Academic Editor: Buddhadeb Dawn
Background: Atrial fibrillation (AF) is associated with an increased
risk of heart failure, death and thromboembolism. AF is prevalent in patients
with cancer. Although current guidelines suggest the application of oral
anticoagulants (OACs) for thromboembolic event prevention in high-risk AF
patients, owing to the high thromboembolic and bleeding risks of active-cancer
patients, there is no consensus on the use of OACs in such a population.
Therefore, we conducted this retrospective cohort study to investigate the
applicability of the CHA
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia worldwide. It causes a significant burden to patients, physicians, and the health care system owing to its high morbidity and mortality, including heart failure, death, thromboembolism (e.g., ischemic stroke and systemic emboli), and subsequent anticoagulation-related bleeding events [1, 2, 3, 4]. AF is prevalent in patients with cancer and may be present during anticancer treatment, at the time of diagnosis, or even a period of time after cancer therapy [1]. However, the mechanisms underlying the association between cancer and AF remain unclear [5].
Cancers are associated with an increased risk of thromboembolism due to multiple risk factors, such as hypercoagulability, overproduction of inflammatory cytokines, and compression or invasion of blood vessels [6, 7]. However, patients with active cancers are also at higher risk of bleeding, possibly due to endothelial dysfunction, thrombocytopenia, or thrombocyte dysfunction, especially during anticancer therapies [5]. These opposing characteristics challenge the clinician on how to best manage the hypercoagulable state without causing major bleeding.
Currently, there is no consensus on the use of oral anticoagulants (OACs) to
prevent thromboembolism in patients with active cancer and AF. Additionally, it
is indicated that the popular AF risk-stratification models, namely, the
CHA₂DS₂-VASc and HAS-BLED scores, may not be appropriate for cancer patients with
AF to predict the risk of AF-related thromboembolism and OAC-related major
bleeding events, respectively [8, 9]. Therefore, this retrospective cohort study
was conducted to verify the applicability of the CHA
In this retrospective cohort study, patients with active cancer were recruited at the National Cheng Kung University Hospital from November 2012 to August 2019. The cancer diagnosis of the enrolled patients was based on the medical records of the International Classification of Diseases-9 codes, including liver, colorectal, lung, urologic (kidney, renal pelvis, ureter, and urinary bladder), breast, prostate, upper gastrointestinal (esophageal, gastric, duodenal), biliary tract, pancreatic, and gynecologic (vaginal, vulvar, cervical, and uterine) cancers, hematologic disorder (leukemia and lymphoma), brain tumor, skin, oral, and nasopharyngeal carcinoma. Clinical information, including physiological data and underlying comorbidities, was collected from electronic medical records on the date of enrollment. Demographic characteristics included age, sex, body weight, body height, body mass index, diabetes mellitus, hypertension, dyslipidemia, coronary artery disease, myocardial infarction, heart failure (HF), hypertrophic cardiomyopathy (HCM), stroke, peripheral artery disease, deep vein thrombosis, and chronic kidney disease.
Patients who met all the following criteria were included: (1) age
 Fig. 1.
Fig. 1.Flowchart of the study design and processes.
The primary effectiveness endpoints were all-cause mortality and the composition of stroke/transient ischemic attack (TIA) and systemic emboli (SE). Acute myocardial infarction (AMI), hospitalization for HF, stroke/TIA, and SE were defined as secondary effectiveness endpoints.
Based on the criteria of the International Society on Thrombosis and Hemostasis, the safety outcome was the composite of major bleeding events, including (1) clinically overt gastrointestinal (GI) bleeding accompanied by a decrease in the hemoglobin level of at least 2 g/dL or transfusion of at least 2 units of packed red blood cells, (2) occurrence of intracranial hemorrhage (ICH), or (3) death.
Numeric and dichotomous data are presented as the mean
A total of 2429 patients with active cancer were enrolled in this study. There
were 1060 patients (age, 75.2
| Characteristics | Before propensity score matching | After propensity score matching | ||||||
| Overall N = 1060 | OAC | p | Overall N = 690 | OAC | p | |||
| Yes N = 326 | No N = 734 | Yes N = 225 | No N = 465 | |||||
| Age | 75.16 (10.55) | 74.08 (9.80) | 75.64 (10.84) | 0.007 | 74.94 (10.81) | 74.74 (9.86) | 75.04 (11.24) | 0.44 |
| Male (%) | 627 (59.2%) | 187 (57.4%) | 440 (59.9%) | 0.43 | 414 (60.0%) | 121 (53.8%) | 293 (63.0%) | 0.02 |
| Diabetes mellitus (%) | 401 (37.8%) | 131 (40.2%) | 270 (36.8%) | 0.29 | 284 (41.2%) | 84 (37.3%) | 200 (43.0%) | 0.16 |
| Dyslipidemia (%) | 561 (52.9%) | 219 (67.2%) | 342 (46.6%) | 412 (59.7%) | 149 (66.2%) | 263 (56.6%) | 0.02 | |
| Hypertension (%) | 807 (76.1%) | 543 (74.0%) | 264 (81.0%) | 0.01 | 566 (82.0%) | 193 (85.8%) | 373 (80.2%) | 0.07 |
| Stroke (%) | 199 (18.8%) | 72 (22.1%) | 127 (17.3%) | 0.07 | 146 (21.2%) | 54 (24.0%) | 92 (19.8%) | 0.20 |
| Coronary artery disease (%) | 239 (22.5%) | 84 (25.8%) | 155 (21.1%) | 0.10 | 185 (26.8%) | 60 (26.7%) | 125 (26.9%) | 0.95 |
| Chronic kidney disease (%) | 388 (36.6%) | 105 (32.2%) | 283 (38.6%) | 0.048 | 245 (35.5%) | 77 (34.2%) | 168 (36.1%) | 0.62 |
| Myocardial infarction (%) | 74 (7.0%) | 24 (7.4%) | 50 (6.8%) | 0.75 | 59 (8.6%) | 19 (8.4%) | 40 (8.6%) | 0.95 |
| Heart failure (%) | 349 (32.9%) | 142 (43.6%) | 207 (28.2%) | 262 (38.0%) | 97 (43.1%) | 165 (35.5%) | 0.053 | |
| Peripheral artery disease (%) | 51 (4.8%) | 24 (7.4%) | 27 (3.7%) | 0.01 | 35 (5.1%) | 14 (6.2%) | 21 (4.5%) | 0.34 |
| Deep vein thrombosis (%) | 21 (2.0%) | 2 (0.6%) | 19 (2.6%) | 0.03 | 12 (1.7%) | 2 (0.9%) | 10 (2.2%) | 0.36 |
| CHA |
3.93 (1.83) | 4.15 (1.74) | 3.83 (1.86) | 0.005 | 4.15 (1.81) | 4.30 (1.66) | 4.07 (1.88) | 0.14 |
| CHA |
94 (8.9%) | 20 (6.1%) | 74 (10.0%) | - | 50 (7.2%) | 10 (4.4%) | 40 (8.6%) | 0.06 |
| Aspirin (%) | 161 (15.2%) | 44 (13.5%) | 117 (15.9%) | 0.31 | 109 (15.8%) | 32 (14.2%) | 77 (16.6%) | 0.43 |
| P2Y12 inhibitors (%) | 120 (11.3%) | 44 (13.5%) | 76 (10.3%) | 0.20 | 99 (14.1%) | 32 (14.2%) | 67 (14.4%) | 0.76 |
| ACEI/ARB | 227 (21.4%) | 128 (39.3%) | 99 (13.5%) | 161 (23.3%) | 74 (32.9%) | 87 (18.7%) | ||
| statin | 215 (20.3%) | 108 (33.1%) | 107 (14.6%) | 162 (23.5%) | 71 (31.6%) | 91 (19.6%) | ||
| ACEI/ARB, Angiotensin converting enzyme inhibitor/Angiotensin II receptor blockers; OAC, oral anticoagulant. | ||||||||
| Cancer diagnosis | Prevalence |
| Colorectal cancer | 17% |
| Hepatocellular cancer | 15% |
| Lung cancer | 15% |
| Genitourinary cancer | 10% |
| Oral cancer | 6% |
| Others | 37% |
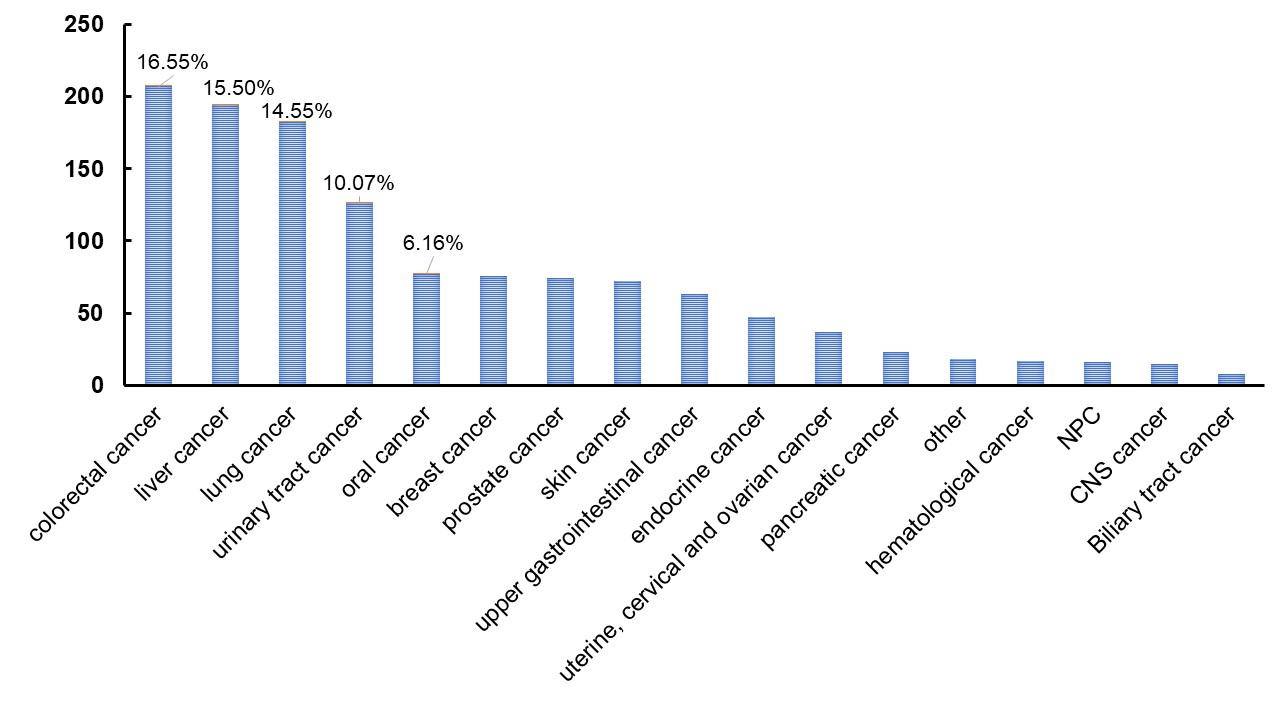 Fig. 2.
Fig. 2.Distribution of cancer diagnoses.
The median follow-up duration was 3.2 (interquartile range, 1.6–5.1) years.
Both groups had high CHA
| Variables | OAC | Hazard ratio (95% CI) | p | ||
| Yes | No | ||||
| Effectiveness endpoints | |||||
| Stroke/SE | 19 (8.4%) | 22 (4.7%) | 1.84 (0.99, 3.40) | 0.06 | |
| Death | 55 (24.4%) | 174 (37.4%) | 0.58 (0.43, 0.78) | ||
| AMI | 7 (3.1%) | 24 (5.2%) | 0.60 (0.26, 1.40) | 0.24 | |
| HF hospitalization | 14 (6.2%) | 27 (5.8%) | 1.09 (0.57, 2.08) | 0.79 | |
| Safety endpoints | |||||
| Composition of major bleeding | 8 (3.6%) | 20 (4.3%) | 0.82 (0.34, 1.83) | 0.64 | |
| Major GI bleeding | 4 (1.8%) | 16 (3.4%) | 0.51 (0.14, 1.4) | 0.23 | |
| ICH | 1 (0.4%) | 2 (0.4%) | 1.03 (0.05, 10.84) | 0.98 | |
| AMI, acute myocardial infarction; GI bleeding, gastrointestinal bleeding; HF, heart failure; ICH, intracranial hemorrhage; OAC, oral anticoagulant; SE, systemic embolism. | |||||
The risk of major bleeding composition (major GI bleeding and ICH) was similar between the two groups (OAC-treated vs. no OAC: 3.6% vs. 4.3%, HR 0.82 [95% CI 0.34–1.83], p = 0.64, Table 3). Neither major GI bleeding nor ICH risk was significantly different between the two groups (Table 3).
Finally, stroke/TIA and SE events were identified in the nontreated group. The
CHA
| CHA |
OAC (+), n = 225 | OAC (-), n = 465 | ||
| Stroke/SE, n (%) | Total (%) | Stroke/SE, n (%) | Total (%) | |
| 0 | 1 (33.3%) | 3 (1%) | 0 (0%) | 17 (4%) |
| 1 | 0 (0%) | 7 (3%) | 1 (4.3%) | 23 (5%) |
| 2 | 1 (6.3%) | 16 (7%) | 0 (0%) | 51 (11%) |
| 3 | 1 (2.2%) | 46 (20%) | 5 (6.1%) | 82 (18%) |
| 4 | 5 (9.4%) | 53 (24%) | 4 (3.7%) | 108 (23%) |
| 5 | 5 (9.8%) | 51 (23%) | 5 (6.0%) | 83 (18%) |
| 6 | 3 (10%) | 30 (13%) | 2 (4.1%) | 49 (11%) |
| 7 | 3 (27.3%) | 11 (5%) | 4 (10.5%) | 38 (8%) |
| 8 | 0 (0%) | 6 (3%) | 1 (10%) | 10 (2%) |
| 9 | 0 (0%) | 2 (1%) | 0 (0%) | 4 (1%) |
| OAC, oral anticoagulant; SE, systemic embolism. | ||||
This study revealed that compared to the active cancer and AF patients without taking OACs, those active cancer and AF patients with OAC treatment did not have lower risk of stroke/TIA and SE. However, these OAC-treated cancer patients with AF had lower all-cause mortality rate. Furthermore, OAC treatment did not increase the risk of major bleeding, such as major gastrointestinal bleeding and ICH.
Given improvements in cancer treatment, the survival rate of cancer patients has increased. The coexistence of cancer and AF is becoming increasingly prevalent, and clinicians are likely to encounter an increasing number of patients with comorbid conditions. The increased risk of thromboembolism and bleeding challenges clinicians in deciding whether to initiate anticoagulation and how to choose an anticoagulant. Nevertheless, there are no guidelines or recommendations for the treatment of this high-risk population. The 2020 European Society of Cardiology guidelines for AF recommended a multidisciplinary team to make decisions regarding thromboprophylaxis in cancer patients because these patients may have multiple comorbidities, such as renal failure, hepatic failure, thrombocytopenia, obesity, or cachexia, and drug–drug interactions between OACs and cancer therapy regimens [4]. Several observational studies investigated the efficacy and safety of direct oral anticoagulants (DOACs) and vitamin K antagonist (warfarin) in patients with active cancer and AF and showed the efficacy and safety of OACs [10, 11, 12, 13]. Major trials regarding stroke prevention for patients with atrial fibrillation carried subgroup analyses. In ARISTOLTE, ROCKET AF, and ENGAGE AF-TIMI 48 trials, the relative efficacy and safety of DOACs compared with warfarin were not significantly different in patients with and without active cancer [10, 11, 14]. However, current existing data have focused on efficacy and safety in the comparison of different OAC treatments in patients with active cancer and AF [11]. The efficacy and safety of using OAC treatment are left to be explored. Moreover, the prescription of OACs is often hindered by the fear of bleeding in our current practice.
Regarding bleeding tendency, the OAC subgroup did not show a significant increase in the risk of GI bleeding and ICH compared to that in AF cancer patients without OAC. The results may be associated with a lack of data to stratify indications and dosage of OACs, site(s) or complexity of cancer, and most importantly, a previous history of bleeding events. The OAC subgroup may have been in better condition, regardless of the comorbidities.
This study has several limitations. First, the study population was limited after propensity score matching. Second, the cancer population enrolled in the study was based on ICD-9 coding because these codes reflect only the sites of cancer. There was no information regarding the timing of cancer diagnosis, the stage of cancer, adoption of anticancer therapies, or therapeutic response in these patients. Moreover, the clinical conditions and indications at the time when OACs were prescribed were not known. Third, the data of index events, including death, stroke/SE, AMI, HF hospitalization, GI bleeding, and ICH, were obtained from the electronic medical records of a single hospital. Medical records from other hospitals were not available. Fourth, AF, particularly paroxysmal AF, may be underdiagnosed. Finally, the possibility of selection bias and incomplete patient records cannot be excluded. Additional prospective studies are warranted to confirm the results of the present study.
OAC treatment may significantly reduce the risk of death, without safety concerns, in active-cancer patients with AF. Further studies are required to determine the optimal use of anticoagulation therapy in this high-risk population.
Conceptualization—LYY, YWL and PYL; methodology—LYY, YWL and PYL; software—YCL and TYC; validation—YWL, PFS and PYL; formal analysis—LYY, YCL and PFS; investigation—YWL; resources—PYL; data curation—YWL and PYL; writing—original draft preparation—LYY; writing—review and editing—YWL and PYL; visualization—YWL; supervision—PYL; project administration—PYL; funding acquisition—PYL. All authors have read and agreed to the published version of the manuscript.
This study was conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the NCKUH Human Research and Ethics Committee (IRB: B-ER-111-052). Because this was a retrospective study and all data were fully anonymized, the Human Research and Ethics Committee of the National Cheng Kung University Hospital waived the requirement for informed consent.
Not applicable.
This study was supported by a research grant from the National Health Research Institutes (NHRI-111A1-CACO-02222211).
The authors declare no conflict of interest. Yen-Wen Liu is serving as one of the Guest Editors of this journal. We declare that Yen-Wen Liu had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Buddhadeb Dawn.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
