1. Introduction
The prevalence of chronic kidney disease (CKD) is increasing steadily around the
world, and a “CKD epidemic” is being warned against [1]. As well, the rate of
complications from renal dysfunction in patients with heart disease is also
rising. In recent reports, the proportion of patients with renal dysfunction was
48% for those with coronary artery disease [2], 41% for heart failure with
reduced ejection fraction [3], and 51% for heart failure with preserved ejection
fraction [4]. In fact, about half of all heart disease patients have renal
dysfunction. These patients have lower peak oxygen uptake (peak V̇O) [5],
and it decreases as renal dysfunction progresses [6]. Lower peak V̇O is a
serious problem in this cohort as it is a predictor of cardiovascular events and
mortality [6, 7, 8]. To improve peak V̇O, it is necessary to verify the cause
of the low peak V̇O and take appropriate countermeasures. However, the
factors that influence low peak V̇O in heart disease patients are diverse
[9], and the addition of renal dysfunction further complicates the search for
causative factors [10]. This problem cannot be overlooked in improving the
prognosis of heart disease patients with renal dysfunction. Since the
pathophysiology of renal dysfunction and cardiorenal syndrome differs depending
on the stage of renal dysfunction [11], it is necessary to verify the
determinants of peak V̇O in heart disease patients by stage of renal
dysfunction. On the basis of the above, we hypothesized that the determinants of
peak V̇O in heart disease patients with renal dysfunction depend on the
stage of renal dysfunction. The determinants of peak V̇O are dividing into
the oxygen delivery and oxygen extraction [9, 10]. It has been clarified that the
contributions of oxygen extraction are greater than those of oxygen delivery in
CKD patients [12]. Therefore, in this study, we focused on end-tidal oxygen
partial pressure (PETO), which has been reported to be associated with
renal dysfunction and to show oxygen extraction capacity in skeletal muscle
[13, 14, 15, 16]. The purpose of this study was to verify the determinants of peak
V̇O for each stage of renal function in heart disease patients, including
PETO.
2. Methods
2.1 Study Design and Patients
This was a retrospective, single-center, observational study. From April 2016 to
August 2021, 250 patients with heart disease (defined as myocardial infarction,
angina, and chronic heart failure) who underwent cardiopulmonary exercise testing
(CPET) in our institution were consecutively enrolled in the study. Exclusion
criteria included patients with a resting respiratory exchange ratio (RER)
1.00 due to resting hyperventilation and abnormal breathing [17] and peak
RER 1.10 during CPET [18], AT impossible to determine, and no laboratory
data measured during CPET. Patients’ characteristics and clinical parameters
including age, sex, body mass index, left ventricular ejection fraction (LVEF),
medical history, laboratory values during CPET (estimated glomerular filtration
rate [eGFR (mL/min/1.73 m)], hemoglobin [Hb (g/dL)]), medications, and the
results of CPET were obtained from the electronic medical records by two physical
therapists. Laboratory values at CPET were extracted within 2 weeks around the
date of CPET.
2.2 Definition
eGFR in this study was evaluated with the Japanese version of the following
equation: eGFR = 194 (serum creatinine) – 1.094 age – 0.287
( 0.739 if female) [19].
2.3 Cardiopulmonary Exercise Testing
All patients underwent symptom-limited maximal CPET using a cycle ergometer
(Strength Ergo 8; Mitsubishi Electric Engineering Co., Ltd., Tokyo, Japan) with a
10 watt/min continuous ramp exercise protocol after an initial 3-min rest period
and a 4-min warm-up period. The warm-up wattage was chosen to be 0 watts or 20
watts in consideration of age, sex, cardiac function, and exercise habits. During
CPET, analysis of expired gas was performed with an AE-310S analyzer (Minato
Medical Science, Osaka, Japan). The patients were encouraged to perform a maximal
or near maximal effort by monitoring the RER at 1.10 [18]. Peak V̇Owas defined as the mean value of V̇O during the last 15 s of the test, and
%peak V̇O was also calculated. AT was determined using the V-slope,
ventilatory equivalents, and end-tidal pressure methods based on the statement
from the American Heart Association [17] by at least two experts in CPET.
Resting PETO was determined as the mean value during the last 30 s of the
rest, and AT PETO was the PETO at AT. PETO was the
difference between the resting PETO and AT PETO. Peak oxygen pulse
(peak O pulse), minute ventilation-carbon dioxide production linear
regression slope (V̇E vs. V̇CO slope), and minimum ventilatory equivalent for
carbon dioxide (V̇E/V̇CO) were also obtained. Peak work rate was defined as
the work rate at peak V̇O.
2.4 Statistical Analysis
Patients were stratified according to their eGFR into three clinically
meaningful strata: 45, 45–59, and 60 mL/min/1.73 m [20]. Data
are expressed as mean values standard deviation (SD) or median
(interquartile range) for continuous variables, as appropriate. Normality of
distribution was verified using the Shapiro-Wilk test. Categorical variables are
presented as numbers and percentages. One-way ANOVA test and the Kruskal-Wallis
test were used for comparison between groups, and the test and
Fisher’s exact test were used for comparing categorical variables. We used the
Bonferroni test as post hoc test. Multivariate linear regression analysis was
performed to evaluate independent determinants of peak V̇O after adjusting
for all significant determinants on univariate linear regression analyses. In
addition, resting PETO was also included as a confounding factor to rule
out the effect of resting PETO on PETO. Univariate linear
regression analyses were performed to evaluate the contribution of each
determinant to peak V̇O. A p-value of 0.05 was considered to
indicate statistical significance. The statistical analyses were performed with
EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is
a graphical user interface for R (The R Foundation for Statistical Computing,
Vienna, Austria).
3. Results
Of the 250 heart disease patients who underwent CPET, 49 patients were excluded
because of rest RER 1.00 (n = 6), peak RER 1.10, judgement of AT
impossible (n = 8), and no laboratory data (n = 4). Finally, 201 patients were
enrolled in the analysis. All patients were divided into three groups by eGFR
level: eGFR 45 group (n = 30, 14.9%), eGFR 45–59 (n = 59, 29.4%), and eGFR
60 group (n = 112, 55.7%). Table 1 shows the clinical characteristics
and CPET parameters of the three groups. The patients in the eGFR 45 group
were older and had a higher proportion of chronic heart failure and lower LVEF
and Hb. There was a significant difference in peak V̇O between the three
groups (eGFR 45, 16.2 3.9 mL/min/kg vs. eGFR 45–59, 19.7 4.7
mL/min/kg vs. eGFR 60, 23.0 4.5 mL/min/kg, p 0.002).
PETO decreased with the deterioration of renal function (eGFR
45, 0.1 mmHg vs. eGFR 45–59, 2.4 mmHg vs. eGFR 60, 5.2 mmHg,
p 0.001) (Fig. 1). There was no significant difference in peak RER
and rest PETO between the three groups.
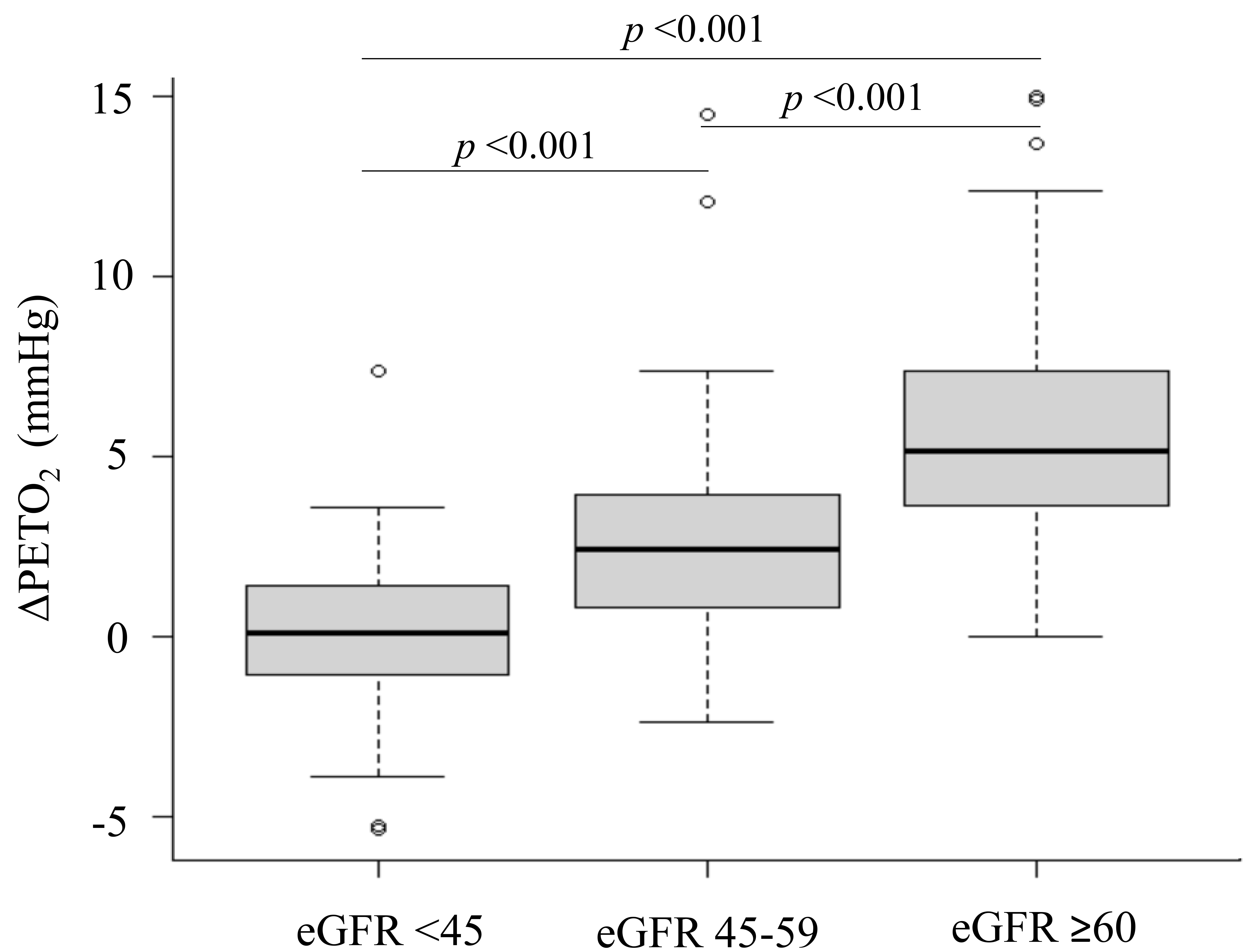 Fig. 1.
Fig. 1.
Comparison of the change in end-tidal oxygen partial pressure
(PETO) at different values of estimated glomerular filtration
rate (eGFR).
Table 1.Patient characteristics and CPET parameters.
|
eGFR 45 |
eGFR 45–59 |
eGFR 60 |
p-value |
|
(n = 30) |
(n = 59) |
(n = 112) |
eGFR 45 vs. eGFR 45–59 |
eGFR 45–59 vs. eGFR 60 |
eGFR 45 vs. eGFR 60 |
| Age, years |
71.4 7.7 |
67.8 7.8 |
61.2 10.8 |
0.300 |
0.001 |
0.0001 |
| Male, n (%) |
29 (96.7) |
50 (84.7) |
104 (92.9) |
0.46 |
0.33 |
1 |
| Body mass index, kg/m |
23.2 2.8 |
23.1 3.2 |
23.8 2.9 |
1 |
0.45 |
0.88 |
| MI, n (%) |
19 (63.3) |
43 (72.9) |
86 (76.8) |
1 |
1 |
0.63 |
| AP, n (%) |
0 (0) |
4 (6.8) |
16 (14.3) |
0.887 |
0.632 |
0.071 |
| CHF, n (%) |
24 (80.0) |
19 (32.2) |
25 (22.3) |
0.001 |
0.666 |
0.001 |
| LVEF, (%) |
51.2 (38.9–54.4) |
58.7 (49.0–65.4) |
59.3 (51.5–68.2) |
0.090 |
0.384 |
0.001 |
| Hypertension, n (%) |
24 (80.0) |
36 (61.0) |
74 (66.1) |
0.35 |
1 |
0.64 |
| Diabetes, n (%) |
17 (56.7) |
20 (33.9) |
36 (32.1) |
0.201 |
1 |
0.073 |
| Laboratory values |
|
|
|
|
|
|
|
eGFR, mL/min/1.73 m |
36.8 (32.2–40.5) |
54.6 (51.7–57.0) |
71.2 (65.2–80.0) |
0.001 |
0.001 |
0.001 |
|
Hemoglobin, g/dL |
12.7 1.8 |
13.3 1.5 |
14.2 1.3 |
0.221 |
0.001 |
0.001 |
| Medications |
|
|
|
|
|
|
|
Beta blockers, n (%) |
23 (76.7) |
45 (77.6) |
72 (64.9) |
1 |
0.38 |
0.95 |
|
ACE-I, n (%) |
8 (26.7) |
9 (15.3) |
33 (29.5) |
0.94 |
0.19 |
1 |
|
ARB, n (%) |
15 (50.0) |
24 (40.7) |
39 (34.8) |
1 |
1 |
0.57 |
|
CCB, n (%) |
9 (30.0) |
6 (10.2) |
21 (18.8) |
0.12 |
0.64 |
0.83 |
|
Diuretics, n (%) |
19 (63.3) |
16 (27.1) |
14 (12.5) |
0.006 |
0.088 |
0.001 |
|
Statin, n (%) |
19 (63.3) |
49 (83.1) |
95 (84.8) |
0.212 |
1 |
0.054 |
| CPET parameters |
|
|
|
|
|
|
|
Peak V̇O, mL/min/kg |
16.2 3.9 |
19.7 4.7 |
23.0 4.5 |
0.002 |
0.001 |
0.001 |
|
%Peak V̇O, % |
70.6 16.4 |
82.8 18.7 |
92.8 19.0 |
0.011 |
0.003 |
0.001 |
|
AT V̇O, mL/min/kg |
10.9 2.1 |
12.4 2.5 |
14.0 2.6 |
0.029 |
0.001 |
0.001 |
|
Peak RER |
1.20 0.05 |
1.20 0.06 |
1.18 0.06 |
1 |
0.086 |
0.382 |
|
AT RER |
0.96 0.02 |
0.96 0.03 |
0.95 0.04 |
1 |
0.14 |
0.11 |
|
Peak WR, watts |
86.2 17.2 |
102.3 28.6 |
122.9 28.3 |
0.028 |
0.001 |
0.001 |
|
V̇E vs. V̇CO slope |
33.9 (30.8–38.5) |
30.6 (27.9–33.5) |
29.2 (26.3–31.7) |
0.007 |
0.137 |
0.001 |
|
Minimum V̇E/V̇CO |
36.1 (33.5–39.9) |
33.9 (30.9–37.4) |
30.8 (28.8–34.5) |
0.173 |
0.002 |
0.001 |
|
Peak O pulse |
8.6 2.0 |
9.6 2.5 |
11.1 2.2 |
0.181 |
0.001 |
0.001 |
|
ΔV̇O/ΔWR |
8.1 1.6 |
8.8 1.4 |
9.4 1.3 |
0.051 |
0.030 |
0.001 |
|
Rest PETO, mmHg |
107.2 5.5 |
107.8 4.9 |
108.1 4.2 |
1 |
1 |
0.88 |
|
AT PETO, mmHg |
107.1 5.4 |
105.0 5.5 |
102.4 4.7 |
0.216 |
0.004 |
0.001 |
|
ΔPETO, mmHg |
0.1 (–1.1–1.4) |
2.4 (0.8–4.0) |
5.2 (3.7–7.4) |
0.001 |
0.001 |
0.001 |
| CPET, cardiopulmonary exercise testing; eGFR, estimated glomerular filtration
rate; MI, myocardial infarction; AP, angina pectoris; CHF, chronic heart failure;
LVEF, left ventricular ejection fraction; ACE-I, angiotensin converting enzyme
inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker;
V̇O, oxygen uptake; AT, anaerobic threshold; RER, respiratory exchange
ratio; WR, work rate; V̇E, expiratory minute volume; V̇CO, carbon dioxide
output; V̇E/V̇CO, ventilatory equivalent for carbon dioxide; O, oxygen;
PETO, end-tidal oxygen partial pressure. Values shown are % (n), mean
standard deviation, or median (interquartile range). |
The results of univariate and multivariate linear regression analysis in all
subjects showed that age ( = –0.142, p = 0.023), LVEF
( = 0.150, p = 0.006), eGFR strata (
= 0.154, p = 0.026), Hb ( = 0.167, p =
0.005), and PETO ( = 0.356, p
0.001) were significantly associated with peak V̇O (Table 2).
Table 2.Univariate and multivariate linear regression analyses for peak
V̇O in all subjects.
|
Univariate |
Multivariate |
| β |
p-value |
β |
95% CI |
p-value |
| Age |
–0.451 |
0.001 |
–0.142 |
–0.128, –0.009 |
0.023 |
| LVEF |
0.113 |
0.001 |
0.150 |
0.018, 0.110 |
0.006 |
| eGFR strata |
0.508 |
0.001 |
0.154 |
0.128, 2.000 |
0.026 |
| Hb |
0.148 |
0.001 |
0.167 |
0.164, 0.914 |
0.005 |
| ΔPETO |
0.552 |
0.001 |
0.356 |
0.308, 0.690 |
0.001 |
| Rest ΔPETO |
0.121 |
0.020 |
–0.194 |
–0.335, –0.092 |
0.001 |
| R |
|
|
|
|
0.462 |
| V̇O, oxygen uptake; CI, confidence interval; LVEF, left ventricular
ejection fraction; eGFR, estimated glomerular filtration rate; Hb, hemoglobin;
PETO, end-tidal oxygen partial pressure. |
The results of univariate and multivariate linear regression analyses differed
between the eGFR strata. In the eGFR 45 group, LVEF and Hb were significantly
associated with peak V̇O ( = 0.518, p 0.001
and = 0.567, p 0.001, respectively). In the eGFR
45–59 group, age, Hb, and PETO showed a significant association
with peak V̇O ( = –0.354, p = 0.006;
= 0.258, p = 0.007; =
0.501, p 0.001; respectively). In the eGFR 60 group,
PETO was significantly associated with peak V̇O ( = 0.308, p = 0.003) (Table 3).
Table 3.Univariate and multivariate linear regression analyses for peak
V̇O by eGFR strata.
|
Univariate |
Multivariate |
|
|
p-value |
|
95% CI |
p-value |
| eGFR 45 group |
|
|
|
|
|
|
Age |
–0.358 |
0.052 |
|
|
|
|
LVEF |
0.572 |
0.001 |
0.518 |
0.086, 0.229 |
0.001 |
|
Hb |
0.616 |
0.001 |
0.567 |
0.728, 1.766 |
0.001 |
|
ΔPETO |
0.175 |
0.356 |
|
|
|
|
Rest ΔPETO |
0.121 |
0.059 |
|
|
|
| R |
|
|
|
|
0.620 |
| eGFR 45–59 group |
|
|
|
|
|
|
Age |
–0.521 |
0.001 |
–0.354 |
–0.297, –0.052 |
0.006 |
|
LVEF |
0.183 |
0.166 |
|
|
|
|
Hb |
0.365 |
0.004 |
0.258 |
0.241, 1.449 |
0.007 |
|
ΔPETO |
0.523 |
0.001 |
0.501 |
0.402, 1.013 |
0.001 |
|
Rest ΔPETO |
0.062 |
0.058 |
–0.181 |
–0.384, –0.016 |
0.035 |
| R |
|
|
|
|
0.538 |
| eGFR 60 group |
|
|
|
|
|
|
Age |
–0.236 |
0.012 |
–0.215 |
–0.140, 0.017 |
0.125 |
|
LVEF |
0.198 |
0.036 |
0.146 |
–0.014, 0.137 |
0.113 |
|
Hb |
0.078 |
0.416 |
|
|
|
|
ΔPETO |
0.314 |
0.001 |
0.308 |
0.154, 0.716 |
0.003 |
|
Rest ΔPETO |
0.036 |
0.045 |
–0.193 |
–0.154, –0.013 |
0.037 |
| R |
|
|
|
|
0.194 |
|
V̇O, oxygen uptake; CI, confidence interval; eGFR, estimated glomerular
filtration rate; LVEF, left ventricular ejection fraction; Hb, hemoglobin;
PETO, end-tidal oxygen partial pressure. |
Fig. 2 summarizes the coefficients of determination of age, LVEF, Hb, and
PETO for peak V̇O by eGFR level. In the eGFR 45–59 group,
the coefficient of determination for peak V̇O was higher in age and
PETO than in the other groups (R = 0.241,
p 0.001; R = 0.247, p 0.001;
respectively). The eGFR 45 group showed higher coefficients of determination
for peak V̇O in LVEF and Hb than in the other groups (R =
0.327, p 0.001; R = 0.380, p 0.001;
respectively). The p value for interaction analysis of the slope
difference was 0.001.
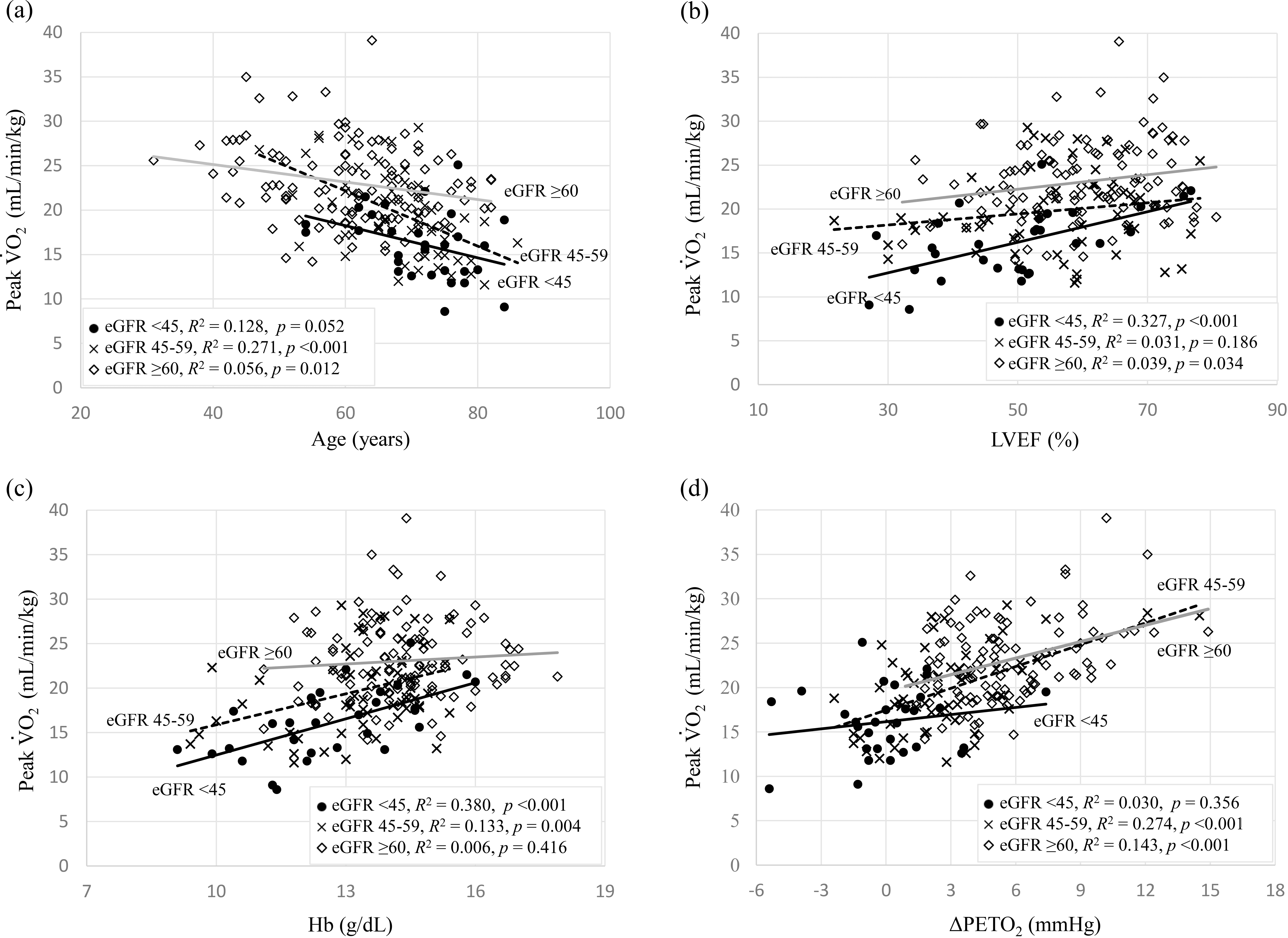 Fig. 2.
Fig. 2.
Coefficients of determination for peak V̇O for each group. Coefficients of determination of the (a) age, (b) left
ventricular ejection fraction (LVEF), (c) hemoglobin (Hb), and (d) change in
end-tidal oxygen partial pressure (PETO) to peak V̇O for
each group.
4. Discussion
This study revealed that the determinants of peak V̇O depend on the stage
of renal function in heart disease patients. In the group with eGFR 45, the
determinants of peak V̇O were LVEF and Hb. In the group with eGFR 45–59,
PETO was the most influential determinant of peak V̇O. As a
result of examining the determinants of peak V̇O in all subjects, age, LVEF,
Hb, and PETO were all determinants independently of eGFR strata.
Thus, the present study clarified that in patients with heart disease with renal
dysfunction, it is necessary to investigate the determinants for each level of
renal dysfunction. The decrease in peak V̇O is observed in the stage of mild
renal dysfunction [21]. In this study as well, peak V̇O was significantly
decreased even in the eGFR 45–59 group. In this group, age, Hb and
PETO were the determinants of peak V̇O, and both oxygen
delivery capacity and oxygen extraction capacity affected peak V̇O in this
group. Among these factors, multivariate analysis showed that
PETO had the highest for peak V̇O. Furthermore, in
the univariate analysis, this group showed the highest contribution of
PETO to peak V̇O.
PETO reflects the oxygen extraction capacity of skeletal muscle during
incremental exercise up to AT [13, 14], it is also reported to reflect
mitochondrial oxygen uptake [15]. Although the subjects of these prior studies
were mainly healthy individuals, in our study of patients with myocardial
infarction, PETO at AT was affected by abnormal ventilation, whereas
PETO from rest to AT reflected peripheral factors of peak
V̇O [16].
Therefore, it is highly possible that PETO represents the oxygen
extraction capacity of skeletal muscle, that is, mitochondrial function. This
study showed that PETO decreased as renal dysfunction progressed.
Heart disease patients have decreased mitochondrial function due to oxidative
stress, inflammation, and insulin resistance due to heart disease [22, 23]. In
addition, heart disease risk factors such as hyperglycemia, hyperlipidemia, and
smoking also reduce mitochondrial function [24]. With the addition of renal
dysfunction in these patients, oxidative stress, inflammation, and uremic toxins
from the renal dysfunction cause further mitochondrial dysfunction [25, 26, 27].
Furthermore, in heart failure patients with renal dysfunction, the relation
between the cardiac and renal dysfunction of cardiorenal syndrome, which
adversely affect each other [11], may contribute to a further decline in
mitochondrial function. A report that mitochondrial dysfunction worsens as renal
dysfunction progresses also supports this result [28].
The most interesting finding in this study was that although
PETO, which represents oxygen extraction capacity, decreased as
renal dysfunction progressed, PETO was not a determinant of peak
V̇O in the eGFR 45 group. As peak V̇O is composed of the product of
oxygen delivery capacity times oxygen extraction capacity, there is no doubt that
a decrease in oxygen extraction capacity will lead to a decrease in peak
V̇O. However, in this study, the determinants of peak V̇O in the eGFR
45 group were LVEF and Hb, which are mainly related to oxygen delivery
capacity. In this regard, as the eGFR 45 group had significantly lower LVEF
and Hb than the other two groups, decreased oxygen delivery capacity may be the
main contributor to the decrease in peak V̇O. A previous study also reported
that LVEF is not a determinant of peak V̇O [29]. However, LVEF in the
present study was the determinant in heart disease patients with moderate to
severe renal dysfunction. The mechanism for this is unknown, but it may be a
characteristic of heart disease patients with an eGFR 45. This result also
contrasted with recent reports that low oxygen extraction capacity is the main
factor for low peak V̇O in CKD patients [5, 12]. The PETO of
the eGFR 45 group was very low at 0.1 mmHg, and it is estimated that oxygen
extraction of skeletal muscle would hardly increase during incremental exercise
in these patients. As skeletal muscle oxygen extraction cannot be increased, it
may be necessary for these patients to rely on oxygen delivery to increase oxygen
uptake. Thus, this may be the reason why the only determinants of peak V̇O
were the factors related to oxygen delivery capacity. This also supports the
finding that the contribution of LVEF and Hb to peak V̇O in this group was
higher than that in the other groups. The effects of exercise training aimed at
improving mitochondrial function and oxygen extraction capacity to improve peak
V̇O have been reported [30], but in heart disease patients with eGFR 45,
such interventions may not lead to improvement in peak V̇O. Improving oxygen
delivery capacity may be more important. Further verification is needed on
interventions to improve peak V̇O in this group.
Heart disease patients with eGFR 45 experience increased cardiovascular
events [31]. One of the causes is suggested to be that cardiac load is increased
due to the abnormally low value of oxygen extraction capacity being compensated
for by oxygen delivery capacity.
Regarding the clinical implication of this study, the first was that decrease in
PETO with the progression of renal dysfunction revealed that the
oxygen extraction capacity of skeletal muscle decreased as renal dysfunction
progressed. Second, in the eGFR 45 group, PETO was not a
determinant, and the determinant of peak V̇O was different depending on the
degree of renal dysfunction. Therefore, intervention strategies for improving
peak V̇O in heart disease patients should be considered for each stage of
renal dysfunction. The effects of exercise training aimed at improving
mitochondrial function and oxygen extraction capacity to improve peak V̇O
have been reported [31], but in heart disease patients with eGFR 45, such
interventions may not lead to improvement in peak V̇O. Improving oxygen
delivery capacity may be more important. A meta-analysis has been reported that
Fe therapy improved peak V̇O in patients with heart failure with reduced EF
[32]. Further verification is needed on interventions to improve peak V̇O in
this group. On the other hand, in heart disease patients with eGFR 45–59,
interventions that improve skeletal muscle oxygen extraction, i.e., mitochondrial
function, may be effective. In recent years, it has been reported that exercise
improves mitochondrial function in heart disease patients [33, 34].
Study Limitations
This study has several limitations. First, this was a single-center,
retrospective study consisting of a relatively small number of patients. Second,
there is potential selection bias as patients who were unable to undergo CPET due
to frailty and sarcopenia were excluded. Because these factors themselves are
associated with mitochondrial dysfunction [35, 36], further investigation of
these patients is needed. Third, eGFR calculated with serum creatinine is
affected by skeletal muscle mass and may not accurately reflect renal function
[37]. Fourth, renal dysfunction is classified into acute kidney injury, CKD, and
worsening renal function [38]. Further studies are needed to determine whether
each clinical status may have different effects on skeletal muscle oxygen
extraction capacity. Fifth, there was a significant difference in the etiology
between the groups. Future studies will need to be validated for association with
etiology. Finally, we could not evaluate cardiac output, vascular function
including that of the capillaries, and skeletal muscle mass, which are additional
determinants of peak V̇O.
5. Conclusions
PETO, which indicates the oxygen extraction capacity of skeletal
muscle, decreased with the progression of renal dysfunction. In the eGFR 45–59
group, PETO was the strongest determinant of peak V̇O, but
the determinants in the eGFR 45 group were LVEF and Hb, and
PETO was not included. This study suggests that intervention
strategies should be considered for each stage of renal dysfunction to improve
peak V̇O in heart disease patients.
Abbreviations
CKD, chronic kidney disease; CPET, cardiopulmonary exercise testing; eGFR,
estimated glomerular filtration rate; LVEF, left ventricular ejection fraction;
peak V̇O, peak oxygen uptake; PETO, end-tidal oxygen partial pressure;
RER, respiratory exchange ratio; V̇CO, carbon dioxide production; V̇E, minute
ventilation.
Author Contributions
AO—Conceptualization, Methodology, Formal analysis, Investigation,
Writing-Original Draft. KPI—Conceptualization, Methodology, Writing-Review &
Editing, Supervision. SS—Conceptualization, Methodology. HT—Investigation,
Writing-Review & Editing. FK—Investigation, Writing-Review & Editing.
MW—Formal analysis, Investigation. MK—Writing-Review & Editing.
IK—Writing-Review & Editing. RY—Supervision. YM—Project administration.
All authors approved the manuscript for submission.
Ethics Approval and Consent to Participate
This study complied with the Declaration of Helsinki with respect to
investigation in humans and was approved by the Ethics Committee of Sanda City
Hospital (approval number: 2021009). Written informed consent was obtained from
each patient.
Acknowledgment
The authors would like to thank all of the participating patients in Sanda City
Hospital. We thank the staff members of Kobe University who collaborated in this
study. This study was also benefitted by the support and encouragement of Ryo
Yoshihara of the Faculty of Health Sciences, Kobe University, and by Kodai
Ishihara, Masahiro Kitamura, Yuji Kanejima, Masato Ogawa, and Shinichi Shimada,
all of the Graduate School of Health Sciences, Kobe University. We also thank Dr.
Minato Nakazawa, Department of Public Health, Graduate School of Health Sciences,
Kobe University, for statistical support of the present study.
Funding
This work was supported by JSPS KAKENHI Grant Number JP22K11392.
Conflict of Interest
The author declares no conflict of interest. Kazuhiro P. Izawa is serving as one
of the guest editor of this journal. We declare that Kazuhiro P. Izawa had no
involvement in the peer review of this article and has no access to information
regarding its peer review. Full responsibility for the editorial process for this
article was delegated to Brian Tomlinson.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2.