE diagnosis may be suspected due to elevated CRP,
ESR, anemia, and microscopic hematuria, etc. However, the above indicators lack
specificity and have not been included in modern clinical diagnostic criteria.
The negative blood culture rate of 31.8% (7/22) in our IE cases with
 Fig. 4.
Fig. 4.TTE of IE with very large vegetations are susceptible to misdiagnosis. Arrow: vegetations. (A1) The pedunculated vegetation in Case 1 protruded into the mitral valve orifice during diastole, (A2) and returns to the left atrium during systole. (B1) In Case 15, TTE showed fixed fuzzy echoes of the right atrium near the tricuspid valve orifice, surrounded by electrodes, (B2) and incarcerated in the tricuspid valve orifice.
 Fig. 5.
Fig. 5.Tricuspid valve vegetations in IE. (A) The gross appearance of a large vegetation on the tricuspid valve in Case 16, as measured in centimeters. (B) Hematoxylin and eosin staining of a microscopic cross section of a vegetation. White arrow: bacteria embedded within the vegetation. Black arrow: inflammatory cells.
In conclusion, in terms of improving early diagnosis, our study suggests that IE accompanied by giant vegetation has a wide variety of first appearances, diverse imaging features, dangerous clinical condition, and a high negative blood culture rate. Clinicians should be more vigilant about IE when a large space-occupying mass is found by TTE. High suspicion of IE in the early stage of clinical admission is appropriate for patients with the following manifestations. (a) With underlying structural heart disease or with intracardiac implants, (b) with vascular manifestations such as arterial embolism, pulmonary embolism, and intracranial hemorrhage, (c) with consumptive disease manifestations such as anemia and hypoalbuminemia, (d) with unexplained manifestations of acute heart failure, (e) with fever and arterial embolism. Patients with unexplained spleen, brain, or kidney infarction as the first symptom should also be alerted to the possibility of IE.
Compared with the 10–20 mm vegetation group, the diagnosis of IE with very large vegetation is more difficult as it is often mixed with basic heart diseases or implantations. Compared with TTE, transesophageal echocardiography (TEE) can more accurately assess the hemodynamic effects of location, attachment site, size and shape of vegetation and valve damage (Fig. 6A1,A2,B), especially in the case of prosthetic valve endocarditis (PVE) and perivalvular abscess. However, cardiac computed tomography/computed tomography angiography (CT/CTA) is more suggestive in detecting perivalvular complications (abscess/pseudoaneurysm) in native valve endocarditis (NVE) and PVE, and non-cardiac abnormalities can be found in a single examination [28]. Case 6 was admitted to hospital due to abdominal pain for 9 days, with no fever and negative blood culture. TTE showed echo mass in the left atriun, and aortic CTA showed mesenteric artery pseudoaneurysm (Fig. 6D). Thrombosis and abscess were seen during aneurysmectomy, and MVR was performed after oral antibiotics for 2 weeks. Mitral valve biopsy was consistent with IE, so CTA was of great significance for prompting diagnosis of IE with atypical clinical features. Case 16 was admitted with negative blood cultures and a paravalvular aortic abscess detected by cardiac CT (Fig. 6D). CT can also be used to differentiate between benign and malignant tumors (Fig. 6E). Magnetic resonance imaging (MRI), on the other hand, can better differentiate tissue components (solid, liquid, hemorrhage, fat, and thrombus), and is especially useful in differentiating giant vegetations from thrombus (Fig. 6F) or myxomas (Fig. 6G). Myocardial perfusion ultrasonography is also useful in excluding thrombus (Fig. 6H). Because there are no lead artifacts in positron emission tomography (PET), its diagnostic ability for CDRIE is higher than CT. Silbiger and his coworkers [29] reported that PET reclassifies 90% of Duke-possible patients with suspected device infections, and PET was also superior in finding primary and extracardiac sepsis [30]. Doctors should be aware of the possibilities offered by the multimodal imaging approach when appropriate.
 Fig. 6.
Fig. 6.Multimodality imaging in IE. (A) TEE showed a very large mitral valve vegetation in the left atrium. Yellow arrows: vegetations. (A1) TEE of Case 2 showed a pedicle connection to the mitral valve rather than the atria, which was confirmed to be IE by biopsy. (A2) Left atrial vegetation of Case 6. (B) TEE demonstrates pulmonary valve vegetations in a Case of patent ductus arteriosus. (C) Aortic CTA shows superior mesenteric artery aneurysm. Red arrow: rupture of pseudoaneurysm in Case 6. (D) Cardiac CT revealed a perivalvular aortic abscess (blue arrow) of Case 16. (E) CT showing cardiac metastases (primary gastric inflammatory myofibroblastic tumor). Yellow arrow: uneven enhancement. (F) MRI shows abnormal signal foci in the right atrium, with no obvious enhancement after enhancement. Blue arrow: thrombus in the right atrium. (G) Most of the lesions did not enhance during delayed MRI enhancement. Red arrow: Myxoma. (H) Myocardial perfusion ultrasound showed two moderate echoic masses in left ventricle, and there was no contrast agent filling in the irregular masses. Blue arrow: thrombus.
In addition to the emphasis on improving early diagnosis, our study also
supports early surgical intervention in IE with
There are few reported cases of giant IE vegetations worldwide. Radcliffe
et al. [2] summarized all twenty-three IE cases with
| Year/Location | Age/Sex | Size of Vegetation (mm) | Pathogen | Area of involvement | Antimicrobial Therapy | Length of antimicrobial Therapy | Surgery | Outcome |
| 2020/USA [37] | 27/F | 34 |
MSSA, S. marcescens | TV | VAN/TZP | 6 wk | Transcatheter aspiration | Success |
| 2020/USA [38] | 27/M | 35 |
MRSA | TV | NR | 8 d | Percutaneous Debulking of Vegetation | Worsening of TR |
| 2020/USA [39] | 55/M | 50 |
MSSA | TV | NR | 8 wk | CIED extraction + transcatheter aspiration + PE | Success |
| 2020/USA [40] | 54/M | 39 |
No growth | TV | CRO | 8 wk | AVR + TVR | Success |
| 2021/Iran [41] | 58/M | 31 |
No growth | PV | CZO/TEC | 7 wk | None | Success |
| 2021/USA [42] | 31/M | 50 (length) | MRSA | TV | NAF | 6 wk | TVP | Success |
| 2022/Belgium [43] | 61/M | 45 (length) | MSSA | ATVL | CZO | 6 wk | TVP | Success |
| Abbreviations: NR, not reported; MRSA, methicillin-resistant staphylococcus aureus; MSSA, methicillin-sensitive staphylococcus aureus; VAN, vancomycin; TZP, piperacillin-tazobactam; CRO, ceftriaxone; CZO, cefazolin; TEC, teicoplanin; NAF, nafcillin; CIED, cardiac implantable electronic device; PE, pulmonary embolism; AVR, aortic valve replacement; TVR, tricuspid valve replacement; TVP, tricuspid valve repair; TR, tricuspid regurgitation. | ||||||||
In our study, patients with
In terms of the perioperative period, surgical timing, surgical method, details
and prognosis, we summarized some experiences of IE with
| 10–20 mm | ||
| Surgery within 1 week of admission (cases) | 16 | 20 |
| The number of valve replacements | 24 | 23 |
| The number of biological valves | 9 | 4 |
| Postoperative embolism/shock/ICH | 10 | 2 |
| Surgical reintervention | 2 | 2 |
| The number of patients with ICU-LOS |
8 | 2 |
| Death/recurrence | 0 | 2 |
(1) In terms of preoperative examination, although routine brain imaging
screening is reasonable in patients with left-sided IE (IIa B), the 2016 AATS
consensus guidelines emphasizes the necessity of preoperative cranial examination
in IE with very large vegetations. Because
(2) Because patients with
(3) As for the timing of surgery, the decision of a multidisciplinary team is
particularly significant in patients with
(4) The choice of surgical incision and the surgical difficulty are different
between the 10–20 mm group and the
(5) According to 2016 AATS Guidline, when valvular stenosis and regurgitation
occur, MVP is the optimal choice instead of MVR, while AVR is better than
aortic valve plasty (AVP). Generally
speaking, TVP is recommended. But in the patients with
(6) According to the 2015 ESC IE Guidelines [67], as predictors of poor
prognosis in admission assessment, compared patients with 10–20 mm vegetations,
those with
For IE complicated with
AATS, American Association for Thoracic Surgery; APTT, activated partial thromboplastin time; AMP, ampicillin; AVP, aortic valve plasty; CAG, coronary arteriography; CRE, carbapenem-resistant Enterobacter; CDRIE, cardiac device-related infective endocarditis; CRTD, cardiac resynchronization-defibrillator device; cTn I, cardial troponin I; CKD5, chronic kidney disease stage 5; CHD, congenital heart disease; CRP, c-reactive protein; CT/CTA, computed tomography/computed tomography angiography; CK, creatine kinase; CK-MB, creatine kinase MB; DCM, dilated cardiomyopathy; DBIL, direct bilirubin; DIC, disseminated intravascular coagulation; DAA, double aortic arch; ESR, erythrocyte sedimentation rate; EuroSCORE II, european System for Cardiac Operative Risk Evaluation II; FDP, fibrinogen degradation product; GAS, group A streptococci; GBS, group B streptococci; HCM, hypertrophic cardiomyopathy; IE, infective endocarditis; ICU-LOS, intensive care unit length of stay; ICH, intracerebral hemorrhage; LDH, lactate dehydrogenase; LOS, length of stay; LY%, lymphocytes percentage; MRI, magnetic resonance imaging; MRSA, methicillin-resistant staphylococcus aureus; MSSA, methicillin-sensitive staphylococcus aureus; NE%, neutrophils percentage; NT-proBNP, N-terminal pro-brain natriuretic peptide; NVE, native valve endocarditis; TEE, transesophageal echocardiography; TZP, piperacillin-Tazobactam; PCT, procalcitonin; PET, positron emission tomography; PT, prothrombin time; PVE, prosthetic valve endocarditis; PE, pulmonary embolism; SP, severe pneumonia; Q1–Q3, the 25th–75th percentiles; ACC, the American College of Cardiology; ESC, the European Society of Cardiology; TBIL, total bilirubin; TTE, transthoracic echocardiography; VAN, vancomycin; VVI, ventricular demand pacemaker; WBC, white blood cells.
FZ and JM designed the research study. XYC performed the research. YQC provided help and advice on data collection. FZ analyzed the data. XYC, JM and YQC wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
This study involved human data only. The name of the ethics committee: medical ethics committee of Xiangya Hospital of Central South University. The reference number: 202203076.
Thanks to all the peer reviewers for their opinions and suggestions.
This study was supported by the National Natural Science Foundation of China (No. 81873585, No. 82090020, No. 82090024, No. 82073918 and No. 82070070) and Natural Science Foundation of Hunan Province (No.2020SK2088, 2018JJ3835).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
