†These authors contributed equally.
Academic Editor: Carlo Briguori
Background: Covered stents are effective in treating coronary artery
perforation (CAP), however, the high rate of immediate device deployment failure
and in-stent restenosis have limited the application of the currently covered
stents. Methods: We designed a covered stent system consisting of a
single layer of drug-eluting stent and a layer of polytetrafluoroethylene (PTFE)
membrane wrapped at the outer layer of the stent. The immediate sealing effect of
our novel covered stent was observed by using an Ellis type III CAP model. The
device’s success was defined as its ability to seal the perforation, assessed by
visual estimation and final thrombolysis in myocardial infarction (TIMI) 3 flow.
The antiproliferative effect was evaluated in 12 swine, which were randomly
assigned to treatment (sirolimus-eluting covered stents) and control (bare metal
covered stents) groups. Coronary angiography and optical coherence tomography
(OCT) were performed at index procedure, 1- and 6-month after stent implantation.
All swine were sacrificed for histopathological analyses at 6-month.
Results: The device success rate was 100%. All swine were alive at
6-month follow-up. At 1-month, the treatment group had a larger minimal luminal
diameter (MLD) (1.89
Coronary artery perforation (CAP) is a rare but life-threatening complication of percutaneous coronary intervention (PCI) [1]. Covered stents are considered an effective bailout strategy for CAP [2, 3]. Currently, the most widely used covered stent, GRAFTMASTER stent (Abbott Vascular), is designed in a “sandwich” fashion. A Polytetrafluoroethylene (PTFE) membrane is placed between two layers of 316L stainless steel stents [4]. However, such design has led to a large profile and the flexibility and trackability of the device are compromised. Reports have suggested that the device failure rate was 14.6% in complex lesions, such as calcified and torturous lesions [5]. Moreover, at 3-year follow-up, the incidence of target vessel revascularization (TVR) and definite stent thrombosis post this double-layer cover stents implantation was 26% [6] and 3.5% [7], respectively. To reduce the adverse events and improve the deliverability, newer generation of covered stents, such as BeGraft (Bentley InnoMed GmbH, Hechingen, Germany) and PK Papyrus (Biotronik, Lake Oswego, OR, USA) stents, have adopted the design to a single layer metal stent [8]. In comparison to the GRAFTMASTER, the BeGraft and PK Papyrus were associated with higher device success rates [9]; however, both stents did not lower the incidence of in-stent restenosis (ISR) and TVR [10], as compared with the GRAFTMASTER.
The underlying mechanism of ISR after covered stent implantation has remained elusive. A putative reason is that PTFE, as a foreign body, can stimulate intimal proliferation [11, 12, 13]. To resolve this issue, some covered stents [6] use the pericardium, a collagen-rich biological tissue, to replace the PTFE membrane. Disappointingly, the pericardium-covered stent was not associated with a lower incidence of ISR than that of the PTFE or polyurethane stent [6]. Previous studies [12, 14] showed that the combination of a PTFE-covered stent and an underlying long sirolimus-eluting stent implantation is associated with better angiographic follow-up results, as evidenced by a reduction in the incidence of ISR. Such data indicate that antiproliferative drugs could decrease covered-membrane induced intimal proliferation. Therefore, we designed a covered stent system consisting of a single layer of drug-eluting stent and a layer of PTFE membrane wrapped at the outer layer of the stent. In this study, we aimed to evaluate the safety and efficacy of this drug-eluting covered stent in a porcine coronary perforation model.
Study animals were swine, 8–9 months old, weighing 25–30 kg. These animals
were pretreated with aspirin (100 mg/day) and clopidogrel (75 mg/day) for 5 days
prior to the procedure. On the day of the procedure, swine were anesthetized with
propofol, and received continuous supplemental oxygen through an oxygen mask. The
femoral artery was punctured using the Seldinger method [15]. Throughout the
entire procedure, continuous hemodynamic and surface electrocardiographic
monitoring were maintained. Heparin (200 IU/kg) was administered via the sheath
to achieve activated clotting time
Two experimental steps were conducted (Fig. 1). First, to evaluate the immediate closure effect, a CAP model was constructed in 10 swine and subsequently, drug-eluting covered stents were immediately implanted. The success of the device was defined by its ability to seal the perforation, which was assessed by visual estimation and final thrombolysis in myocardial infarction (TIMI) 3 flow [9]. Second, to evaluate the ISR, 12 swine were divided into the treatment (drug-eluting covered stents) and control (bare mental covered stents) groups. In the present study, we recommended the covered stent should be deployed at the nominal pressure of 8 atm for 10–20 seconds. The stent implantation was optimized and evaluated by optical coherence tomography (OCT). Post-dilation with a non-compliant balloon was performed according to the OCT expert consensus recommendations [18]. Coronary angiography and OCT were performed 1- and 6-months post stent implantation. If the stented artery was not occluded, the OCT examination was conducted. Aspirin (100 mg/day) and clopidogrel (75 mg/day) were administered for 6 months. All swine were sacrificed for histopathological analysis at 6-month.
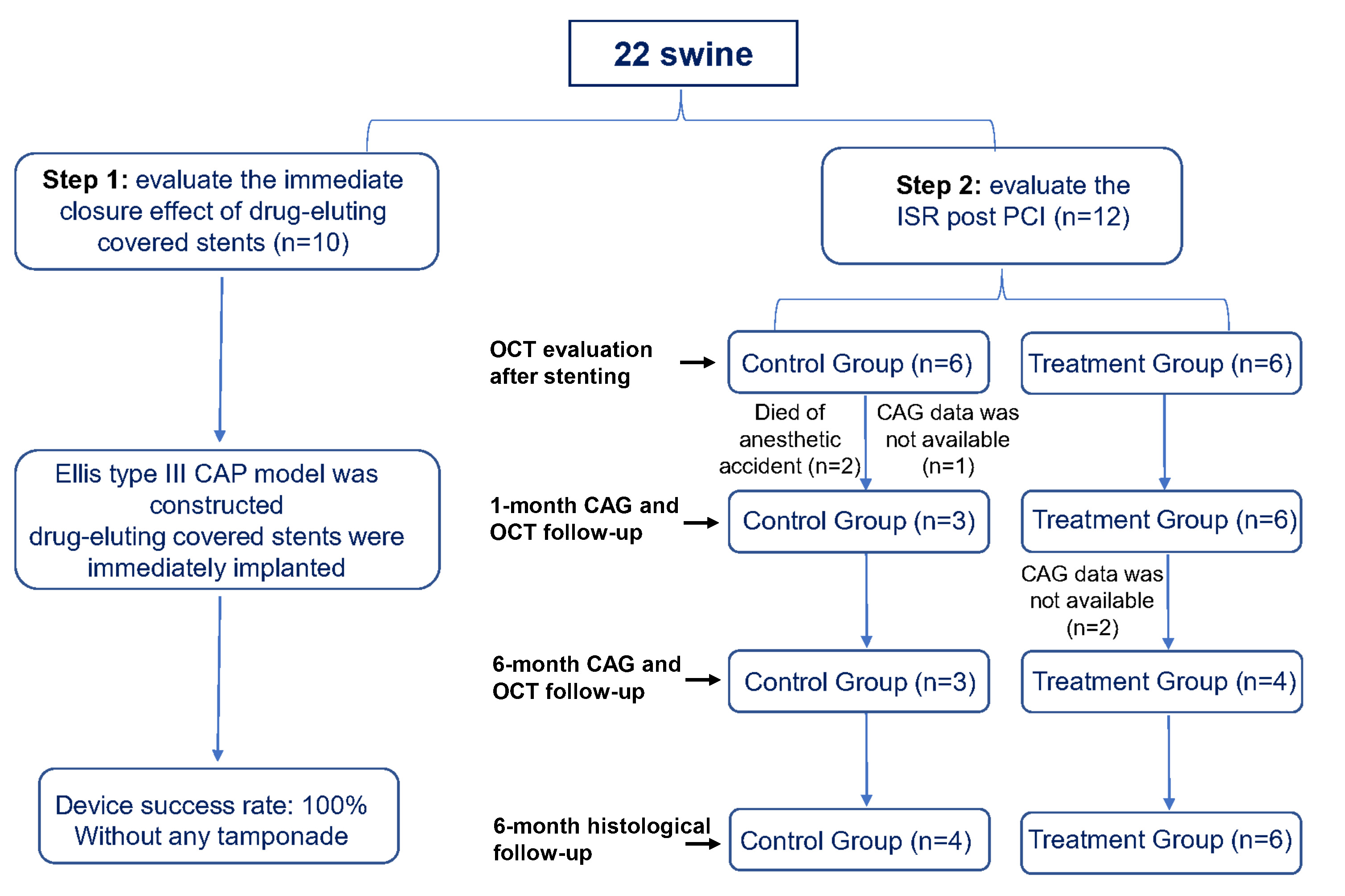 Fig. 1.
Fig. 1.The flow chart of the study. CAG, Coronary angiography; CAP, coronary perforation; ISR, in-stent restenosis; OCT, optical coherence tomography; PCI, percutaneous coronary interventions.
The covered stent system in the present study is a balloon-expandable stent
system, consisting of a single layer sirolimus-eluting stent and an expanded
polytetrafluoroethylene (ePTFE) membrane wrapped at the outer surface of the
stent. The platform of the stent is 316L stainless steel with Poly
Lactic-co-Glycolic Acid (PLGA) biodegradable polymer. In the control group, a
single layer bare mental without sirolimus-eluting covered stents were used. The
covered stent system has a small crimped profile. The crimped profile of the
covered stent system used in this study ranged between 1.1 and 1.3 mm. The size
of the device implanted in the study was 2.75 mm in diameter and 18 mm in length
for all the swine. The dosage of sirolimus on the covered stent was 225
Ellis type III CAP model, defined as frank streaming of contrast through a
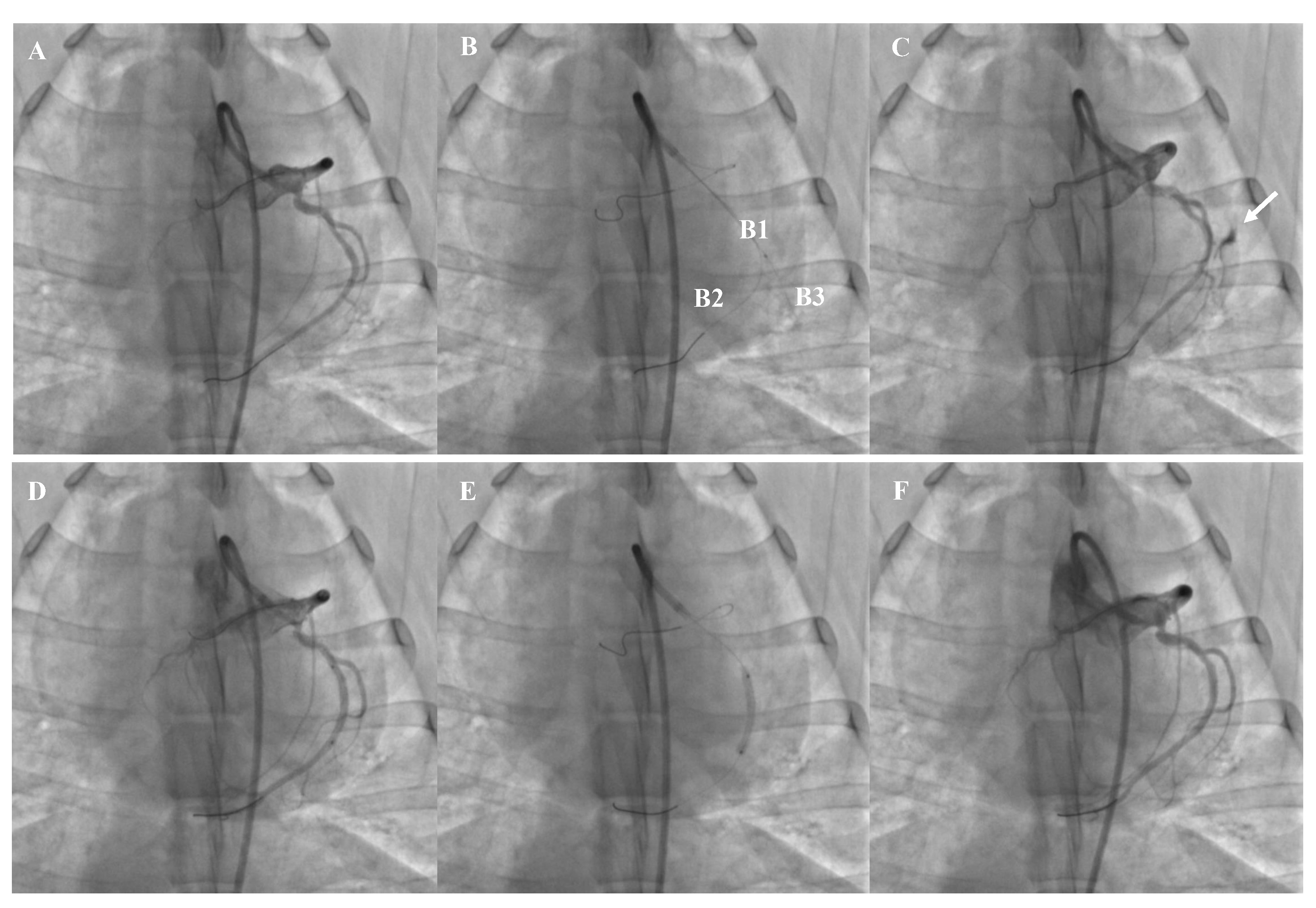 Fig. 2.
Fig. 2.The representative example of CAP model and covered stent implantation. (A) Baseline CAG. (B) Construction of CAP model (B1, micro-catheter, B2, soft guidewire, B3, stiff guidewire). (C) Streaming of contrast through an exit hole (white arrow). (D) Stent positioning. (E) Stent dilation. (F) Complete closure of the perforation. CAP, coronary perforation; CAG, Coronary angiography.
Loaded with the soft tip guidewire, the covered stent was released using 8–12
atm single expansion for 5–10 s to seal the perforation. Angiography was
repeated to ensure that the perforation was completely occluded (Fig. 2). OCT was
used to determine whether any post-dilation was needed and to ensure that no
serious dissection or thrombosis occurred. Finally, the femoral sheath was
withdrawn, and the puncture site was compressed for 20–30 min to stop the
bleeding. An intramuscular injection of penicillin at a dose of 8
The Medis QCA-CMS 6.0 system (Raleigh, NC, USA) was used for quantitative
coronary angiography (QCA) measurements. In this study, the following parameters
were included: reference luminal diameter (RLD) at preimplantation, minimal lumen
diameter (MLD), late lumen loss (LLL), diameter and area of stenosis at
post-implantation and follow-up. The stenosis diameter was calculated as
OCT was performed using a commercially available C7 OCT system (St Jude Medical, St Paul, MN, USA). OCT was used to optimize the stent implantation and evaluate the safety index such as any incidence of dissection and in-stent thrombosis.
Stented segments of the coronary arteries were fixed with 10% formalin, dehydrated in a graded series of ethanol and embedded in polymethyl methacrylate. After polymerization, 5 sections were sliced from the proximal to distal portion in each stented segment. Sections were stained with methylene blue and eosin, and examined by light microscopy. The plaque area was calculated by the Image J software. The mean plaque area derived from 5 sections was compared between the treatment and control groups.
Variables with normal distribution are expressed as the mean
The CAP model was successfully developed in all 10 swine. In experimental step 1, all covered stents were successfully deployed, and the device success rate was 100% without any incidence of tamponade. A representative case showing the development of the CAP model and subsequent sealing of the perforation by a covered stent is presented in Fig. 2.
Among the 12 swine, two died of anesthetic accident in the control group. As
shown in Table 1, the RLD was similar between the treatment and control groups
(2.59
| Control group | Treatment group | p value | |
| Day 0 | |||
| N = 4 | N = 6 | ||
| RLD, mm | 2.63 |
2.59 |
0.549 |
| MLD, mm | 2.52 |
2.44 |
0.200 |
| 1-month | |||
| N = 3 | N = 6 | ||
| MLD, mm | 0.63 |
1.89 |
0.004 |
| LLL, mm | 1.80 |
0.47 |
|
| DS% | 75.70 |
26.69 |
0.002 |
| AS% | 90.35 |
45.56 |
0.001 |
| 6-month | |||
| N = 3 | N = 4 | ||
| MLD, mm | 0.26 |
0.94 |
0.230 |
| LLL, mm | 2.17 |
1.43 |
0.215 |
| DS% | 90.54 |
62.53 |
0.207 |
| AS% | 97.31 |
79.29 |
0.232 |
| RLD, Reference Luminal Diameter; MLD, Minimal Luminal Diameter; LLL, Late Luminal Loss; DS, Diameter Stenosis; AS, Area Stenosis. | |||
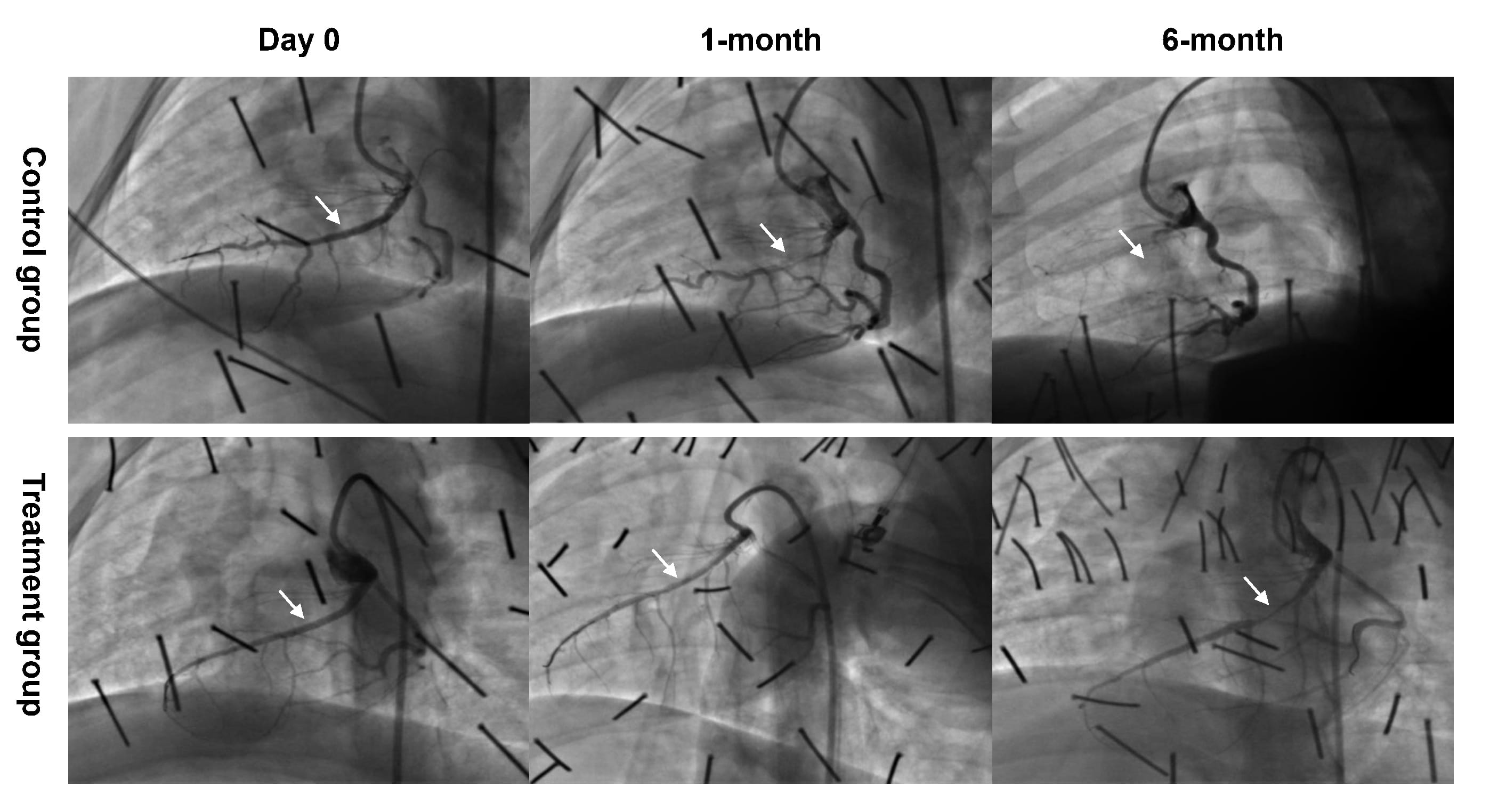 Fig. 3.
Fig. 3.Representative examples of CAG follow-up at all time points. Two swine died of anesthetic accident in the control group. The CAG data was not available in 1 swine from the control group and 2 swine from the treatment group, respectively. CAG, Coronary angiography.
No in-stent thrombosis was observed for all the available cases in any group at 1-month and 6-month OCT follow-up. Representative examples of such cases undergoing control and drug-eluting covered stents implantation are presented in Fig. 4. According to OCT analysis, at 1-month and 6-month follow-up, complete endothelialisation on the stents were observed for all the available cases in both groups.
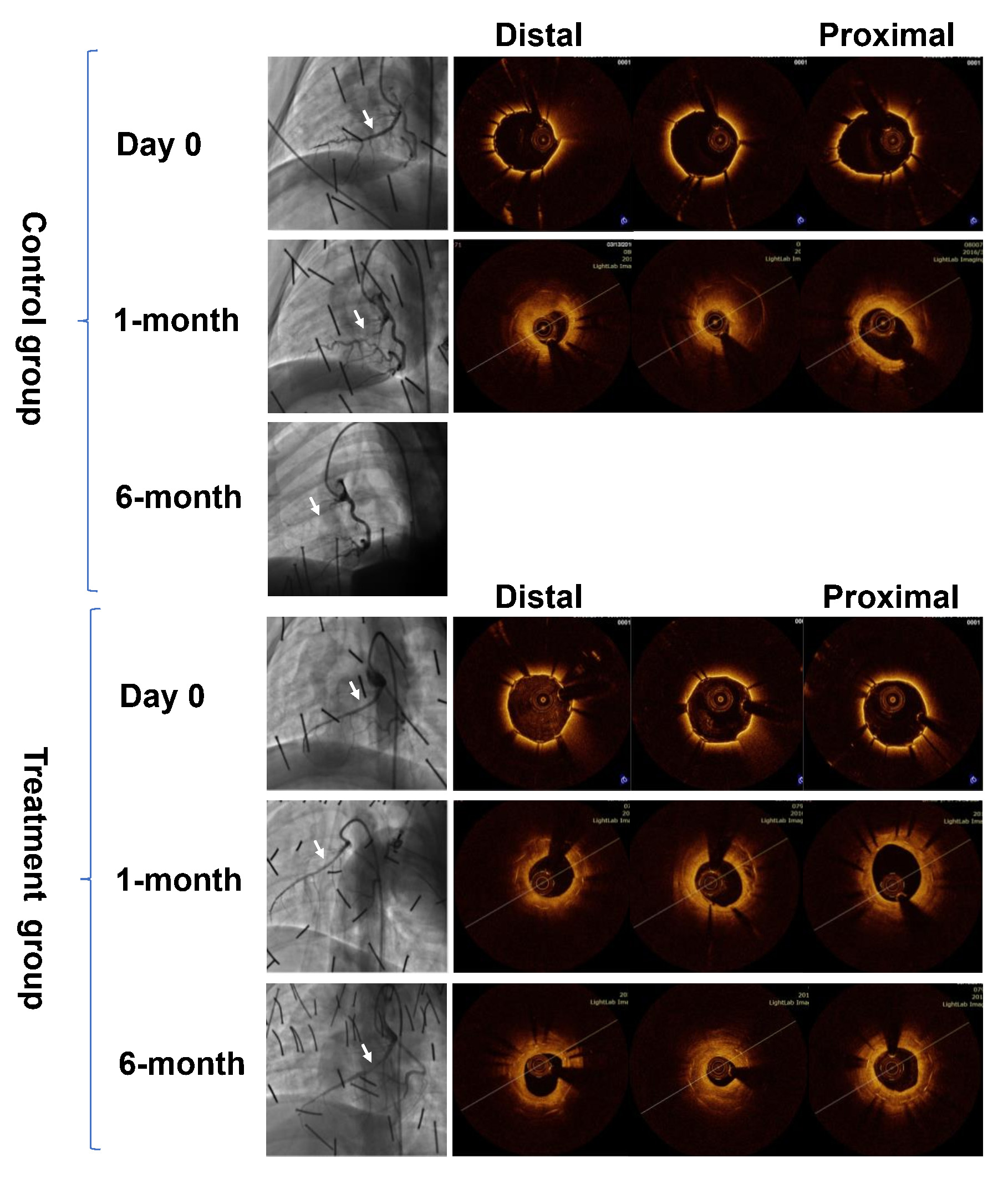 Fig. 4.
Fig. 4.Representative examples of OCT follow-up at all time points. OCT, Optical Coherence Tomography.
All the swine underwent histological analysis. Histomorphometric analysis
results are shown in Fig. 5. At 6-month follow-up, histopathological analysis
showed complete endothelialisation for all the cases in both groups (n = 4 in the
control group and n = 6 in the treatment group). The mean plaque area was lower
in the treatment group than in the control group (2.99
 Fig. 5.
Fig. 5.Representative examples of histological follow-up.
*p
In the present study, we investigated the safety and efficacy of a novel covered stent in a swine coronary perforation model. The findings from the present study are summarized as follows: (1) A controllable and safe CAP model was developed using guidewire assisted by micro-catheter. (2) The novel drug-eluting covered stent had a high success rate in sealing the coronary perforation without any incidence of cardiac tamponade. (3) The novel drug-eluting covered stent demonstrated a relatively sustained antiproliferative effect up to 6 months post implantation.
Coronary artery perforation (CAP), especially Ellis type III CAP, is a life-threatening complication of PCI, and is associated with a high incidence of cardiac tamponade (42.9%) [19], however, the technique of creating an animal model of CAP has not been previously addressed. To evaluate the efficacy of covered stents, we developed a CAP animal model. Our model has two salient features. First, the perforation was controllable. We used a micro-catheter to control the position and size of the perforation. Second, the model was relatively secured. A protective soft tip guidewire allowed an immediate deployment of the covered stent, thereby avoiding cardiac tamponade and percutaneous pericardial drainage. In this study, all Ellis type III perforations were successfully established using guidewire guided by micro-catheter without inducing any cardiac tamponade required treatment. We believe this coronary perforation model is promising for a variety of applications such as vascular injury, cardiac tamponade, and device evaluation in cases with coronary perforation.
Covered stents are considered an effective bailout strategy for Ellis types III coronary perforation which cannot be salvaged with a prolonged balloon inflation [5]. Covered stents with a “sandwich-like” structure have a device failure rate in the range of 16.7% [20] to 23.8% [21] in complex lesions. Compared to the GRAFTMASTER stent, the new generation PK Papyrus stent is associated with a shorter delivery time (8 vs. 15 min, p = 0.001), and a higher procedural success rate (86% vs. 69%, p = 0.216) [9]. Similar to the BeGraft and PK Papyrus stents, the covered stent established in the present study adopted the design of a single layer metal stent with a single layer of PTFE membrane, and has a small crimped profile that ranged between 1.1 and 1.3 mm. In our study, the device success rate was 100% without any incidence of cardiac tamponade and cardiac arrest.
In-stent restenosis (ISR) is a major concern of covered stents that needs to be
resolved. Current data suggest that the rate of ISR in PTFE covered stent-treated
patients range between 29.2% [20] and 54.6% [22]. Although the deliverability
of the newer generation covered stents has greatly improved due to the adoption
of a single layer design of metal stent, the incidence of ISR remains high.
Kufner et al. reported outcomes from 61 coronary perforation patients
treated with the BeGraft covered stent. During a follow-up of 192.8
The underlying mechanism of ISR post PTFE covered stent implantation is considered to be associated with the following reasons [11, 12, 13, 21]: (1) delayed endothelialization, (2) thrombus formation, (3) intimal proliferation stimulated by PTFE, and (4) stent malapposition or vascular injury such as dissection. To avoid such causes of ISR, intensive antiplatelet treatment, OCT or IVUS guided stent implantation and specific histocompatible materials could be used. To improve the biocompatibility, the asneugraft® Dx stent used pericardium, a collagen-rich biological tissue, to replace the PTFE membrane. Unfortunately, the pericardium covered stent demonstrated a higher occurrence of ISR than the PTFE covered stent [6].
Papafaklis et al. [14] and Hou et al. [12] reported that the
combination of PTFE-covered stents and an underlying long sirolimus-eluting stent
implantation offered better angiographic follow-up results, as evidenced by a
decrease in the stent-edge or stent-segment binary restenosis. Therefore, we
hypothesized that antiproliferative drugs could decrease neointimal proliferation
induced by the PTFE. In the present study, we used a stent platform with PLGA
biodegradable polymer. The dosage of sirolimus on the covered stent was 225
Covert stent was mainly used for the treatment of CAP, but also could be used for other indications such as coronary artery aneurysms (CAA), pseudoaneurysms, arterial-venous fistula, etc. [27, 28, 29]. Previous studies showed that CAA was the second most frequent indication for covered stents, but with a relatively high rate of stent thrombosis [2, 27]. Using intravascular imaging or a computed tomography scan to achieve accurate landing zone assessment and stent sizing during CAA with covered stents is recommended [27]. Post-dilatation and procedural guidance with intravascular imaging may lead to optimal apposition and expansion of the stent, which could improve the long-term outcomes. Besides CAP and CAA, covert stent also could be used to treat pseudoaneurysm, arterial-venous fistula, saphenous vein graft, etc. [27, 28, 29, 30]. Information regarding covered stents in these off-label use was heterogeneous and limited to small sample size studies, highlighting the unmet need for large-scale trials in these settings.
First, we used porcine coronary arteries with no atherosclerosis, calcification, or tortuous lesions. However, in clinical cases, covered stents are usually used to treat perforation in the atherosclerotic and calcified arteries. Therefore, the results should be interpreted with caution. Second, during the follow-up, the CAG data was not available in 3 swine, which decreased the sample size that was used to evaluate CAG results. However, these 3 swine underwent histological analysis. Third, pharmacokinetic studies on the release of sirolimus were not conducted. In addition, optimal antiplatelet treatment duration after covered stent implantation has not been well studied, and no specific recommendations are available. In real-life scenarios CAP often occurs in patients without or delayed dual antiplatelet therapy due to the fear of recurrent bleeding. In our study, no in-stent thrombosis was observed for all the available cases which could be possibly explained by the dual antiplatelet therapy administration during the whole study period. Therefore, our findings should not be extrapolated to a real-world setting and should be confirmed in future studies. Finally, the underlying mechanisms of the antiproliferative effect were not investigated. However, our study provides preliminary data on a novel drug-eluting covered stent that warrants further investigations.
In the present study, the swine coronary perforation mode which was developed using guidewire assisted by micro-catheter seemed to be a controllable and safe CAP model. In this CAP model, implantation of a novel drug-eluting covered stent was proven to be safe and effective with a high device success rate and without any incidence of stent thrombosis or delayed endothelialization. Moreover, the novel drug-eluting covered stent demonstrated a relatively sustained antiproliferative effect up to 6 months post implantation. Our findings need to be confirmed in future studies with a larger sample size and long-term follow-up.
LT had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. YL, JZ, XW and RW contributed to the data collection, analysis and interpretation, and the writing of the manuscript. CG, FM, WY, RW and LT contributed substantially to study design, the data analysis and interpretation, and the critical revision of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The animal study was approved by the Ethics Committee of Xijing Hospital, Fourth Military Medical University (Xi’an, China) (No. IACUC-20150511).
We thank our research staff at the Heart Center of XijingHospital, and the nurses and technologists at the cardiac catheterization laboratories of the Heart Center of Xijing Hospital. We thank Yinyi (Liaoning) Biotech Co., Ltd (Dalian, China) for providing technical support on the design and production of the novel covered stent in our study. Thanks to all the peer reviewers for their opinions and suggestions.
This work was financially supported by the National Key R&D Program of China (Grant No. 2018YFA0107400), Program for National Science Funds of China (Grants No. 82070853, 81730011, 81970721 and 81927805), Program for Changjiang Scholars and Innovative Research Team in University (Grant No. PCSIRT-14R08).
Yinyi (Liaoning) Biotech Co., Ltd (Dalian, China) kindly assisted us on the design and production of the novel covered stent. We declare that Yinyi (Liaoning) Biotech Co., Ltd had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results. The authors declare no conflicts of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
